Abstract
Recently, two Drosophila melanogaster models of infection, fly feeding and fly nicking, have been developed that allow a determination of pathogenic potential of Pseudomonas aeruginosa isolates. In this study, control strains, isolates from burn wounds, and isolates from the sputa of cystic fibrosis (CF) patients were used to compare the two infection models to determine whether any of the isolates might be better adapted to either of the models. In addition, our goal was to determine the variability of isolates from individual CF patients. Three of four control strains (PAO1, PAK, and PA14) caused significant mortality in the flies in both models of infection. The remaining control strain, PA103, was lethal to flies in the nicking model but lacked significant lethality in the feeding model. The burn wound isolates had a high level of lethality in both models. Interestingly, the CF isolates had the largest diversity of lethality in both models of infection. The range of pathogenic potentials of the CF isolates occurred across a cohort of patients, both at the patient level and down to the level of individual sputum samples. The majority of all isolates had similar levels of lethality in both fly infection models. However, two CF isolates were significantly more lethal in the nicking model, and three CF isolates were significantly more lethal in the feeding model. In conclusion, the two Drosophila infection models were useful for the analysis of the diversity of pathogenic potentials of P. aeruginosa isolates.
Pseudomonas aeruginosa is a ubiquitous opportunistic pathogen that can infect virtually any tissue (26). It is an important pathogen in lung infections, especially the chronic lung infections associated with cystic fibrosis (CF), burn wound infections, and eye infections (26). In CF patients P. aeruginosa accounts for nearly 60% of the lung infections (1) and contributes significantly to the morbidity and mortality of the disease (11). Individuals as young as 3 years of age can acquire P. aeruginosa, resulting in the obstruction of small airways and bronchiolar inflammation (4). Once the lungs are colonized, the infections tend to become chronic in nature.
P. aeruginosa isolates from early CF infections are similar to environmental isolates in phenotype. However, P. aeruginosa isolates from chronic CF lung infections are phenotypically quite distinct from those causing acute infections in other settings (29). The phenotypic variation of CF isolates may arise from the development of mutator strains during the chronic infection (30) or as a result of adaptation to the harsh environmental conditions of the lung (38). The specific adaptations by P. aeruginosa to the CF lung environment include the ability to grow as a biofilm (20), development of a mucoid phenotype (17), and the loss of virulence (38). Other phenotypic variation can also occur. For example, isolates from prolonged infections are more resistant to antibiotics (17); there is the loss of O-side chains on lipopolysaccharide, making the strains nonreactive with typing sera (14); a distinctive acylation of lipopolysaccharide (14); and the loss of flagellum-dependent motility (6, 27). These characteristics appear to be selected within CF airways and tend to occur increasingly with long-term infections.
Genotypic diversity has also been observed in CF isolates. For instance, the presence of multiple genotypes of P. aeruginosa and the persistence of the same bacterial lineage with some variation has been well documented in chronic infections of CF patients (34). Recent studies suggest that this bacterial genotypic diversity may be due to conditions in the lung which select for loss-of-function mutations (38). Taken together, these phenotypic and genotypic changes may allow P. aeruginosa to adapt in order to persist in the lungs of patients with CF.
There have been a number of different infection models adapted to the study of P. aeruginosa pathogenesis. The most commonly used animal models include the mouse and the rat, which mimic either chronic or acute infections (5, 39). Alternative model hosts have also been explored. A positive correlation between insect and mouse models for studying P. aeruginosa virulence was found, suggesting that insect models are a reasonable alternative to traditional animal models (19). Boman et al. (3) was the first to demonstrate that P. aeruginosa is exceptionally virulent in Drosophila melanogaster (3); subsequently, the model has been adapted to study the bacterium's pathogenic potential (9). There have been two different models of bacterial infection used in D. melanogaster. The fly nicking model, which may represent an acute infection, involves pricking flies with a needle dipped into bacterial culture. Subsequently, the bacteria grow exponentially even after fly death has occurred (9). The second model, fly feeding, involves feeding starved flies with bacterial cultures and represents a longer-term infection model (8). Our hypothesis was that P. aeruginosa isolates from chronic CF lung infections may be better adapted to the fly feeding model than the fly nicking model since the former may be a slower and more drawn out infection. Furthermore, we hypothesized that isolates from acute infections, such as burn wounds, might be better adapted to the fly nicking model of infection. The present study assessed the infectivity of P. aeruginosa control strains, CF isolates, and burn wound isolates in the two D. melanogaster infection models in order to determine the variability of pathogenic potential between the different isolates.
MATERIALS AND METHODS
Strains and growth conditions.
P. aeruginosa strains and isolates were cultured with aeration at 37°C in Luria-Bertani (LB) broth or brain heart infusion (BHI) broth (Difco). Strains used in the present study were PAO1, the prototypical strain (18); PAK (41); ATCC 27853, a blood culture isolate from a burn wound infection (28); and Utah3 and Utah4, which were isolated from invasive burn wound infections. PA14, a human isolate, was initially designated UCBPP-PA14 and is virulent in both a mouse full-skin-thickness burn infection model and an Arabidopsis thaliana leaf infiltration model (32). PA103, a hypertoxigenic strain, was initially isolated by Liu (25). CF isolates were provided courtesy of the University of Calgary Medical Clinic or the Adult Cystic Fibrosis Clinic at the Foothills Hospital, Calgary, Alberta, Canada. Voluntary consent was given by all patients for participation in these studies. Approval of the design and purpose of the present study was granted by the Conjoint Research Ethics Board of the University of Calgary. CF P. aeruginosa isolates 4384, 5154, and 5166 were previously reported by Raivio et al. (33); isolates 5552, 5585, and 5588 were previously reported by Gallant et al. (15) and isolates 6106 and 14655 were previously reported by Erickson et al. (12).
Maintenance of D. melanogaster.
D. melanogaster Oregon R flies were maintained as described in Chugani et al. (8). Flies were maintained at 27°C on standard cornmeal agar.
D. melanogaster feeding assays.
D. melanogaster flies were infected orally as described by Chugani et al. (8). For fly feeding assays, flies were anesthetized by using a cold tile; 3- to 5-day-old male flies were then separated into vials (10 per vial) and starved without food or water for 5 h. In parallel, 2.3-cm Whatman filter disks were placed into fly vials containing 6 ml of 5% sucrose agar. After overnight growth in BHI, 2 ml of bacterial culture was normalized to an optical density at 600 nm of 3.0 and centrifuged, and the resulting pellet was resuspended in 175 μl of sterile 5% sucrose and then added onto filters in the sucrose feeding vials. Vials inoculated with 175 μl of 5% sucrose were used as negative controls in each experiment. Starved flies were transferred to the sucrose feeding vials containing bacterial suspensions and incubated at 27°C with moderate humidity. Fly mortality was monitored daily for 14 days, and each isolate was tested in triplicate.
D. melanogaster nicking assays.
Healthy 2- to 4-day-old female flies were used in the fly nicking assays according to a modified method of D'Argenio et al. (9). Flies were anesthetized and separated on a cold tile. The female flies were carefully nicked in the dorsal thorax with a 27.5-gauge needle (BD Biosciences) which was dipped in bacterial culture normalized to an optical density at 600 nm of 1.0 in BHI broth. After nicking, 10 flies were placed into each vial of standard cornmeal medium and maintained at room temperature. Each isolate was tested in triplicate. Fly survival was monitored and recorded from 12 to 36 h postinoculation.
Viable bacterial counts.
Bacterial infection was monitored throughout D. melanogaster fly feeding experiments. For enumeration of viable bacteria in fly feeding assays, control sucrose vials, and test vials inoculated with P. aeruginosa strains/isolates were harvested every 2 days up to 14 days for the duration of the infection. Flies were washed four times in 50 ml of 50% LB medium and then homogenized by using pestles (Diamed) and a cordless motor (VWR). The homogenate was resuspended in 1 ml of LB medium. Serial dilutions of the homogenate were made, and 30 μl was plated onto LB agar. Filters from the vials were removed, placed in 5 ml of LB medium, and vortex mixed for 15 s; the LB medium was then serially diluted, and 30 μl was plated onto LB agar. After incubation at 37°C for 24 h, the colonies appearing on the plates were counted.
Cluster analysis.
Cluster 3.0 was used to assign CF isolates, burn wound isolates and control strains into groups based on the percentage of fly survival at each time point (10). For each time point in fly feeding and fly nicking experiments, the data for all isolates was averaged and used as a baseline for comparison. Uncentered complete clustering was performed with a GWEIGHT of 0.5 for fly feeding and 0.25 for fly nicking. GWEIGHT in this instance takes into account the greater number of time points in the fly nicking experiment than in the fly feeding experiment. Java Treeview 1.1.1 was used to visualize the cluster alignments (35).
Statistics.
Survival curves were generated and analyzed by using the log-rank test of Prism 3 software (GraphPad Software, Inc.).
RESULTS
Comparison of P. aeruginosa strains in two models of Drosophila infection.
The Drosophila feeding and nicking models of infection were used to evaluate the pathogenic potential of P. aeruginosa strains. The models are distinct. For instance, in the feeding model the bacteria have to be able to cross the gut barrier and the infection is slower to develop (time to death measured over 14 days). In contrast, in the nicking model the surface of the fly is breached and so the infection proceeds more quickly than the fly feeding model (time to death measured over 36 h). Hence, we speculated that in both models the bacteria have to circumvent the innate immune systems of the fly, but the flies may respond differently depending on how the bacteria are presented.
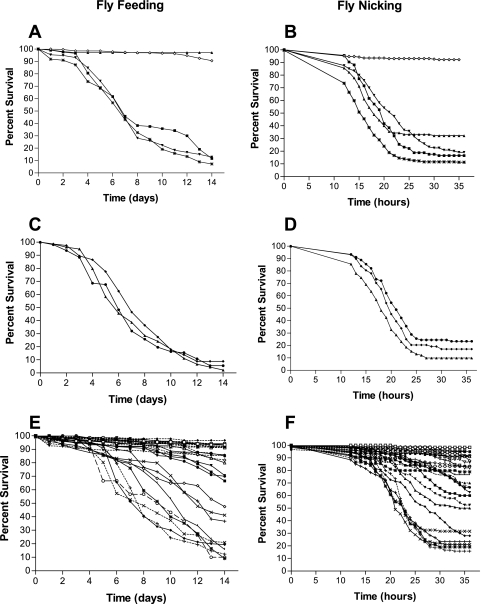
In order to assess the infectivity of each isolate, the percent survival of each P. aeruginosa strain was averaged at each time point to generate survival curves for comparison. Figure 1A showed that for strains PAO1, PAK, and PA14 there was not a significant difference between the survival curves in the fly feeding model (P = 0.2262). In contrast, there was a significant difference between the survival curves for the control strains PAO1, PAK, and PA14 and control strain PA103, since PA103 showed very little ability to kill the flies (Fig. 1A) (P < 0.0001). In the fly nicking model (Fig. 1B) the control strains (PAO1, PAK, PA14, and PA103) all displayed an ability to kill the flies but had significantly different survival curves (P < 0.0001). In general, except for strain PA103, the control strains showed a pronounced ability to kill flies in both models.
FIG. 1.
Survival curves for D. melanogaster flies challenged with Pseudomonas isolates. D. melanogaster fly feeding is shown in panels A, C, and E, and fly nicking is shown in panels B, D, and F. The percent survival of PAO1 (*), PAK (▪), PA14 (▾), PA103 (▴), and medium control (⋄) was graphed over time in days (A) and hours (B). The Pseudomonas strains from acute infections graphed in panels C and D are Utah4 (▴), Utah3 (⧫), and ATCC 27853 (•). In panels E and F, the percent survival values for the CF isolates are indicated as follows: 14649 (*), 14673 (⧫), 14671 (⋄), 7307 (▪), 14684 (▴), 14670 (•), 14716 (□), 14683 (▵), 5588 (▾), 5552 (-⧫-), 14715 (▾), 5585 (○), 14661 (-×-), 14690 (-+-), 14703 (▪), 14651 (-•-), 14656 (-□-), 14655 (-▿-), 14672 (-▾-), 14685 (-▴-), 4384 (×), 14660 (-⋄-), 14717 (-*-), 14650 (|), 5166 (+), 5154 (-○-), and 6106 (-|-). Symbols without dashes indicate symbols connected by a solid line on the graph, and symbols with dashes indicate symbols connected by a dashed line on the graph. Fly mortality was measured hourly in the fly nicking assays and daily in the fly feeding assays using sets of 30 flies. The data are representative of the means of three or more replicate experiments performed in triplicate.
Human burn wound isolates ATCC 27853, Utah3 and Utah4 were all obtained from highly invasive infections. Their survival curves in the fly feeding model were not significantly different (P = 0.239), showing a pronounced ability to kill flies (Fig. 1C). In the fly nicking model (Fig. 1D) the burn wound isolates all displayed some ability to kill the flies, but the survival curves were significantly different from each other (P = 0.0092).
A greater diversity in the ability to kill flies in both models was observed among the CF isolates compared to the other strains tested (Fig. 1). In the fly feeding model, we observed that the CF isolates formed five groups based on their survival curves (Fig. 1E). The groups ranged from one with essentially no killing to a group that killed >80% of the flies. Within each of the groups there was not a significant difference among the survival curves of the isolates, but between all groups there were significant differences. In the fly nicking model, the survival curves for some CF isolates, such as isolates 14690, 14649, 14661, and 4384, were not significantly different (P = 0.3661) (Fig. 1C). However, the CF isolates overall showed much greater diversity in terms of their survival curves in the fly nicking model.
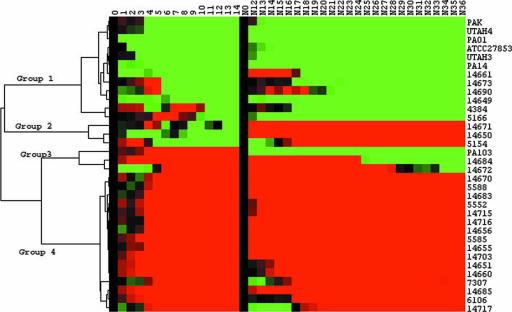
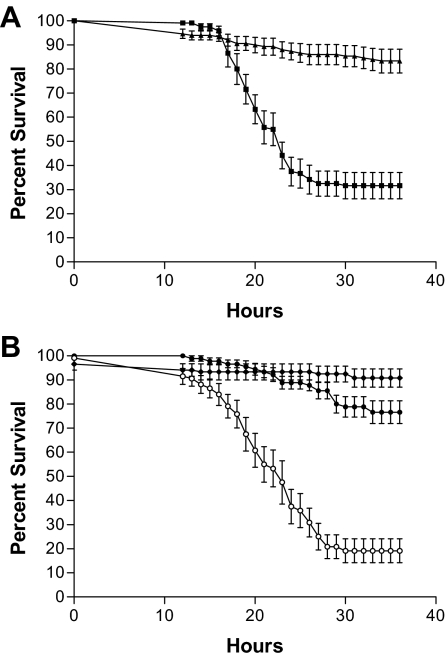
Strain PA103 exhibited distinct survival profiles in each of the infection models (Fig. 1A and B). As such, we wanted to analyze how the isolates performed in each model to determine whether trends might exist among the isolates. To carry out this analysis, we first averaged the results for all strains (control, burn wound, and CF isolates) in each infection model. This baseline average was used to separate fly survival for each isolate at every time point as either above, below, or at the baseline average level. These results were used in cluster analysis to further assess each isolate's ability to kill flies in both models (Fig. 2). This analysis separated the strains into four distinct groupings (Fig. 2). Group 1 consisted of isolates that elicited above-average lethality (less-than-average fly survival) in both infection models. This group included strains PAO1, PAK, and PA14; the burn wound isolates; and six CF isolates. Group 2 was made up of three CF isolates (14671, 14650, and 5154) that elicited above-average lethality in the fly feeding model and less-than-average lethality in the fly nicking infection model. Figure 3 shows a comparison of the survival curves for each group 2 isolate in the two models. Group 3 consists of isolates that elicited above-average lethality in the fly nicking infection model and less-than-average lethality in the fly feeding model (Fig. 2). Strain PA103 and CF isolates 14684 and 14672 fall into this group. Group 3 isolates elicited <15% lethality in the feeding model but, in contrast, showed >40% lethality when used to challenge D. melanogaster in the fly nicking model (ca. 50% survival) (Fig. 4). Group 4 consists solely of CF isolates that demonstrate less-than-average lethality (greater-than-average survival) in both models (Fig. 2). This group also contained 11 of 16 isolates which had <10% killing in one or both models of infection. The majority (28 of 34) of all isolates and strains examined fell into groups 1 or 4; in other words, these isolates showed no preference for either Drosophila infection model, since they demonstrated similar fly lethality in both models.
FIG. 2.
Cluster analysis of fly lethality. Survival curves of Drosophila infected with Pseudomonas are compared by using Cluster 3.0 and visualized by using Java Treeview 1.1.1. The average fly survival of all strains, in the present study, was used as the baseline for comparison. Green boxes indicate where fewer flies were alive compared to the average, red boxes indicate where more flies were alive than the average, and black boxes indicate that the number of flies alive were equal to the average. Four groups were identified in clustering; isolates that had above-average lethality in both models (group 1), above-average lethality in fly feeding and less-than-average lethality in fly nicking (group 2), and below-average lethality in fly feeding and above-average lethality in fly nicking (group 3) and isolates that had less-than-average lethality in both models (group 4).
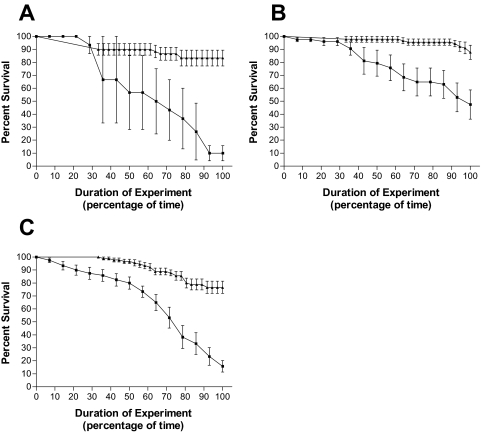
FIG. 3.
Pseudomonas CF isolates in cluster group 2 exhibit above-average lethality in the fly feeding model. D. melanogaster survival curves plotted against percentage of time in the experiment for challenge with CF isolates 5154 (A), 14671 (B), and 14650 (C). Results for fly feeding experiments (▪) and fly nicking experiments (▴) are shown. The data are representative of assays performed three times in triplicate, with error bars representing the standard error of the mean.
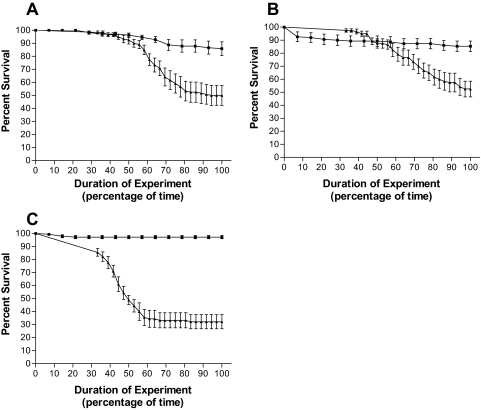
FIG. 4.
Pseudomonas CF isolates in cluster group 3 exhibit above-average lethality in the fly nicking model. Survival curves are shown for D. melanogaster when challenged with isolates 14684 (A), 14672 (B), and PA103 (C). Results for fly feeding experiments (▪) and fly nicking experiments (▴) are shown. Survival curves were plotted as a percentage of time since the duration of each experiment was different. The data are representative of assays performed three times in triplicate, with error bars representing the standard error of the mean.
Fly survival on the filters during the Drosophila feeding model.
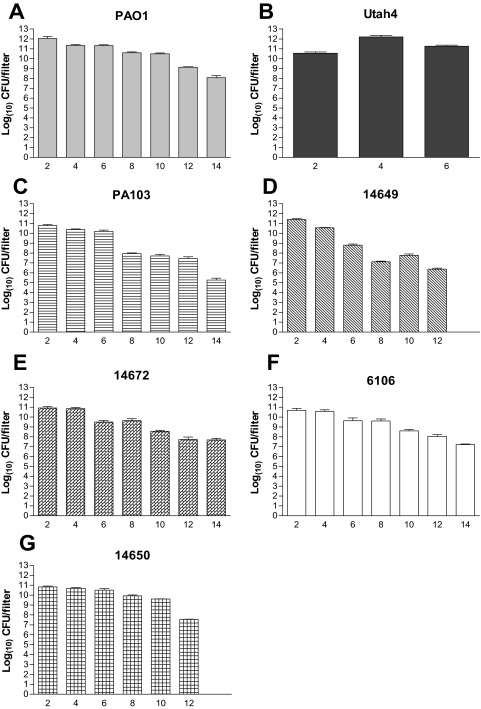
Differences in the isolates' ability to kill flies in the feeding model may have been due to an altered ability of the isolates to survive on the filters during the experiment. To address this possibility, we assayed filter survival of three highly lethal isolates (PAO1, Utah4, and 14649), two less-lethal isolates (6106 and 14672), and three isolates that exhibited different levels of lethality between the two models (PA103, 14650, and 14671). In all strains but one, a decrease of at least 101 CFU per filter in viability over the 14-day period of the experiment was seen (Fig. 5). The highly lethal isolates PAO1, 14649, and 14650 showed decreases of 104, 103, and 105 CFU per filter, respectively (Fig. 5A, D, and G). Likewise, the less lethal strains PA103 and 6106 showed 105 and 103 decreases, respectively, in cell numbers on the filters over a 14-day period (Fig. 5C and F). The one exception was Utah4, which showed very consistent numbers of bacteria on the filters for 6 days (Fig. 5B). However, on the eighth day all of the flies had died, so the experiment was not continued for this isolate. Overall, these results suggested that the differences in the survival of the isolates on the filters could not account for the differences in fly killing among isolates, since strains from each of the four groups from Fig. 2 were able to maintain similar filter colonization.
FIG. 5.
Pseudomonas survival on sucrose filters in fly feeding assays. Viable bacterial counts per filter were determined for bacterial filters on sucrose agar every second day. Strains: PAO1 (A), Utah4 (B), PA103 (C), 14649 (D), 14672 (E), 6106 (F), 14650 (G). The data are representative of the assay performed twice in triplicate, with the standard error of the mean graphed as error bars.
Establishing bacterial colonization in the D. melanogaster fly feeding model.
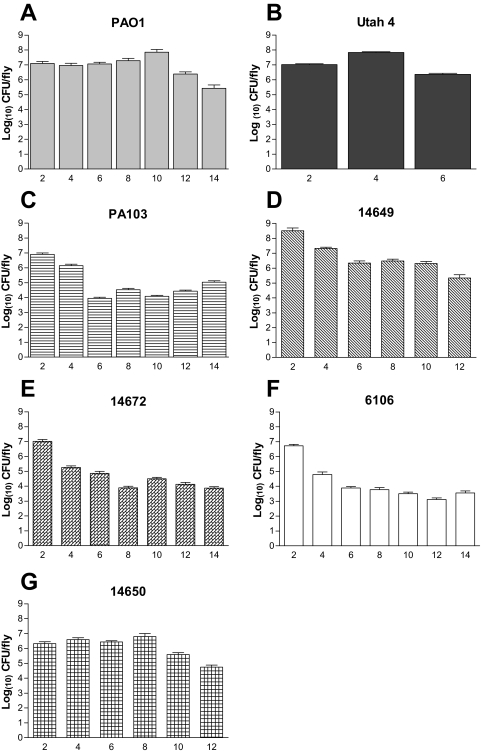
Since the survival of bacteria on the filters was similar, the bacterial load in the flies was also monitored. Bacterial CFU per fly was assessed at the same intervals as described above to determine the bacterial load during infection. Figure 6 shows that for all representative isolates and strains significant P. aeruginosa loads were detected until the experimental endpoint was reached or all of the flies had died. The isolates (PA103, 14672, and 6106) that elicited below-average lethality in the feeding model exhibited an overall drop in the bacterial load per fly (Fig. 6C, E, and F). In particular, by day 4 the bacterial load of these strains had dropped significantly compared to day 2 (P < 0.001) and remained lower than bacterial counts of the strains that tended to induce greater than average lethality in the flies (Fig. 6C, E, and F). The trend for the group 1 strains (PAO1, 14649, and 14650 [Fig. 6A, D, and G]) was that the bacterial load per fly remained at a higher level until the twelfth day of the experiment. The bacterial loads in all three of these strains appeared to drop for the last 2 days (Fig. 6A, D, and G). These results demonstrate that PAO1 and some of the CF isolates were able to establish an infection in Drosophila and maintain at least 106 bacteria per fly, whereas the strains that elicited below-average lethality exhibited a decrease in the levels of bacteria in the flies. The burn wound isolate Utah4 had a more consistent bacterial load of about 106 to 107 bacteria per fly but killed the flies early in the experiment such that the experiment could not be continued after the sixth day of infection. These results indicated that the isolates that were more lethal to the flies (group 1) maintained higher bacterial loads per fly. However, the isolates that were less lethal to the flies were still able to maintain a considerable bacterial load in the flies.
FIG. 6.
Pseudomonas viability in Drosophila during fly feeding assays. Viable bacterial counts were determined for fly homogenates every second day. Strains: PAO1 (A), Utah4 (B), PA103 (C), 14649 (D), 14672 (E), 6106 (F), 14650 (G). The data are representative of the assay performed twice in triplicate, with the standard error of the mean graphed as error bars.
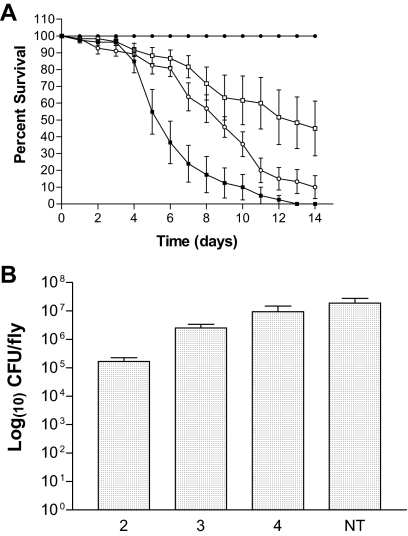
As shown by the average CFU per fly and CFU per filter, there did appear to be a correlation with bacterial load and the ability to cause death in the fly. However, since the flies were kept on the bacteria for the duration of the experiment, the ability of P. aeruginosa to establish and maintain an infection without continuous exposure to bacteria required assessment. To carry out this experiment, PAO1 was used to infect flies as described in Materials and Methods, with the modification that the flies were transferred to sterile sucrose 2, 3, or 4 days postinfection. Fly mortality was monitored daily, and the bacterial CFU per fly was assessed at day 8 (Fig. 7). Increasing the duration of time for which the fly was exposed to PAO1 increased fly mortality (Fig. 7A). All survival curves for PAO1 were significantly different (P < 0.0001). Exposure of the flies to PAO1 for 2 days did not result in fly mortality even though the bacterial load at 6 days postinfection (4 days after transfer to sucrose) was on the order of 105 CFU per fly (Fig. 7). Flies incubated on PAO1 for 3 or 4 days showed increasing fly mortality since 4 days of exposure to PAO1 resulted in more fly mortality than 3 days of exposure (Fig. 7A). The bacterial CFU per fly also showed a correlation to the time of exposure to strain PAO1, but CFU counts were not significantly different (P > 0.05) (Fig. 7B). Figures 6 and 7B together suggested that ca. 106 bacteria per fly were needed for the development of a lethal infection in the Drosophila feeding model. Taken together, these experiments also demonstrated that the fly feeding model was representative of a chronic colonization model since the bacterial loads in the fly were maintained in the absence of continual exposure to P. aeruginosa. Furthermore, the data suggested that even the group 4 CF isolates, which did not elicit much fly death, can maintain colonization in the fly, albeit at a lower level.
FIG. 7.
Colonization of D. melanogaster during P. aeruginosa infection. (A) Fruit flies were infected with PAO1 (▪) and transferred to sucrose at 2 (•), 3 (□), and 4 (○) days postinfection. (B) Bacterial CFU per fly was determined on day 8 for PAO1. The days of exposure to Pseudomonas are shown on the x axis. NT, flies maintained on filters inoculated with PAO1 for the entire duration of the experiment.
Pseudomonas CF isolates from individual patients and within single sputum samples exhibit a diversity of lethality in the two Drosophila models of infection.
In the present study, three burn wound isolates and three control strains were all highly infectious and killed the majority of flies in both models. The one exception was strain PA103, which was lethal to flies only in the fly nicking model of infection. In contrast, the 27 CF isolates showed a wide range of fly lethality in both infection models, ranging from essentially 0 to ≥80% lethality. Since these CF isolates all came from adult CF patients who had been stably colonized for many years, it was of interest to determine the distribution of the isolates' pathogenic potential in samples from individual patients.
Table 1 shows that the 27 CF isolates were isolated from 13 patients. The data set encompasses multiple isolates from individual sputum samples, single isolates from sputum samples, and some isolates from individual patients collected over time.
TABLE 1.
Lethality of P. aeruginosa isolates
| Strain | Patient | Collection datea | Mean % survivalb ± SEM
|
Cluster groupc | |
|---|---|---|---|---|---|
| Fly feeding | Fly nicking | ||||
| PAO1 | 1979 | 7.3 ± 3.5 | 11.3 ± 2.5 | 1 | |
| PA103 | 1966 | 97.2 ± 1.2 | 32.2 ± 5.5 | 3 | |
| PAK | 1966 | 11.7 ± 6.7 | 16.6 ± 2.9 | 1 | |
| PA14 | 1995d | 12.8 ± 6.1 | 19.2 ± 4.8 | 1 | |
| ATCC 27853 | 1971 | 5.5 ± 3.7 | 23.3 ± 5.5 | 1 | |
| UTAH3 | NA | 8.9 ± 8.9 | 17.0 ± 4.7 | 1 | |
| UTAH4 | NA | 2.2 ± 2.2 | 9.9 ± 4.1 | 1 | |
| 4384 | 35 | 02/02/87 | 41.1 ± 12.6 | 21.1 ± 4.5 | 1 |
| 5154 | 35 | 02/11/88 | 10.0 ± 5.7 | 83.3 ± 6.1 | 2 |
| 5166 | 35 | 02/18/88 | 36.7 ± 12.8 | 23.3 ± 4.1 | 1 |
| 5552 | 34 | 10/17/88 | 96.7 ± 1.7 | 91.7 ± 2.1 | 4 |
| 5588 | 34 | 10/27/88 | 70.7 ± 8.1 | 62.5 ± 7.0 | 4 |
| 5585 | 88 | 10/24/88 | 93.3 ± 2.3 | 92.0 ± 1.4 | 4 |
| 7307 | 29 | 11/18/91 | 66.5 ± 6.8 | 60.0 ± 7.7 | 4 |
| 14655 | 29 | 04/30/98 | 92.1 ± 1.9 | 86.7 ± 6.0 | 4 |
| 14656 | 29 | 04/30/98 | 91.3 ± 3.4 | 95.6 ± 3.4 | 4 |
| 6106 | 26 | 01/10/90 | 94.1 ± 1.9 | 67.1 ± 5.7 | 4 |
| 14649 | 89 | 04/29/99 | 9.1 ± 6.0 | 19.2 ± 5.0 | 1 |
| 14650 | 89 | 04/29/99 | 15.8 ± 4.3 | 76.7 ± 4.7 | 2 |
| 14651 | 89 | 04/29/99 | 85.0 ± 4.4 | 90.8 ± 3.8 | 4 |
| 14660 | 57 | 05/04/98 | 82.0 ± 7.3 | 83.3 ± 4.9 | 4 |
| 14661 | 57 | 05/04/98 | 20.9 ± 9.2 | 31.7 ± 5.5 | 1 |
| 14670 | 90 | 05/07/98 | 70.0 ± 11.7 | 66.5 ± 5.6 | 4 |
| 14671 | 90 | 05/07/98 | 47.6 ± 11.2 | 87.8 ± 5.5 | 2 |
| 14672 | 91 | 05/07/98 | 85.3 ± 4.0 | 52.5 ± 6.0 | 3 |
| 14673 | 91 | 05/07/98 | 19.3 ± 8.9 | 28.0 ± 6.6 | 1 |
| 14683 | 92 | 05/13/98 | 80.0 ± 8.7 | 91.1 ± 3.9 | 4 |
| 14684 | 92 | 05/13/98 | 85.8 ± 5.3 | 50.0 ± 7.7 | 3 |
| 14685 | 92 | 05/13/98 | 93.9 ± 2.9 | 70.0 ± 5.4 | 4 |
| 14690 | 93 | 05/14/98 | 12.3 ± 5.6 | 15.8 ± 3.4 | 1 |
| 14703 | 33 | 05/20/98 | 91.9 ± 2.6 | 95.0 ± 2.9 | 4 |
| 14715 | 38 | 05/26/98 | 94.2 ± 2.3 | 82.0 ± 3.4 | 4 |
| 14716 | 38 | 05/26/98 | 93.3 ± 1.9 | 98.3 ± 1.1 | 4 |
| 14717 | 38 | 05/26/98 | 91.7 ± 1.7 | 78.9 ± 7.0 | 4 |
The date is expressed as the year or as the month/day/year. NA, not available.
Lethality was measured as the percent survival of D. melanogaster flies on the last day for fly feeding and at the last hour for fly nicking experiments.
Groupings were determined by using Cluster 3.0 and are depicted in Fig. 2.
First published by Rahme et al. (32).
We had two or more isolates from nine patients collected over a short period of time. As mentioned above, these patients had been chronically colonized for years with P. aeruginosa. As such, we might have expected that different isolates from the same patient would display similar levels of lethality in the fly models of infection. However, in all but one patient we found that the survival curves generated from their isolates were significantly different (P ≤ 0.028). This would suggest that the diversity seen for the entire set of CF isolates is also found within isolates from individual patients.
For seven patients there were at least two P. aeruginosa isolates from the same sputum. In five of these patient samples (from patients 57, 89, 90, 91, and 92) there was a range of lethality for the P. aeruginosa isolates, with at least one isolate falling into one of the more lethal groups 1, 2, or 3 (Table 1). Isolates from each of these patients showed significant differences in fly survival in both model systems (P < 0.029). This suggested diversity in pathogenic potentials for isolates even within a single patient sample. This was best illustrated by patients 89 and 57 (Fig. 8), where the isolates from patient 57 fell into group 1 (lethal in both model systems) and group 4 (low lethality in both model systems). Again, for patient 89 the three isolates each fell into different groups (groups 1, 2, and 4). In two sputum samples, from patients 29 and 38, only isolates from group 4, which had lower lethality in both models, were found. For patient 29 there was not a significant difference in the survival curves between isolates 14655 and 14656 in either the fly feeding model (P = 0.1632) or the fly nicking model (P = 0.8278). Likewise, for patient 38 there was no significant difference between the three isolates in the fly feeding model (P = 0.8273). There was, however, a significant difference in the fly nicking model between the three isolates of patient 38 (P = 0.002). Taken together, our results suggested that isolates with a wide spectrum of lethality occurred even within individual patients. The diversity of lethality among the Pseudomonas isolates suggested a degree of heterogeneity in the CF lung bacterial population even within a single patient sample.
FIG. 8.
Lethality of strains from CF patients simultaneously infected with multiple Pseudomonas strains. (A and B) The lethality of Pseudomonas CF isolates from patient 57 (A) and patient 89 (B) was graphed. The pathogenic potential of strains was determined by fly nicking. The strains shown in panel A are 14660 (▴) and 14661 (▪), and the strains shown in panel B are 14649 (○), 14651 (⧫), and 14650 (•). The data are representative of the assay performed twice in triplicate, with error bars representing the standard error of the mean.
DISCUSSION
As a pathogen, P. aeruginosa is able to adapt to various hosts and cause either acute or chronic infections (14). In CF, chronic and persistent lung infections occur that are interspersed with periods of worsened symptomatology that could be characterized as acute exacerbations. In acute burn wounds, P. aeruginosa can cause fulminant infections especially if the strains are invasive. In the present study, we wanted to compare the pathogenic potential of P. aeruginosa isolates from acute burn wound infections and from chronic CF lung infections in two Drosophila infection models. Previously, the D. melanogaster models have been used separately to assay the pathogenic potential of many Pseudomonas strains, based on lethality in the fly (9, 13, 23), but a direct comparison of the models has not been done. We were particularly interested in these two models because the fly nicking model likely represents a faster, more-acute infection, wherein the host's external barriers are compromised, and the fly feeding model represents a slower, more-chronic long-term infection.
A comparison of the two infection models revealed that the majority of strains (82%) had similar but not identical levels of lethality in both models of infection. These included all of the burn wound isolates; strains PAO1, PAK, and PA14; and 81% of the CF isolates. Thus, the majority of the isolates and strains did not show a predilection for one model or the other. There was a subtle difference between the infection models, where the survival curves for the all of the isolates seemed to form groups in the fly feeding model but displayed more of a continuum in the fly nicking model. This might suggest that the nicking model was slightly more sensitive to differences between strains.
Some strains exhibited dramatically different pathogenic potentials in the two fly infection models. One group (Fig. 2, group 3) consisting of the strain PA103 and CF isolates 14672 and 14684 was more lethal in the fly nicking model of infection and less lethal in the fly feeding model. Interestingly, strain PA103 lacks a functional quorum-sensing system (12, 16, 31). Therefore, quorum sensing or its target genes may play an important role in the fly feeding model of infection. Quorum sensing has previously been implicated as playing a role in virulence of P. aeruginosa in both Drosophila models of infection (8, 13, 36). Additional experiments are needed to determine whether quorum sensing is required for lethality in fly feeding but not in fly nicking.
In contrast to group 3, the three CF isolates 14650, 5154, and 14671 (belonging to group 2) elicited greater killing in the Drosophila feeding model compared to the nicking model and are therefore of particular interest. It was unexpected that they would be unable to set up an infection when the Drosophila defenses were breached (nicking model) but would be able to cause an infection when defenses were intact (feeding model). This difference may be due to different interactions with the Drosophila immune system. In the nicking model of infection, P. aeruginosa causes a strong immune response compared to other pathogenic organisms (24). The immune response may vary depending on the strain used. For example, in the nicking model of infection a CF isolate, CF5, induces a robust immune response that gives the fly protection against subsequent infections (2). In contrast, PA14 quickly overwhelms the fly immune response and sets up a fatal infection (2). In the Drosophila feeding assay, P. aeruginosa may not induce as strong an immune response as that induced by other bacterial pathogens (43). Thus, a possible explanation for the lack of lethality of isolates 14650, 5154, and 14671 in the nicking model is that these isolates may induce a strong immune response and as a result cannot set up an infection. In the feeding model, these three isolates may be able to get around the hostile environment in the gut but may not elicit a strong enough immune response and were able to set up an infection. Further work is needed to establish the nature of these differences.
It was previously reported that P. aeruginosa isolates from human infections demonstrate a spectrum of pathogenic potential, from 47 to 100% mortality in the Drosophila nicking model (21). Such a range of pathogenic potential of human isolates is also found using the Caenorhabditis elegans infection model (22). Interestingly, Lee et al. (22) tested four CF isolates and found that these had the widest spectrum of pathogenic potential of any human isolates tested. Our results with 27 CF isolates, in the two fly models, confirmed the heterogeneity of the pathogenic potential of CF isolates (Fig. 1 and 2, Table 1). Other studies have shown that some P. aeruginosa CF isolates produce fewer virulence factors over time (14, 38), which would eventually result in a loss of pathogenic potential but may allow adaptation to a chronic infection in the lung.
Notably, the CF isolates showed a diversity of pathogenic potentials both at the patient level (Table 1, patients 89, 57, 90, 91, and 92) and at the level of individual sputum samples (Fig. 8 and Table 1). In the selection of our isolates from sputa only a small number of isolates were picked by the technologists. However, each isolate was selected based on its distinctive morphotype. As such, our isolates represent at least some of the diversity of the bacterial population in a sputum sample. This may be reflective of other studies that examined the distribution of bacterial populations in the lungs of CF patients which showed that multiple morphotypes of P. aeruginosa are present and represent a diverse population (37, 42). More recent studies indicate that in the majority of cases, but not all samples, multiple clonal variants can simultaneously coexist with the population being genomically homogeneous (40). We are currently examining our strains to determine whether they are genomically homogeneous or heterogeneous.
In two sputum samples from CF patients 29 and 38, respectively, only isolates with less-than-average lethality were detected. Furthermore, of the five isolates (14655, 14556, 14715, 14716, and 14717) from these two samples, all had low levels of lethality (<10% killing) in at least one model, and two isolates lacked significant lethality in both models (Table 1). These isolates and others that lack the ability to kill flies in both models may individually lack the pathogenic potential to establish infections, or they may elicit a strong immune response in the flies and so were unable to establish an infection. These results raise the question of whether the isolates that are not lethal to the flies could themselves maintain an infection in CF patients. It is possible that these strains, while not lethal in the fly models of infection, might retain pathogenicity in human infections or at least retain the ability to colonize the lung. Our research on isolate 6106 suggested that the isolate could colonize the flies at a low level but could not go on to cause an infection and death of the fly (<10% lethality overall). Thus, even CF isolates with very low levels of lethality may still retain the ability to colonize a host. Finally, since all of our patients are adults who have been chronically infected for years with P. aeruginosa, it is also possible that the host defenses have degraded to such a point where the infecting bacteria may no longer need their virulence determinants to maintain the infection.
At the other end of the spectrum, we have found a number of CF isolates, burn wound isolates, and control strains that are highly lethal to the flies in both model systems (Fig. 2, group 1). The CF isolates in this group are particularly interesting since our isolates have come from adult patients that have been chronically colonized with P. aeruginosa for years. As such, we might not have expected to find as many isolates that have such a high degree of lethality in one or both fly infection models. These highly lethal isolates may contribute to some aspects of CF infections. For instance, the strains which had high lethality in the Drosophila models, and hence high pathogenic potential, may be responsible for the spread of P. aeruginosa between CF patients. This concept is supported by research on the Liverpool epidemic strain, which has been shown to be highly lethal in the Drosophila feeding infection model (36) and is notorious for its spread among CF patients and across continents (7). Another possible role that these isolates may play in CF lung infections could occur during exacerbations of the lung disease. It has been reported that lethality in the Drosophila nicking infection model correlates to lethality in a mammalian infection model (21). As such, it is possible the CF isolates that are highly lethal in the flies may be at least partly responsible for the pathology during chronic colonization or during an exacerbation.
This research has many implications for the chronic lung infections associated with CF. Our findings suggested that within bacterial populations in the lung there may be distinct subpopulations that have various degrees of pathogenic potentials. It is not clear what role the isolates with different pathogenic potentials might play in the chronic lung infections associated with CF. However, based on the characterizations in the present study, we speculate that the isolates with higher pathogenic potential may be involved in establishment of infection, cross-infection and pathogenic processes. On the other hand, the CF isolates with lower or no pathogenic potential in the flies may play a role more in chronic colonization and perhaps in evasion of the immune response. Alternatively, in CF patients that have been colonized for many years, the host immune response may have degraded to a point where the colonizing strains no longer need their pathogenic potential in order to maintain the infection. Further research is needed to define the characteristics of the subpopulations and to determine the factors involved in differences of these subpopulations between patients.
In summary, our results demonstrate that the majority of strains (82%) have similar pathogenic potential in both Drosophila infection models, regardless of the type of infection from which they were isolated. The CF isolates examined in the present study displayed a wide spectrum of lethality in both fly infection models, whereas P. aeruginosa isolates from burn wounds and most of the control strains were highly lethal in both models. Differences in bacterial load in the flies suggested that lethal strains were able to establish and maintain an infection in Drosophila, whereas less-lethal strains were able to colonize the flies at a lower level but were not able to establish an infection that killed the flies. Alternatively, the less lethal strains and isolates may elicit an immune response that blocks their progress to a lethal infection. Finally, differences between the Drosophila models allowed the discrimination of some isolates that were better adapted to one of the infection models. Taken together, our results suggested that the two Drosophila infection models were useful for analysis of the diversity of the pathogenic potentials of P. aeruginosa isolates.
Acknowledgments
This research was supported by a grant to D.G.S. from the Canadian Cystic Fibrosis Foundation. E.I.L. was supported by a Summit Foundation Studentship.
Editor: V. J. DiRita
Footnotes
Published ahead of print on 19 February 2008.
REFERENCES
- 1.Anonymous. 2002. Patient registry 2001 annual report. Cystic Fibrosis Foundation, Bethesda, MD.
- 2.Apidianakis, Y., M. N. Mindrinos, W. Xiao, G. W. Lau, R. L. Baldini, R. W. Davis, and L. G. Rahme. 2005. Profiling early infection responses: Pseudomonas aeruginosa eludes host defenses by suppressing antimicrobial peptide gene expression. Proc. Natl. Acad. Sci. USA 1022573-2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boman, H. G., I. Nilsson, and B. Rasmuson. 1972. Inducible antibacterial defense system in Drosophila. Nature 237232-235. [DOI] [PubMed] [Google Scholar]
- 4.Burns, J. L., R. L. Gibson, S. McNamara, D. Yim, J. Emerson, M. Rosenfeld, P. Hiatt, K. McCoy, R. Castile, A. L. Smith, and B. W. Ramsey. 2001. Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. J. Infect. Dis. 183444-452. [DOI] [PubMed] [Google Scholar]
- 5.Cash, H. A., D. E. Woods, B. McCullough, W. G. Johanson, Jr., and J. A. Bass. 1979. A rat model of chronic respiratory infection with Pseudomonas aeruginosa. Am. Rev. Respir. Dis. 119453-459. [DOI] [PubMed] [Google Scholar]
- 6.Chang, Y. S., J. Klockgether, and B. Tummler. 2007. An intragenic deletion in pilQ leads to nonpiliation of a Pseudomonas aeruginosa strain isolated from cystic fibrosis lung. FEMS Microbiol. Lett. 270201-206. [DOI] [PubMed] [Google Scholar]
- 7.Cheng, K., R. L. Smyth, J. R. Govan, C. Doherty, C. Winstanley, N. Denning, D. P. Heaf, H. van Saene, and C. A. Hart. 1996. Spread of beta-lactam-resistant Pseudomonas aeruginosa in a cystic fibrosis clinic. Lancet 348639-642. [DOI] [PubMed] [Google Scholar]
- 8.Chugani, S. A., M. Whiteley, K. M. Lee, D. D'Argenio, C. Manoil, and E. P. Greenberg. 2001. QscR, a modulator of quorum-sensing signal synthesis and virulence in Pseudomonas aeruginosa. Proc. Natl. Acad. Sci. USA 982752-2757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Argenio, D. A., L. A. Gallagher, C. A. Berg, and C. Manoil. 2001. Drosophila as a model host for Pseudomonas aeruginosa infection. J. Bacteriol. 1831466-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Hoon, M. J., S. Imoto, J. Nolan, and S. Miyano. 2004. Open source clustering software. Bioinformatics 201453-1454. [DOI] [PubMed] [Google Scholar]
- 11.Emerson, J., M. Rosenfeld, S. McNamara, B. Ramsey, and R. L. Gibson. 2002. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr. Pulmonol. 3491-100. [DOI] [PubMed] [Google Scholar]
- 12.Erickson, D. L., R. Endersby, A. Kirkham, K. Stuber, D. D. Vollman, H. R. Rabin, I. Mitchell, and D. G. Storey. 2002. Pseudomonas aeruginosa quorum-sensing systems may control virulence factor expression in the lungs of patients with cystic fibrosis. Infect. Immun. 701783-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erickson, D. L., J. L. Lines, E. C. Pesci, V. Venturi, and D. G. Storey. 2004. Pseudomonas aeruginosa relA contributes to virulence in Drosophila melanogaster. Infect. Immun. 725638-5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ernst, R. K., K. N. Adams, S. M. Moskowitz, G. M. Kraig, K. Kawasaki, C. M. Stead, M. S. Trent, and S. I. Miller. 2006. The Pseudomonas aeruginosa lipid A deacylase: selection for expression and loss within the cystic fibrosis airway. J. Bacteriol. 188191-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gallant, C. V., T. L. Raivio, J. C. Olson, D. E. Woods, and D. G. Storey. 2000. Pseudomonas aeruginosa cystic fibrosis clinical isolates produce exotoxin A with altered ADP-ribosyltransferase activity and cytotoxicity. Microbiology 146(Pt. 8)1891-1899. [DOI] [PubMed] [Google Scholar]
- 16.Gambello, M. J., and B. H. Iglewski. 1991. Cloning and characterization of the Pseudomonas aeruginosa lasR gene, a transcriptional activator of elastase expression. J. Bacteriol. 1733000-3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Govan, J. R., and G. S. Harris. 1986. Pseudomonas aeruginosa and cystic fibrosis: unusual bacterial adaptation and pathogenesis. Microbiol. Sci. 3302-308. [PubMed] [Google Scholar]
- 18.Holloway, B. W., V. Krishnapillai, and A. F. Morgan. 1979. Chromosomal genetics of Pseudomonas. Microbiol. Rev. 4373-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jander, G., L. G. Rahme, and F. M. Ausubel. 2000. Positive correlation between virulence of Pseudomonas aeruginosa mutants in mice and insects. J. Bacteriol. 1823843-3845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lam, J., R. Chan, K. Lam, and J. W. Costerton. 1980. Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect. Immun. 28546-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lau, G. W., B. C. Goumnerov, C. L. Walendziewicz, J. Hewitson, W. Xiao, S. Mahajan-Miklos, R. G. Tompkins, L. A. Perkins, and L. G. Rahme. 2003. The Drosophila melanogaster toll pathway participates in resistance to infection by the gram-negative human pathogen Pseudomonas aeruginosa. Infect. Immun. 714059-4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee, D. G., J. M. Urbach, G. Wu, N. T. Liberati, R. L. Feinbaum, S. Miyata, L. T. Diggins, J. He, M. Saucier, E. Deziel, L. Friedman, L. Li, G. Grills, K. Montgomery, R. Kucherlapati, L. G. Rahme, and F. M. Ausubel. 2006. Genomic analysis reveals that Pseudomonas aeruginosa virulence is combinatorial. Genome Biol. 7R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee, J. S., Y. J. Heo, J. K. Lee, and Y. H. Cho. 2005. KatA, the major catalase, is critical for osmoprotection and virulence in Pseudomonas aeruginosa PA14. Infect. Immun. 734399-4403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lemaitre, B., J. M. Reichhart, and J. A. Hoffmann. 1997. Drosophila host defense: differential induction of antimicrobial peptide genes after infection by various classes of microorganisms. Proc. Natl. Acad. Sci. USA 9414614-14619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu, P. V. 1966. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. II. Effects of lecithinase and protease. J. Infect. Dis. 116112-116. [DOI] [PubMed] [Google Scholar]
- 26.Lyczak, J. B., C. L. Cannon, and G. B. Pier. 2000. Establishment of Pseudomonas aeruginosa infection: lessons from a versatile opportunist. Microbes Infect. 21051-1060. [DOI] [PubMed] [Google Scholar]
- 27.Mahenthiralingam, E., M. E. Campbell, and D. P. Speert. 1994. Nonmotility and phagocytic resistance of Pseudomonas aeruginosa isolates from chronically colonized patients with cystic fibrosis. Infect. Immun. 62596-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medeiros, A. A., T. F. O'Brien, W. E. Wacker, and N. F. Yulug. 1971. Effect of salt concentration on the apparent in-vitro susceptibility of Pseudomonas and other gram-negative bacilli to gentamicin. J. Infect. Dis. 124(Suppl.)S59-S64. [DOI] [PubMed] [Google Scholar]
- 29.Oliver, A., R. Canton, P. Campo, F. Baquero, and J. Blazquez. 2000. High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science 2881251-1254. [DOI] [PubMed] [Google Scholar]
- 30.Oliver, A., B. R. Levin, C. Juan, F. Baquero, and J. Blazquez. 2004. Hypermutation and the preexistence of antibiotic-resistant Pseudomonas aeruginosa mutants: implications for susceptibility testing and treatment of chronic infections. Antimicrob. Agents Chemother. 484226-4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pearson, J. P., E. C. Pesci, and B. H. Iglewski. 1997. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J. Bacteriol. 1795756-5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rahme, L. G., E. J. Stevens, S. F. Wolfort, J. Shao, R. G. Tompkins, and F. M. Ausubel. 1995. Common virulence factors for bacterial pathogenicity in plants and animals. Science 2681899-1902. [DOI] [PubMed] [Google Scholar]
- 33.Raivio, T. L., E. E. Ujack, H. R. Rabin, and D. G. Storey. 1994. Association between transcript levels of the Pseudomonas aeruginosa regA, regB, and toxA genes in sputa of cystic fibrosis patients. Infect. Immun. 623506-3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Romling, U., B. Fiedler, J. Bosshammer, D. Grothues, J. Greipel, H. von der Hardt, and B. Tummler. 1994. Epidemiology of chronic Pseudomonas aeruginosa infections in cystic fibrosis. J. Infect. Dis. 1701616-1621. [DOI] [PubMed] [Google Scholar]
- 35.Saldanha, A. J. 2004. Java Treeview: extensible visualization of microarray data. Bioinformatics 203246-3248. [DOI] [PubMed] [Google Scholar]
- 36.Salunkhe, P., C. H. Smart, J. A. Morgan, S. Panagea, M. J. Walshaw, C. A. Hart, R. Geffers, B. Tummler, and C. Winstanley. 2005. A cystic fibrosis epidemic strain of Pseudomonas aeruginosa displays enhanced virulence and antimicrobial resistance. J. Bacteriol. 1874908-4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seale, T. W., H. Thirkill, M. Tarpay, M. Flux, and O. M. Rennert. 1979. Serotypes and antibiotic susceptibilities of Pseudomonas aeruginosa isolates from single sputa of cystic fibrosis patients. J. Clin. Microbiol. 972-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smith, E. E., D. G. Buckley, Z. Wu, C. Saenphimmachak, L. R. Hoffman, D. A. D'Argenio, S. I. Miller, B. W. Ramsey, D. P. Speert, S. M. Moskowitz, J. L. Burns, R. Kaul, and M. V. Olson. 2006. Genetic adaptation by Pseudomonas aeruginosa to the airways of cystic fibrosis patients. Proc. Natl. Acad. Sci. USA 1038487-8492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stotland, P. K., D. Radzioch, and M. M. Stevenson. 2000. Mouse models of chronic lung infection with Pseudomonas aeruginosa: models for the study of cystic fibrosis. Pediatr. Pulmonol. 30413-424. [DOI] [PubMed] [Google Scholar]
- 40.Syrmis, M. W., M. R. O'Carroll, T. P. Sloots, C. Coulter, C. E. Wainwright, S. C. Bell, and M. D. Nissen. 2004. Rapid genotyping of Pseudomonas aeruginosa isolates harboured by adult and paediatric patients with cystic fibrosis using repetitive-element-based PCR assays. J. Med. Microbiol. 531089-1096. [DOI] [PubMed] [Google Scholar]
- 41.Takeya, K., and K. Amako. 1966. A rod-shaped Pseudomonas phage. Virology 28163-165. [DOI] [PubMed] [Google Scholar]
- 42.Thomassen, M. J., C. A. Demko, B. Boxerbaum, R. C. Stern, and P. J. Kuchenbrod. 1979. Multiple of isolates of Pseudomonas aeruginosa with differing antimicrobial susceptibility patterns from patients with cystic fibrosis. J. Infect. Dis. 140873-880. [DOI] [PubMed] [Google Scholar]
- 43.Vodovar, N., M. Vinals, P. Liehl, A. Basset, J. Degrouard, P. Spellman, F. Boccard, and B. Lemaitre. 2005. Drosophila host defense after oral infection by an entomopathogenic Pseudomonas species. Proc. Natl. Acad. Sci. USA 10211414-11419. [DOI] [PMC free article] [PubMed] [Google Scholar]