Abstract
Cervical cancer, the second leading cause of cancer deaths in women, is the consequence of high-risk human papillomavirus (HPV) infections. Toward the development of therapeutic vaccines that can induce both innate and adaptive mucosal immune responses, we analyzed intravaginal (ivag) vaccine delivery of live attenuated Salmonella enterica serovar Typhimurium expressing HPV16L1 as a model antigen. Innate immune responses were examined in cervicovaginal tissues by determining gene expression patterns by microarray analysis using nylon membranes imprinted with cDNA fragments coding for inflammation-associated genes. At 24 h, a wide range of genes, including those for chemokines and Th1- and Th2-type cytokine and chemokine receptors were up-regulated in mice ivag immunized with Salmonella compared to control mice. However, the majority of transcripts returned to their steady-state levels 1 week after immunization, suggesting a transient inflammatory response. Indeed, cervicovaginal histology of immunized mice showed a massive, but transient, infiltration of macrophages and neutrophils, while T cells were still increased after 7 days. Ivag immunization also induced humoral and antitumor immune responses, i.e., serum and vaginal anti-HPV16VLP antibody titers similar to those induced by oral immunization, and significant protection in tumor protection experiments using HPV16-expressing C3 tumor cells. These results show that ivag immunization with live attenuated Salmonella expressing HPV16 antigens modulates the local mucosal gene expression pattern into a transient proinflammatory profile, elicits strong systemic and mucosal immunity against HPV16, and confers protection against HPV16 tumor cells subcutaneously implanted in mice. Examination of the efficacy with which ivag HPV16E7E6 Salmonella induces regression of tumors located in cervicovaginal tissue is warranted.
Cervical cancer is the second leading cause of cancer deaths among women worldwide, with half a million new cases per year, of which 83% occur in developing countries (54). Cervical cancer is caused by infection with a subset of human papillomavirus (HPV), of which type 16 is found most frequently (62). HPV vaccines based on highly purified virus-like particles (VLP) from HPV have been shown to be safe, highly immunogenic, and effective in preventing the development of HPV16 and -18 infections and associated cervical neoplasia (53, 70). However, these prophylactic vaccines can only prevent ca. 70% of all cervical cancers if administered to young adolescents before sexual activity. During the decades necessary to implement prophylactic strategies, millions of women will be or have already been infected by HPVs and may develop associated malignant lesions that could be treated by therapeutic vaccines. Up to now, different therapeutic vaccines, usually targeting the HPV E6 and/or E7 oncogenes and administered parenterally, have shown only limited clinical success (39). Cell-mediated immune responses are important in controlling both HPV infections and HPV-associated neoplasms, as shown by the increased prevalence of these diseases when impaired cell-mediated immunity occurs, such as in transplant recipients or in human immunodeficiency virus-infected patients (38, 61, 63). The immunosuppressive microenvironment of the cervical mucosa favors the development of high-grade squamous intraepithelial lesions and/or cervical cancer, which correlates with local type II cytokines (24), absence of gamma interferon (IFN-γ) (23), and reduced density and function of Langerhans cells (25), as well as an increased proportion of regulatory T cells in the draining lymph nodes of cervical cancer patients (17, 67). In addition, there is little or no inflammation at the site of primary HPV infection and HPV may be able to suppress the host immune response (35), in particular, type 16, which was recently shown to down-regulate the expression of Toll-like receptor 9 (TLR9) in human primary keratinocytes (28). We hypothesize that local induction of an inflammatory response in the vaginal microenvironment would promote recruitment and/or activation of immune cells in the cervicovaginal mucosa, which may, in turn, favor tumor regression. Here we have therefore explored the possibility of using the intravaginal (ivag) route of administration of a bacterial vector in order to deliver HPV heterologous antigens and concomitantly induce local innate immune responses. Live attenuated Salmonella strains are effective antigen delivery systems (9) by different routes of administration, including ivag administration in mice that are in the diestrus stage of the menstrual cycle (31). In addition, Salmonella has the potential to induce a proinflammatory response with its bacterial components lipopolysaccharide (LPS) (59) and flagellin (22) through TLR4 (41) and TLR5 (21), respectively. The immune response to Salmonella infection by the oral route has been extensively studied (reviewed in references 14 and 47). Several cytokines, in particular, tumor necrosis factor (TNF), IFN-γ, and interleukin-12, have been shown to be essential in controlling Salmonella during infection (42). However, the cytokine and chemokine responses induced by Salmonella in the mouse female genital tract are unknown. Here, we have used attenuated Salmonella enterica serovar Typhimurium strains that (i) expressed the L1 HPV16 capsid gene as a model antigen, (ii) induced high HPV16-neutralizing titers in the serum and vaginal secretions of mice after a single oral immunization (2, 19), and (iii) were capable of preventing the growth of HPV16-expressing C3 tumor cells in mice after nasal immunization (59).
As a first step, we analyzed the gene expression patterns of cytokine and chemokine responses in the mouse cervicovaginal mucosa after ivag immunization with two differently attenuated S. enterica serovar Typhimurium strains, i.e., an auxotrophic AroA mutant strain that depends on p-aminobenzoic acid and 2,3-dihydroxybenzoate for the synthesis of aromatic amino acids and growth (30) and a PhoPc strain that has mutations in the two-component regulatory system phoP/phoQ, which controls more than 40 different genes required for intracellular survival and resistance to innate immune defense mechanisms (16, 46). As a second step, we examined the abilities of these HPV16 L1 recombinant attenuated Salmonella strains to induce humoral and antitumor responses against HPV16 L1 after ivag immunization.
MATERIALS AND METHODS
Immunization of mice, analysis of anti-HPV16 VLP antibodies, and recovery of Salmonella.
Six-week-old female BALB/c and C57BL/6 mice (Iffa Credo, France) were used throughout, and the ethical directives of the Swiss veterinary authorities were followed. Twenty microliters of bacterial inoculum was administered orally (109 CFU) to awake mice or by the nasal (106 to 107 CFU) or ivag (109 CFU) route under anesthesia as previously described (20, 31, 51). Nasal immunization is performed under deep anesthesia to allow ca. one-third of the inoculum to be inhaled into the lung, which results in greater immunogenicity (2, 49). Prior to ivag immunization, mice were synchronized in a diestrus-like state by subcutaneous (s.c.) injection with 0.1 μg β-estradiol and 24 h later with 2.5 mg DepoProvera (Pfizer AG, Zurich, Switzerland). Vaginal lavage was performed with 40 μl of phosphate-buffered saline (PBS), and collected cells were observed under a microscope to determine the stage of the estrous cycle. Sampling of blood and vaginal washes and determination of anti-HPV16 VLP antibody titers by enzyme-linked immunosorbent assay were performed as reported earlier (20). Endpoint dilutions of all samples were carried out. Specific immunoglobulin A (IgA) or IgG titers were expressed as the reciprocal of the highest dilutions that yielded an optical density at 492 nm four times that of preimmune samples. The optical densities of preimmune samples are <0.010 for dilutions starting at 1/50 for serum or 1/5 for vaginal washes. These titers were normalized to the amount of total IgA or IgG in vaginal washes. Recovery of S. enterica serovar Typhimurium was determined in organs from euthanized mice by plating on agar plates with and without antibiotic as previously described (51).
C3 challenge and tumor growth monitoring.
The C3 cell line is a tumorigenic HPV16- and EJ-ras-transformed C57BL/6 mouse embryo cell line (18). The C3 cell line was maintained in RPMI medium supplemented with 10% heat-inactivated fetal calf serum (Gibco-Invitrogen), 2 mM l-glutamine (Gibco-Invitrogen), 50 mM 2-mercaptoethanol (Gibco-Invitrogen), 100 U/ml penicillin, and 100 μg/ml streptomycin (Gibco-Invitrogen). Cells were incubated at 37°C in 5% CO2. C3 cells grown to 70 to 80% confluence were trypsinized, washed, and passed through a 70-μm cell strainer (BD Falcon) to obtain single-cell suspensions. Cells were resuspended in serum-free Hanks balanced salt solution at 5 × 106/ml. C57BL/6 female mice were s.c. injected in the right flank with 5 × 105 cells. Tumor growth was monitored and measured twice a week by using the following formula: length × width2. For ethical reasons, mice were sacrificed when tumor volumes reached 3,400 mm3 in volume (∼10% of mouse body size).
RNA isolation, cDNA synthesis, and cDNA labeling.
A GEArray Q Series Mouse Gene Array (GEArray MM-015; SuperArray Bioscience Corporation) was used to identify changes in the expression of 96 cDNA fragments associated with mouse inflammatory cytokine and receptor genes. The list of cDNA fragments spotted on the gene array is publicly available at the following web address: http://www.superarray.com/gene_array_product/HTML/MM-015.html.
Each cDNA fragment in the Q Series is printed in a tetraspot format. All steps were performed according to the manufacturer's recommendations. Briefly, cervicovaginal tissues from individual or pooled mice (n = 2) were immediately rinsed and collected in RNA stabilization reagent (RNAlater; Qiagen) and total RNA was isolated by using the RNeasy Mini Kit (Qiagen). Then, 1.5 μg of RNA was reverse transcribed with Biotin-16-dUTP (Roche) with the GEArray AmpoLabeling-LPR kit (SuperArray Bioscience Corporation) according to the manufacturer's instructions. The cDNA-probe mixture was hybridized to the Q Series Mouse Inflammatory Cytokines and Receptors cDNA array at 60°C overnight. The array was analyzed by using CDP-Star chemiluminescent substrate, and a charged-coupled device camera was used to capture incoming light. Data image files were analyzed according to the GEArray Expression Analysis Suite provided by the Superarray Bioscience Corporation and standardized to two housekeeping genes (β-actin and glyceraldehyde-3-phosphate dehydrogenase) to minimize variability between tests. Output signals were then converted and scaled from 1 to 10.
Immunohistochemistry.
Mouse tissues were stained with primary antibodies against CD4+ T cells (anti-mouse CD4; BD Biosciences), CD8+ T cells (anti-mouse CD8; BD Biosciences), B cells (anti-mouse CD45R/B220; Caltag Laboratories), macrophages (anti-mouse F4/80; Serotec), and neutrophils/granulocytes (antibody to myeloperoxidase [MPO]; DAKO). Formalin-fixed sections provided the best immunological and morphological staining results with all of the antibodies used, except for anti-CD4 and anti-CD8 antibody staining, which was performed on cryosections. Frozen sections were air dried before use for 30 min, fixed in acetone at −20°C for 10 min, air dried again for 30 min, and then washed in Tris buffer (pH 7.6). Formalin-fixed sections were dewaxed in xylene twice for 5 min, followed by rehydration in graded ethanol. Endogenous peroxidase activity was blocked in the paraffin sections by incubation in 1% (vol/vol) hydrogen peroxidase in methanol for 15 min. For CD4, CD8, and CD45R staining, slides were incubated with a biotin blocking kit (Vector SP-2001). For F4/80 staining, slides were treated with proteinase K (DAKO S3020) for 10 min at room temperature, and in the case of MPO staining, a standard epitope retrieval method based on a microwave heating technique in 10 mM citrate buffer (pH 6.0) was used. In all cases, nonspecific binding was blocked by incubation in 10% normal goat serum in Tris buffer (pH 7.6). Both formalin-fixed and cryopreserved slides were then incubated for 45 min at room temperature in the presence of primary antibodies diluted in Tris-0.5% bovine serum albumin (pH 7.6) at 1/250 (anti-mouse CD4 and anti-CD8), 1/100 (anti-mouse CD45R and anti-F4/80), and 1/500 (anti-MPO). Tissue sections were rinsed in Tris buffer (pH 7.6) and incubated for 20 min with streptavidin-horseradish peroxidase (DAKO) (anti-CD4, anti-CD8, and anti-CD45R) or for 30 min with either EnVision anti-rabbit (DAKO) (anti-MPO) or goat anti-rat-horseradish peroxidase (anti-F4/80). Binding was visualized by incubation with a DAB kit (Vector) for 7 min. Slides were washed in tap water, counterstained with hemalun, and mounted in Eukitt. Quantification of infiltrating cells (CD4, CD8, CD45R/B220, F4/80, and MPO) was determined manually by counting the stained cells with a light microscope (Zeiss Axiophot). Counting was performed at a magnification of ×250 (25× objective lens, 10× ocular). The positive (brown) cells were counted in six randomly chosen fields within the entire cervicovaginal tissue sections from two individual mice. The data were expressed as the mean number of positive cells per square millimeter ± the standard error of the mean (SEM).
Statistics.
Comparison analyses were performed with the Student t test by using GraphPad Prism (GraphPad Software).
RESULTS
Inflammatory gene expression profiles in mouse female cervicovaginal tissue following ivag infection with two attenuated S. enterica serovar Typhimurium strains expressing HPV16 L1.
To determine whether ivag immunization with live attenuated recombinant salmonellae could locally modulate the mucosal pattern of cytokine and chemokine responses, we compared the profiles of constitutive and Salmonella-induced cytokine and chemokine genes in mouse cervicovaginal tissue. A prolonged diestrus-like stage was induced prior to ivag administration to enhance Salmonella infection (31). The levels of mRNA expression of 96 mouse inflammation-associated genes in cervicovaginal tissue were determined by SuperArray analysis of cDNAs after ivag delivery of PBS or Salmonella strains PhoPc kanL1S and AroA kanL1S (20) (Table 1 shows the different strains and abbreviations used in the present study). Total isolated RNA pooled from paired tissue samples at 24 h and 7 days after PBS injection was used to determine the gene expression patterns at a steady-state level in mice in a diestrus-like state (Table 2). Although some differences in mRNA levels could be observed for a very limited number of transcripts between 24 h and 7 days, the large majority of genes exhibited similar mean expression levels at 24 h and 7 days (data not shown). Our results showed that the mouse cervicovaginal microenvironment during diestrus is characterized by the constitutive expression of a wide range of genes coding for both pro- and anti-inflammatory cytokines, as well as cytokine receptors and chemokines (for reviews, see references 6 and 60). The majority of these genes showed a moderate expression level (<4; Table 2), with the exception of two chemokines (CCL21a and MIP-2) and one anti-inflammatory cytokine receptor (interleukin-10Rb [IL-10Rb]), which exhibited a stronger expression level (>4), as well as a member of the TNF ligand family (Ltb) and an anti-inflammatory cytokine (IL-13), which were expressed at a particularly high level (>6; Table 2).
TABLE 1.
Salmonella strains used in this study
TABLE 2.
Gene expression in cervicovaginal tissuesa from uninfected control mice
| Group and gene | Product | Expression levelb |
|---|---|---|
| Proinflammatory cytokines | ||
| Il-1a | IL-1α | 2.67 ± 0.85 |
| Il-1b | IL-1β | 2.12 ± 0.69 |
| Il-1R1 | IL-1 receptor type I | 2.59 ± 0.92 |
| Il-6 | IL-6 | 2.03 ± 0.61 |
| Il-18 | IL-18 | 3.73 ± 0.42 |
| Il-21 | IL-21 | 1.69 ± 0.82 |
| Il-25 | IL-25 | 1.75 ± 0.79 |
| Anti-inflammatory cytokines | ||
| Il-1R2 | IL-1 receptor type II | 3.85 ± 0.63 |
| Il-9 | IL-9 | 2.25 ± 0.57 |
| Il-10Rb | IL-10 receptor, β | 4.69 ± 0.62 |
| Il-11 | IL-11 | 1.52 ± 0.46 |
| Il-11Ra1 | IL-11 receptor, α chain 1 | 2.18 ± 1.14 |
| Il-13 | IL-13 | 6.99 ± 1.22 |
| Il-13Ra1 | IL-13 receptor, α chain 1 | 1.69 ± 0.58 |
| Il-16 | IL-16 | 2.09 ± 0.63 |
| Il-20 | IL-20 | 2.15 ± 1.27 |
| T-cell growth factors | ||
| Il-2 | IL-2 | 3.60 ± 0.87 |
| Il-2Ra | IL-2 receptor, α chain | 1.00 ± 0.00 |
| Il-2Rb | IL-2 receptor, β chain | 2.81 ± 0.68 |
| Il-15Ra | IL-15 receptor, α chain | 2.46 ± 0.86 |
| TGF ligands | ||
| TGF-a | TGF-α | 3.13 ± 0.34 |
| TGF-b3 | TGF-β3 | 2.88 ± 0.75 |
| TNF ligands and receptors | ||
| Tnf | TNF | 3.90 ± 1.10 |
| Ltb | Lymphotoxin B | 6.06 ± 1.28 |
| LtbR | Lymphotoxin B receptor | 2.43 ± 0.71 |
| Chemokines | ||
| Ccl9 (MIP-1g) | Chemokine (C-C motif) ligand 9 | 1.33 ± 0.33 |
| Ccl3 (MIP-1a) | Chemokine (C-C motif) ligand 3 | 1.88 ± 0.79 |
| Ccl4 (MIP-1b) | Chemokine (C-C motif) ligand 4 | 1.47 ± 0.47 |
| Ccl7 (MCP-3) | Chemokine (C-C motif) ligand 7 | 2.80 ± 0.66 |
| Ccl12 (MCP-5) | Chemokine (C-C motif) ligand 12 | 1.34 ± 0.34 |
| Ccl8 (MCP-2) | Chemokine (C-C motif) ligand 8 | 1.00 ± 0.00 |
| Ccl11 (Eotaxin) | Chemokine (C-C motif) ligand 11 | 2.13 ± 0.87 |
| Ccl20 (MIP-3a) | Chemokine (C-C motif) ligand 20 | 2.79 ± 0.65 |
| Ccl21a (Scya21a) | Chemokine (C-C motif) ligand 21a | 4.64 ± 1.47 |
| Ccl25 (TECK) | Chemokine (C-C motif) ligand 25 | 3.71 ± 1.01 |
| Cxcl2 (MIP-2) | Chemokine (C-X-C motif) ligand 2 | 4.14 ± 1.02 |
| Cxcl9 (MIG) | Chemokine (C-X-C motif) ligand 9 | 2.00 ± 1.15 |
| Cxcl10 (IP-10) | Chemokine (C-X-C motif) ligand 10 | 1.64 ± 0.45 |
| Cxcl11 (IP-9) | Chemokine (C-X-C motif) ligand 11 | 1.18 ± 0.23 |
| Cxcl14 (MIP-2g) | Chemokine (C-X-C motif) ligand 14 | 1.73 ± 0.25 |
| Xcl1 (Lymphotactin) | Chemokine (C motif) ligand 1 | 2.24 ± 1.24 |
| Cx3cl1 (Fractalkine) | Chemokine (C-X3-C motif) ligand 1 | 2.04 ± 1.04 |
| Chemokine receptors | ||
| Ccr1 | Chemokine (C-C motif) receptor 1 | 1.00 ± 0.00 |
| Ccr5 | Chemokine (C-C motif) receptor 5 | 3.33 ± 0.83 |
| Ccr2 | Chemokine (C-C motif) receptor 2 | 3.82 ± 0.38 |
| Ccr7 | Chemokine (C-C motif) receptor 7 | 2.45 ± 0.68 |
| Ccr8 | Chemokine (C-C motif) receptor 8 | 1.66 ± 0.66 |
| Ccr9 | Chemokine (C-C motif) receptor 9 | 2.67 ± 1.06 |
| Cxcr3 | Chemokine (C-X-C motif) receptor 3 | 1.00 ± 0.00 |
| Cxcr1 | Chemokine (C-X-C motif) receptor 1 | 2.71 ± 1.00 |
| Cx3cr1 | Chemokine (C-X3-C motif) receptor 1 | 1.86 ± 0.56 |
| Cxcr4 | Chemokine (C-X-C motif) receptor 4 | 1.00 ± 0.06 |
To minimize interindividual variability within mouse groups, total isolated RNA was pooled from paired tissue samples at 24 h and 7 days after PBS injection.
Gene expression levels were scaled from 1 to 10 as described in Materials and Methods, and results are expressed as the mean expression level of the mRNA signal obtained from cervicovaginal tissues harvested 24 h and 7 days after PBS injection ± the SEM.
Modulation of inflammation-associated gene expression following ivag infection with Salmonella was considered significant if the mean expression level of transcripts was at least 2 standard deviations out of the mean expression values obtained with PBS-infected mice. Results were then filtered for a change greater than 1.5-fold. As shown in Table 3, AroA kanL1S and PhoPc kanL1S immunizations induced the up-regulation of 24 and 15 genes and the down-regulation of 2 and 5 genes, respectively, compared with uninfected, PBS-treated animals 24 h after infection. Ivag immunization with AroA kanL1S up-regulated the expression of five potent proinflammatory cytokines genes (those for IL-1α, IL-1β, IL-1R1, IL-21, and IL-25), four anti-inflammatory cytokine genes (those for IL-11, its receptor [IL-11Ra1], IL-13Ra1, and IL-16), and three T-cell growth factor-related genes (those for IL-2Rα, IL-2Rβ, and IL-15Rα). Among the chemokines and chemokine receptors, a wide range of genes, including those for macrophage inflammatory proteins (MIP-2γ/CXCL14 and MIP-1γ/CCL9), IFN-γ-inducible proteins (γIP-10/CXCL10 and IP-9/CXCL11), and CC and CXC chemokine receptors (CCR1, CCR7, CCR8, CCR9, CXCR3, and CXCR4), were up-regulated (Table 3). Of these, the genes for proinflammatory cytokine IL-1α, anti-inflammatory cytokines IL-11 and IL-11Ra1, and chemokine receptors CCR8, CXCR3, and CXCR4 were strongly up-regulated (Table 3, footnote c). Meanwhile, the genes for anti-inflammatory cytokine IL-13 and particularly T-cell growth factor IL-2 were down-regulated after ivag infection with AroA kanL1S (Table 3).
TABLE 3.
Changesa in gene expression in cervicovaginal tissues from miceb 24 h and 7 days after ivag infection with live recombinant AroA kanL1S or PhoPc kanL1S
| Group and gene | Product | 24 h
|
7 days
|
||
|---|---|---|---|---|---|
| AroA | PhoPc | AroA | PhoPc | ||
| Proinflammatory cytokines | |||||
| Il-1a | IL-1 α | + (*)c | + (*) | U | U |
| Il-1b | IL-1 β | + | + | + | + |
| Il-1R1 | IL-1 receptor type I | + | + | U | U |
| Il-21 | IL-21 | + | + | U | U |
| Anti-inflammatory cytokines | |||||
| Il-11 | IL-11 | + (*) | + | U | U |
| Il-11Ra1 | IL-11 receptor, α chain1 | + (*) | + | U | U |
| Il-13 | IL-13 | − | U | U | U |
| Il-13Ra1 | IL-13 receptor, α chain1 | + | U | U | U |
| Il-16 | IL-16 | + | − | + | + |
| T-cell growth factors | |||||
| Il-2 | IL-2 | − (*) | − (*) | U | U |
| Il-2Ra | IL-2 receptor, α chain | + | + | U | U |
| Il-2Rb | IL-2 receptor, β chain | + | U | U | U |
| Il-15Ra | IL-15 receptor, α chain | + | U | + | + |
| TGF ligands | |||||
| TGF-a | TGF-α | U | − | U | U |
| Chemokines | |||||
| Ccl9 | Chemokine (C-C motif) ligand 9 | + | + | U | U |
| Ccl4 | Chemokine (C-C motif) ligand 4 | + | U | U | U |
| Ccl12 | Chemokine (C-C motif) ligand 12 | U | U | + | + |
| Ccl8 | Chemokine (C-C motif) ligand 8 | + | U | U | U |
| Ccl21a | Chemokine (C-C motif) ligand 21a | U | − | U | U |
| Cxcl2 | Chemokine (C-X-C motif) ligand 2 | U | U | − | − |
| Cxcl10 | Chemokine (C-X-C motif) ligand 10 | + | U | U | U |
| Cxcl11 | Chemokine (C-X-C motif) ligand 11 | + | U | U | U |
| Cxcl14 | Chemokine (C-X-C motif) ligand 14 | + | − | U | − |
| Chemokine receptors | |||||
| Ccr1 | Chemokine (C-C motif) receptor 1 | + | + | U | U |
| Ccr5 | Chemokine (C-C motif) receptor 5 | U | + | U | U |
| Ccr7 | Chemokine (C-C motif) receptor 7 | + | U | U | U |
| Ccr8 | Chemokine (C-C motif) receptor 8 | + (*) | + | U | U |
| Ccr9 | Chemokine (C-C motif) receptor 9 | + | + | − | − |
| Cxcr3 | Chemokine (C-X-C motif) receptor 3 | + (*) | + (*) | U | U |
| Cxcr1 | Chemokine (C-X-C motif) receptor 1 | + | + (*) | U | U |
| Cxcr4 | Chemokine (C-X-C motif) receptor 4 | + (*) | + (*) | U | U |
Changes in gene expression in infected mice that exhibited a mean expression value at least 2 standard deviations out of the mean expression values obtained with PBS control mice and a change of >1.5-fold compared to control tissues. U, unchanged; + or −, up- or down-regulated, respectively.
Gene expression changes represent the mean value obtained from two pools of cervicovaginal tissues (two mice per pool).
(*), more-than-threefold change.
Infection with PhoPc kanL1S induced similar expression changes in half of the genes modulated by the AroA kanL1S strain (15 out of 26 genes), yet 14 genes showed differential expression. In contrast to AroA kanL1S, infection with PhoPc kanL1S did not modulate the expression of nine genes, i.e., those for anti-inflammatory cytokines IL-13 and IL-13Ra1, T-cell growth factors IL-2Rb and IL-15Ra, chemokines MIP-1 β, MCP-2, IP-10, and IP-9, and chemokine receptor CCR7. However, a few genes were down-regulated after ivag infection with PhoPc kanL1S, including those for one anti-inflammatory cytokine (IL-16), transforming growth factor α (TGF-α), and two chemokines (CCL21a and MIP-2γ) (Table 3). Finally, the gene for one chemokine receptor transcript (CCR5) showed up-regulation following PhoPc kanL1S infection while remaining unchanged after AroA kanL1S infection. These data indicate that ivag immunization with live attenuated Salmonella modulated the cervicovaginal microenvironment gene expression profile toward a more inflammatory pattern.
We next examined the duration of Salmonella-induced local mucosal inflammation by analyzing gene expression patterns in cervicovaginal tissues at day 7 following ivag infection. In contrast to the wide range of genes that were up-regulated at 24 h after infection, only four genes remained up-regulated at day 7 after AroA kanL1S infection, i.e., those for proinflammatory cytokine IL-1β, anti-inflammatory cytokine IL-16, T-cell growth factor-related IL-15Rα, and chemokine MCP-5 (Table 3). Importantly, most of the genes found to be up-regulated at 24 h returned to a steady-state expression level at day 7 compared to uninfected control mice. Furthermore, the genes for one chemokine (MIP-2) and a chemokine receptor (CCR9) were even down-regulated at day 7 after AroA kanL1S infection. Immunization with PhoPc kanL1S showed gene expression changes identical to those induced by AroA kanL1S, with the exception of the gene for MIP-2γ, which remained down-regulated at day 7 after infection (Table 3). It is worth mentioning that all of the genes strongly up-regulated at 24 h after Salmonella infection were either down-regulated or returned to a steady state at day 7 (Table 3). Thus, with the exception of the gene for proinflammatory cytokine IL-1β, all of the transcripts that were strongly up-regulated early (24 h) after Salmonella immunization returned to a steady state at day 7, suggesting a transient up-regulation of proinflammatory genes following ivag infection with Salmonella.
Immunohistochemical analysis of infiltrating cells in the reproductive tract after ivag infection with AroA kanL1S.
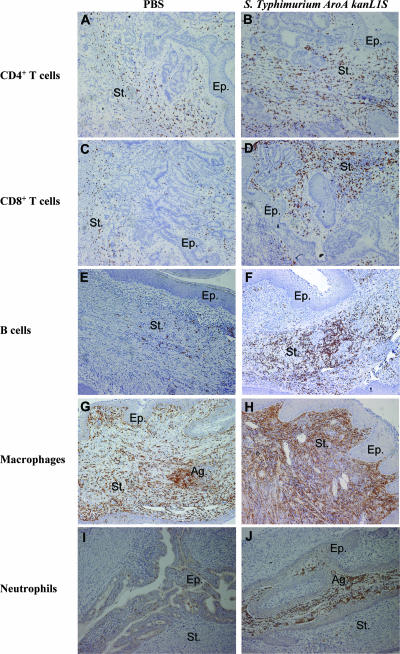
Gene array data strongly suggested infiltration of the cervicovaginal tissue by inflammatory cells upon ivag infection with salmonellae, especially the AroA kanL1S strain. Therefore, the biological relevance of these gene expression profiles was further documented by immunohistochemistry. We stained tissues for CD4+ and CD8+ T lymphocytes, B cells, macrophages, and neutrophils. We analyzed the localization and density of infiltrating immune cells in the reproductive tracts of mice vaginally infected with AroA kanL1S at days 3 and 8 after infection. These two time points were selected to ensure protein-related gene expression and to evaluate the duration of infiltration of immune cells in the cervicovaginal tissue. Staining with F4/80 revealed an early (day 3) massive infiltration of macrophages in the vaginas of AroA kanL1S-infected mice compared to PBS control mice (Table 4, P < 0.001). Infiltrating neutrophils were also clearly more abundant in the vaginas of AroA kanL1S-infected mice than in those of PBS-treated control mice at day 3 (Table 4, P < 0.05). In contrast, while PBS-treated mice showed a reduced influx of CD4+ and CD8+ T cells in the vagina and cervix at day 8, ivag infection with AroA kanL1S was associated with sustained infiltration by both CD4+ and CD8+ T cells at day 8 in the vagina and an increase in CD8+ T cells in the cervix (Table 4). Most likely, the high levels of both CD4+ and CD8+ T cells observed in control mice at day 3, which seemed to resolve afterwards, may be the result of mechanical stress provoked by the contact of the pipette (used to deposit the inoculum or PBS) with the inner part of the vagina. Surprisingly, B cells were barely detected in both AroA kanL1S- and PBS-treated mice at day 3 but were more abundant at day 8, in particular in AroA kanL1S-infected mice, yet the cell densities were not statistically significantly different from those observed in PBS-treated control mice (Table 4). All of the cell types analyzed in the present study were exclusively located in the cervicovaginal region (lower genital tract), with the exception of a few macrophages that were also detected in the uterine horns (data not shown). Analysis of infiltrating cells in the cervical compartment showed results similar to those observed in the vaginal area, with the exceptions of (i) neutrophils that showed similar cell densities in AroA kanL1S- and PBS-treated animals at both time points and (ii) CD8+ T cells that were less abundant in AroA kanL1S-infected mice compared to PBS-treated mice at day 3 (Table 4, P < 0.05). As shown in Fig. 1, all of the cell types analyzed were mostly distributed throughout the stroma, with the exception of neutrophils, which were also sometimes located in the epithelium (Fig. 1I and J). Interestingly, macrophages and neutrophils formed aggregates in some areas (Fig. 1G and J).
TABLE 4.
Cell densities in the mouse vagina and cervix at days 3 and 8 after ivag infection with live recombinant AroA kanL1S
| Subset and origin | Mean no. of cells/mm2 ± SEMa
|
|||
|---|---|---|---|---|
| Day 3
|
Day 8
|
|||
| PBS | AroA kanL1S | PBS | AroA kanL1S | |
| CD4+ T cells | ||||
| Vagina | 767 ± 39 | 469 ± 116 | 393 ± 25f | 697 ± 73c |
| Cervix | 363 ± 57 | 237 ± 86 | 15 ± 7f | 409 ± 42e |
| CD8+ T cells | ||||
| Vagina | 1,015 ± 35 | 902 ± 158 | 429 ± 51f | 912 ± 65d |
| Cervix | 502 ± 45 | 296 ± 32c | 28 ± 7f | 488 ± 44e,g |
| B cells | ||||
| Vagina | 9 ± 5 | 36 ± 23 | 165 ± 83 | 891 ± 406 |
| Cervix | 2 ± 1 | NDb | 6 ± 2 | 143 ± 69 |
| Macrophages | ||||
| Vagina | 558 ± 34 | 1,103 ± 69e | 1,081 ± 109f | 1,933 ± 513 |
| Cervix | 305 ± 64 | 495 ± 53c | 680 ± 112f | 638 ± 277 |
| Neutrophils | ||||
| Vagina | 31 ± 15 | 597 ± 207c | 409 ± 100f | 151 ± 95 |
| Cervix | 170 ± 70 | 43 ± 29 | 82 ± 24 | 164 ± 77 |
Results are densities determined in six random fields from two individual mice.
ND, not detected.
P < 0.05 for AroA kanL1S-treated mice compared with PBS-treated controls.
P < 0.01 for AroA kanL1S-treated mice compared with PBS-treated controls.
P < 0.001 for AroA kanL1S-treated mice compared with PBS-treated controls.
P < 0.01 for PBS-treated controls at day 8 versus day 3.
P < 0.05 for AroA kanL1S-treated mice at day 8 versus day 3.
FIG. 1.
Immunohistochemical localization of immune cell recruitment in cervicovaginal tissue of BALB/c mice 3 and 8 days after ivag infection with 109 CFU of live strain AroA kanL1S (right) compared with that in PBS-injected control mice (left). Cross sections of the entire reproductive tracts were stained with antibodies directed against CD4 (A, B), CD8 (C, D), CD45R/B220 (E, F), F4/80 (G, H), and MPO (I, J). Tissue sections were counterstained with hemalun. Staining of CD4+ and CD8+ T cells and B (CD45R/B220) cells at day 8 and macrophages (F4/80) and neutrophils (MPO) at day 3 to illustrate the clear difference observed between AroA kanL1S-infected and control mice in terms of cell density. Staining of B cells, macrophages, and neutrophils in the vagina and CD4+ and CD8+ T lymphocytes in the cervix is shown. St, stroma; Ep, epithelium; Ag, aggregates. Magnification, ×250 (25× objective, 10× ocular).
These data showed that ivag infection with live attenuated AroA kanL1S induced an early (day 3) recruitment of macrophages and neutrophils and a prolonged (day 8) infiltration of CD4 and CD8 T lymphocytes in the cervicovaginal microenvironment. Thus, with the exceptions of CD4 and CD8 T-lymphocyte densities, which were statistically significantly higher in AroA kanL1S-infected than in PBS-treated mice at day 8, these results suggested a transient infiltration of inflammatory cells following vaginal infection with Salmonella.
Recovery of PhoPc L1S, PhoPc kanL1S, and AroA kanL1S after ivag immunization.
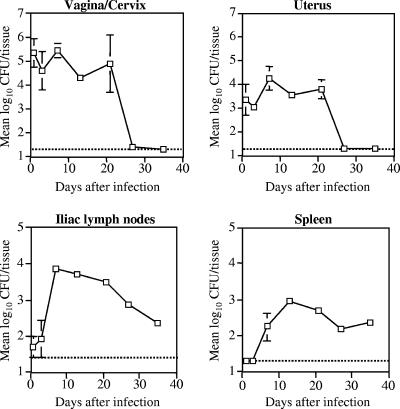
We and others have previously shown that attenuated PhoPc bacteria persisted for at least 3 weeks in mouse organs when two different mucosal routes of immunization, i.e., nasal (5, 31) and oral (32, 51), were used. Here, 6-week-old female BALB/c mice were ivag immunized with 109 CFU of PhoPc and the persistence of the bacteria was determined at different time points in the lower (vagina/cervix) and the upper (uterus) parts of the genital tract, as well as in the draining iliac lymph nodes and the spleen, which was selected as a distant organ. As shown in Fig. 2, PhoPc bacteria were abundant in the genital tissues for at least 3 weeks, while they peaked in the draining lymph nodes and spleen after 1 and 2 weeks, respectively, and were still detected 5 weeks after infection. The stability of the plasmid expressed by different S. enterica serovar Typhimurium strains clearly determines the immunogenicity of the recombinant strain after either nasal or oral immunization (3). High in vitro stability of the kanL1S plasmid was previously observed, with almost 100% of the PhoPc (20) and AroA (data not shown) bacteria still harboring the plasmid after several successive overnight cultures in antibiotic-free medium. Therefore, here we further studied the recovery of both recombinant AroA- and PhoPc-expressing HPV16 L1S plasmids following ivag infection. Strains harboring the HPV16 L1S plasmid encoding either the ampicillin (PhoPc L1S) or the kanamycin (PhoPc kanL1S and AroA kanL1S) resistance marker gene were tested. Both PhoPc kanL1S and AroA kanL1S recovered from the vagina, uterus, and iliac lymph nodes were still harboring the kanL1S plasmid 2 weeks after vaginal infection (Table 5). In contrast, the L1S plasmid harboring the ampicillin resistance marker was less stable, except in the vagina, where all PhoPc bacteria still retained the plasmid. These data suggest better stability of the kanL1S plasmid than the L1S plasmid after ivag immunization, confirming our previous results obtained by using two other mucosal routes of immunization, i.e., nasal and oral (3, 20). In contrast to oral (19) and nasal immunizations, where all PhoPc bacteria recovered from the spleen were still harboring the kanL1S plasmid, ivag immunization with PhoPc, but not with AroA, showed rapid lost of the kanL1S-encoding plasmid in the spleen (4%, Table 5). Plasmid loss in this distant organ may reflect more stringent conditions than in organs close to the site of infection. A similar instability of the L1S plasmid in the spleen was also observed after oral or nasal immunizations with PhoPc (3).
FIG. 2.
Recovery of PhoPc after ivag immunization. Groups of three female BALB/c mice in the diestrus stage were immunized with 109 CFU and sacrificed at different time points following immunization. The different organs were processed and plated on agar plates as described in Materials and Methods. Data are expressed as the geometric mean log10 number of CFU per tissue or organ. The threshold of detection (20 CFU/tissue, or log10 1.3) is indicated by the horizontal dashed line. Error bars indicate the SEM.
TABLE 5.
Recovery of Salmonella PhoPc L1S- and AroA L1S-encoding plasmids carrying either the ampicillin or the kanamycin resistance gene 2 weeks after ivag immunization
| Organ(s) analyzed | Mean log10 no. of Salmonella CFU bearing plasmid ± SEM
|
% of bacteria bearing plasmid
|
||||
|---|---|---|---|---|---|---|
| PhoPc kanL1S | PhoPc L1S | AroA kanL1S | PhoPc kanL1S | PhoPc L1S | AroA kanL1S | |
| Vagina, cervix | 4.23 ± 0.22 | 3.88 ± 0.28 | 4.33 ± 0.04 | 100 | 100 | 100 |
| Uterus | 4.61 ± 0.35 | 2.31 ± 0.48 | 4.72 ± 0.14 | 100 | 21 | 100 |
| Iliac lymph nodes | 3.75 ± 0.15 | 3.18 ± 0.26 | 3.98 ± 0.14 | 100 | 31 | 100 |
| Spleen | 3.53 ± 0.13 | 1.87 ± 0.66 | 1.44 ± 0.30 | 4 | 1 | 100 |
Anti-HPV16 VLP humoral response after ivag immunization with PhoPc kanL1S and PhoPc L1S.
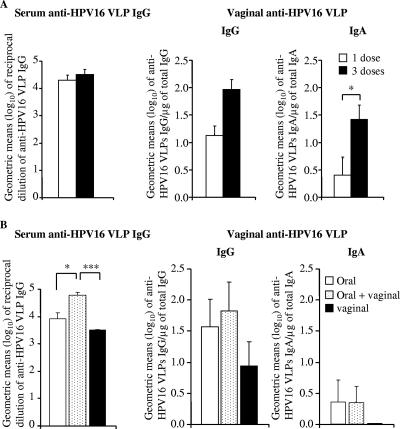
We previously showed that a single oral dose of PhoPc kanL1S (20) or PhoPc L1S (3) induced high anti-VLP antibody titers in serum and in vaginal secretions. Here we further examined the immunogenicity of PhoPc kanL1S and PhoPc L1S by using the ivag route of immunization. Since we previously reported that two or three closely spaced inoculations were more efficient at inducing an HPV16 VLP-specific antibody responses than a single dose when using mucosal routes of immunization, i.e., oral and nasal (2, 51), we compared the HPV16-specific antibody responses in mice ivag immunized with one or three inocula (at 1-week intervals) of PhoPc L1S (109 CFU). HPV16 VLP-specific immune responses in serum and vaginal washes were determined at weeks 0, 4, 6, 8, 10, 14, and 20 after the first immunization and shown at week 8, which corresponded to the peak response in both serum and vaginal secretions. A single vaginal immunization induced high anti-HPV16 VLP IgG titers in serum and vaginal secretions comparable to those induced by three doses (Fig. 3A). In contrast, anti-HPV16 VLP IgA titers in vaginal secretions were significantly higher after three vaginal immunizations (Fig. 3A). A protocol of immunization using a prime-boost strategy was further examined. Three groups of five BALB/c mice were immunized with PhoPc kanL1S (109 CFU) as follows. The first group received an ivag immunization, the second group received an oral immunization, and the third group received a priming immunization given orally, followed by an ivag boost 2 weeks later. The results shown were obtained 14 weeks after the first immunization, which corresponded to the peak response for both serum and vaginal secretions. Interestingly, oral immunization followed by an ivag boost induced significantly higher anti-HPV16 VLP IgG titers in serum than those achieved by oral or ivag immunization alone (Fig. 3B), although specific IgG or IgA levels locally in the vaginal mucosa were not significantly increased. It is worth mentioning that a single ivag immunization with PhoPc kanL1S was as efficient as the oral route of immunization at inducing HPV16 VLP-specific IgG antibodies in serum and vaginal secretions (Fig. 3B). Ivag immunization with the AroA kanL1S strain yielded specific antibody titers similar to those elicited by oral immunization (20) or by ivag immunization with PhoPc kanL1S (a mean serum titer ± SEM of 3.81 ± 0.25 for AroA kanL1S compared to 3.73 ± 0.45 for PhoPc kanL1S at week 11 after immunization).
FIG. 3.
Anti-HPV16 VLP antibody titers in serum and vaginal secretions. (A) Groups of five BALB/c mice were ivag immunized once (white bars) or three times (at 1-week intervals, black bars) with PhoPc L1S (109 CFU). (B) Three groups of five BALB/c mice were immunized with PhoPc kanL1S (109 CFU) as follows. One group received a vaginal immunization (black bars), the second group received an oral immunization (white bars), and the third group received a priming immunization given orally, followed by a vaginal boost 2 weeks later (punctate bars). Shown are anti-HPV16 VLP enzyme-linked immunosorbent assay endpoint titers in serum and vaginal washes at week 8 (A) or 14 (B) after the first immunization. Data are expressed as geometric means (log10) of reciprocal dilutions of specific IgG in serum and specific IgG and IgA per microgram of total IgG and IgA, respectively, in vaginal secretions. Error bars indicate the SEM. Statistical analyses were performed by using Student's t test. *, P < 0.05; ***, P < 0.001.
Antitumor immune response after vaginal immunization with attenuated S. enterica serovar Typhimurium carrying a kanL1S-encoding plasmid.
We previously reported that nasal immunization with a PhoPc strain stably expressing the HPV16 L1 antigen was able to induce antitumor immunity by using the murine C3 tumor model (57). Here, we examined the specific antitumor effect of vaginal immunization with a PhoPc kanL1S or AroA kanL1S strain in the same C3 tumor challenge model. The experimental design used was as previously reported (57). Briefly, mice were vaginally or nasally immunized twice at a 2-week interval with either PhoPc kanL1S or AroA kanL1S and s.c. challenged with C3 tumor cells 4 weeks after the last immunization. Mice were monitored for tumor growth and survival. Tumor volumes were measured twice a week, starting at day 10 following the C3 challenge, and data are shown for day 18 (Fig. 4A), the latest time point before mice bearing large tumors (1.5 cm in diameter or 3,400 mm3 in volume) had to be sacrificed. Tumor-free mice were observed until day 43 post tumor challenge to ensure that they did not develop tumors later. As expected, PhoPc kanL1S administered nasally induced significant tumor protection compared to PBS-treated control animals (Fig. 4A). Comparisons of means tumor volumes showed that vaginal immunization with AroA kanL1S, but not with PhoPc kanL1S, resulted in significant tumor protection compared to the PBS control group (Fig. 4A). Nasal administration of PhoPc kanL1S induced protection similar to that obtained with vaginal AroA kanL1S (survival rate of 70%), while the same PhoPc kanL1S strain given ivag did not improve the outcome (survival rate of 50%) compared to that of uninfected control mice (Fig. 4B). Moreover, tumor-bearing mice vaginally immunized with AroA kanL1S exhibited a significantly smaller tumor volume (mean ± SEM, 703 ± 3 mm3) at day 43 than did PBS-treated control mice (1,775 ± 301 mm3; P < 0.05). These results demonstrated that vaginal immunization with AroA Salmonella expressing the HPV16 L1S antigen was able to induce at least some degree of tumor protection in mice challenged with C3 tumor cells expressing HPV16 antigens.
FIG. 4.
HPV16-specific antitumor effect induced by vaginal immunization with Salmonella-expressing HPV16 L1 antigen in a prophylactic setting. Groups of C57BL/6 female mice (n = 10) were immunized twice at 2-week intervals with a PhoPc kanL1S or a AroA kanL1S strain by either the vaginal or the nasal route and s.c. challenged with 5 × 105 C3 tumor cells in the right flank 4 weeks after the last immunization. Tumor growth was monitored twice a week. (A) The tumor volumes of individual mice are shown at day 18 after the C3 challenge. The mean tumor volumes in each group are represented by horizontal bars. (B) The percent survival rates of PBS-treated control mice (thick solid line) and mice nasally immunized with PhoPc kanL1S (punctate line) or vaginally immunized with PhoPc kanL1S (dashed line) or AroA kanL1S (thin solid line) were monitored for 43 days. Mice sacrificed for ethical reasons when tumors reached 1.5 cm in diameter (3,400 mm3 in volume) were considered dead. Data are representative of two experiments. In panel A, statistical analyses were performed by using Student's t test.
DISCUSSION
Cell-mediated immune responses are important in controlling both HPV infections and HPV-associated neoplasms, as shown by the increased prevalence of these diseases when impaired cell-mediated immunity occurs (38, 61, 63). Spontaneous regression may correlate with the presence of a type 1 (IFN-γ) T-helper memory response against the HPV16 early proteins, which is strongly impaired in patients with high-grade squamous intraepithelial lesions or cancer (12). Interestingly, such a type 1 response, when present in patients suffering from HPV-positive vulvar intraepithelial neoplasia, increased the likelihood of regression upon treatment with imiquimod (a known cytokine inducer (10, 68). To enhance tumor regression, it may thus be necessary to relieve the local immunosuppressive status. Indeed, whereas specific CD8+ T cells that circulate in the blood of melanoma patients were shown to display robust cytotoxic functions, those that reside in tumor lesions were functionally tolerant and thus were unable to prevent tumor progression (71). This functional tolerance was, however, rapidly reversible in vitro, suggesting that improved strategies that combine modification of the tumor microenvironment with induction of tumor antigen-specific immunity may be valuable. In the case of genital lesions, topical application of an immunomodulator and/or a vaccine is feasible and the genital mucosa can generate specific antibody responses after immunization with both inert (27, 36, 52, 55, 65) and live (31, 44, 45) vaccines. Unfortunately, there is no animal model of genital infection by a papillomavirus to assess the importance of local immunity in tumor regression. As preliminary steps, we have thus investigated here the use of live vaccine vectors that can be administered to mice by the vaginal route and are able to induce proinflammatory reactions. First, we analyzed the effect of vaginal delivery of attenuated S. enterica serovar Typhimurium strains on cervicovaginal inflammatory gene expression patterns. We restricted our analysis to mice hormonally treated to remain in a diestrus-like stage of the menstrual cycle for 2 to 3 weeks in order to enhance vaccine uptake and minimize hormone-induced variations in the immune responses. Our microarray analysis of uninfected mice depicted an immunosuppressive cervicovaginal microenvironment. Although such a comprehensive analysis was not previously reported, our data are in agreement with the few studies that partially described the repertoire of the steady-state mucosal immune response in the murine female genital tract, i.e., constitutive expression of chemokines and chemokine receptors (4, 43), as well as Th1- and Th2-type cytokine expression (64). Ivag infection with Salmonella modulated the mucosal gene expression profile toward a more inflammatory pattern by inducing the up-regulation of several key inflammatory mediators. Increased mucosal expression of Th1- and Th2-associated chemokines and chemokine receptors involved in the recruitment of inflammatory cells as described in the present study was also reported during experimental genital Chlamydia trachomatis (4, 43) and Candida albicans (64) infections. Profiling of host gene expression has also been used to examine the host immune response to Salmonella infection, in particular, in cultured host cells such as murine macrophages (59) and human intestinal epithelial cells (15), in both of which Salmonella was shown to induce numerous proinflammatory genes. It was recently shown that, in contrast to the large extent of differentially modulated genes observed in these in vitro studies, oral infection with Salmonella induces few proinflammatory gene expression changes in the rat small intestine (58). Nevertheless, the disparities between the Salmonella strains, cell lines, organs, or animals used, together with the types of genes analyzed in these studies, make direct comparison to our results difficult. However, the changes in the gene expression pattern in the vaginal mucosa in response to invasion by attenuated S. enterica serovar Typhimurium described in the present study, as well as the cited array investigations of host responses to infection with Salmonella, revealed the induction of proinflammatory genes, which is consistent with the common host transcriptional responses to pathogenic bacteria (8). Surprisingly, transcripts of IFN-γ were not detected after ivag Salmonella infection. This may be explained by the fact that message expression was at the limit of the detection level obtained with the SuperArray technique, and thus, other more sensitive methods, such as semiquantitative reverse transcription-PCR, should be used to detect weakly expressed genes. Several sentinel cells, including epithelial cells, macrophages, and dendritic cells, play essential roles in the continuous sensing of pathogenic microorganisms in the mucosal environment. S. enterica serovar Typhimurium has been shown to be taken up by subepithelial dendritic cells in Peyer's patches (32) but also by CD18-expressing phagocytes to disseminate from the gastrointestinal tract to the liver and spleen after oral administration (69). Most likely, similar invasion processes occur in the female genital mucosa following vaginal infection with Salmonella, where mucosal dendritic cells present in the vagina or cervix may internalize and drain the bacteria to the iliac lymph nodes and spleen, as suggested by the presence of Salmonella in these distant organs 5 weeks after vaginal infection. Interestingly, vaginal infection with the auxotrophic AroA mutant strain induced more gene expression changes in the genital tract than did the PhoPc strain. These differential gene expression patterns may be attributable in part to the different LPS structures in the AroA and PhoPc strains. Indeed, the phoP/phoQ system has been shown to regulate structural modifications of lipid A, the major signaling component of LPS, altering the LPS-mediated TNF response (26). Moreover, lipid A modifications can alter TLR4-mediated responses (33, 34), which in turn were shown to affect TLR2 expression in response to S. enterica serovar Typhimurium infection (66), suggesting that although both the AroA and PhoPc strains show attenuated virulence in mice, they differ in their LPS-induced TLR-dependent host immune responses. Finally, the majority of the transcripts that were up-regulated early after Salmonella immunization returned to a steady state later on, suggesting a transient up-regulation of proinflammatory genes induced by vaginal infection with Salmonella. Taken together, these gene array data suggested a recruitment of inflammatory cells in the mucosal genital tract following vaginal infection with Salmonella. Accordingly, histological analysis revealed an early strong infiltration of phagocytes (macrophages and neutrophils) and sustained recruitment of CD4 and CD8 T cells in both the vagina and the cervix similar to that previously reported following vaginal infection with C. trachomatis (48). It is clearly established that chronic inflammation contributes to cancer development (7). Thus, a long-lasting local inflammatory response due to persistent Salmonella infection might have adverse effects in a preexisting tumor microenvironment by promoting malignant progression, as reported in a transgenic mouse model of skin epithelial carcinogenesis (13), where B lymphocytes were shown to mediate chronic inflammation through infiltration of leukocytes into premalignant skin. In the present study, although attenuated Salmonella strains vaginally administered persisted in the genital tract for 3 weeks, the infection was associated with a transient local mucosal inflammatory response.
As a further step, we examined the capacity of the recombinant Salmonella vaccine vectors to induce humoral immune responses against heterologous HPV16 antigens after ivag immunization. Our finding shows that the specific humoral responses induced by this route were similar to those observed after oral immunization. In addition, priming by the oral route, followed by an ivag boost, increased anti-HPV16 VLP titers in serum and, to a lesser extent, in genital secretions, suggesting that such a strategy may enhance the adaptive immune response. As a last step, and in the absence of genital tumors in mice, we examined the ability of ivag immunization with the recombinant Salmonella vaccine strain to induce antitumor responses at distant sites, i.e., regression of s.c. implanted C3 HPV16 tumor cells. Our data show that ivag immunization with AroA kanL1S was able to reduce tumor size and to increase the survival rate, thus suggesting induction of L1-specific cell-mediated immunity (11) and at least partial protection against a challenge with HPV16-expressing tumor cells. The overall ability of the recombinant Salmonella strains to induce distant humoral and cellular responses after ivag immunization may be related to their persistence and plasmid stability not only in genital tissues but also in the draining lymph nodes. Interestingly, in addition to their capacity to induce proinflammatory genes and humoral and cellular responses, salmonellae have the ability to target and replicate within tumors, where they could suppress tumor growth, as reported for mouse tumors s.c. implanted in mice (56). It will be interesting to investigate whether such a property could be useful in an experimental ivag approach to treat local cervical tumors.
In conclusion, our data show that ivag delivery of live attenuated recombinant S. enterica serovar Typhimurium strains may be used to induce transient inflammatory responses in the genital mucosa, as well as specific immunity against heterologous antigens. Whether this strategy can induce regression of HPV tumors located in the genital tract remains to be tested. Caution should also be used in translating this strategy to humans. Attenuated S. enterica serovar Typhimurium has been rarely used as a vaccine strain in humans (1, 29), where bacteria shown to be shed in the feces for a few weeks may be a critical safety issue. Most of the Salmonella-based vaccines are of serovar Typhi (reviewed in reference 40) and are restricted to human hosts. Although oral infection of mice with serovar Typhimurium induces a typhoid disease closely mimicking typhoid fever in humans, it is necessary to confirm that serovar Typhi vaccine strains may induce transient inflammation in the female genital tract. Rectal, but not vaginal, administration of attenuated serovar Typhi strains was tested and shown to be immunogenic in some female volunteers (19, 37, 50). In particular, induction of a specific mucosal immune response in the genital tract was improved by oral priming before a rectal boost with Ty21a, the commercial typhoid vaccine (37). This suggests that oral priming, followed by vaginal boosts with recombinant Ty21a that would express the nononcogenic HPV16 E6 and E7 antigens, may be a testable strategy to treat HPV16-associated cervical lesions.
Acknowledgments
This work was supported by the Fondation Mercier, Oncosuisse (OCS 01403-082003) and the Swiss National Science Foundation (PP00A-104318 and 310000-112406).
Editor: F. C. Fang
Footnotes
Published ahead of print on 10 March 2008.
REFERENCES
- 1.Angelakopoulos, H., and E. L. Hohmann. 2000. Pilot study of phoP/phoQ-deleted Salmonella enterica serovar Typhimurium expressing Helicobacter pylori urease in adult volunteers. Infect. Immun. 682135-2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balmelli, C., S. Demotz, H. Acha-Orbea, P. De Grandi, and D. Nardelli-Haefliger. 2002. Trachea, lung, and tracheobronchial lymph nodes are the major sites where antigen-presenting cells are detected after nasal vaccination of mice with human papillomavirus type 16 virus-like particles. J. Virol. 7612596-12602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baud, D., F. Ponci, M. Bobst, P. De Grandi, and D. Nardelli-Haefliger. 2004. Improved efficiency of a Salmonella-based vaccine against human papillomavirus type 16 virus-like particles achieved by using a codon-optimized version of L1. J. Virol. 7812901-12909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belay, T., F. O. Eko, G. A. Ananaba, S. Bowers, T. Moore, D. Lyn, and J. U. Igietseme. 2002. Chemokine and chemokine receptor dynamics during genital chlamydial infection. Infect. Immun. 70844-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Benyacoub, J., S. Hopkins, A. Potts, S. Kelly, J. P. Kraehenbuhl, R. Curtiss III, P. De Grandi, and D. Nardelli-Haefliger. 1999. The nature of the attenuation of Salmonella typhimurium strains expressing human papillomavirus type 16 virus-like particles determines the systemic and mucosal antibody responses in nasally immunized mice. Infect. Immun. 673674-3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borish, L. C., and J. W. Steinke. 2003. 2. Cytokines and chemokines. J. Allergy Clin. Immunol. 111S460-S475. [DOI] [PubMed] [Google Scholar]
- 7.Coussens, L. M., and Z. Werb. 2002. Inflammation and cancer. Nature 420860-867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cummings, C. A., and D. A. Relman. 2000. Using DNA microarrays to study host-microbe interactions. Emerg. Infect. Dis. 6513-525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curtiss, R., III. 1990. Attenuated Salmonella strains as live vectors for the expression of foreign antigens, p. 161-188. In G. C. Woodrow and M. M. Levine (ed.), New generation vaccines. Marcel Dekker, Inc., New York, NY.
- 10.Dahl, M. V. 2002. Imiquimod: a cytokine inducer. J. Am. Acad. Dermatol. 47S205-S208. [DOI] [PubMed] [Google Scholar]
- 11.De Bruijn, M. L., H. L. Greenstone, H. Vermeulen, C. J. Melief, D. R. Lowy, J. T. Schiller, and W. M. Kast. 1998. L1-specific protection from tumor challenge elicited by HPV16 virus-like particles. Virology 250371-376. [DOI] [PubMed] [Google Scholar]
- 12.de Jong, A., M. I. van Poelgeest, J. M. van der Hulst, J. W. Drijfhout, G. J. Fleuren, C. J. Melief, G. Kenter, R. Offringa, and S. H. van der Burg. 2004. Human papillomavirus type 16-positive cervical cancer is associated with impaired CD4+ T-cell immunity against early antigens E2 and E6. Cancer Res. 645449-5455. [DOI] [PubMed] [Google Scholar]
- 13.de Visser, K. E., L. V. Korets, and L. M. Coussens. 2005. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell 7411-423. [DOI] [PubMed] [Google Scholar]
- 14.Eckmann, L., and M. F. Kagnoff. 2001. Cytokines in host defense against Salmonella. Microbes Infect. 31191-1200. [DOI] [PubMed] [Google Scholar]
- 15.Eckmann, L., J. R. Smith, M. P. Housley, M. B. Dwinell, and M. F. Kagnoff. 2000. Analysis by high density cDNA arrays of altered gene expression in human intestinal epithelial cells in response to infection with the invasive enteric bacteria Salmonella. J. Biol. Chem. 27514084-14094. [DOI] [PubMed] [Google Scholar]
- 16.Ernst, R. K., T. Guina, and S. I. Miller. 1999. How intracellular bacteria survive: surface modifications that promote resistance to host innate immune responses. J. Infect. Dis. 179(Suppl. 2)S326-S330. [DOI] [PubMed] [Google Scholar]
- 17.Fattorossi, A., A. Battaglia, G. Ferrandina, F. Coronetta, F. Legge, V. Salutari, and G. Scambia. 2004. Neoadjuvant therapy changes the lymphocyte composition of tumor-draining lymph nodes in cervical carcinoma. Cancer 1001418-1428. [DOI] [PubMed] [Google Scholar]
- 18.Feltkamp, M. C., H. L. Smits, M. P. Vierboom, R. P. Minnaar, B. M. de Jongh, J. W. Drijfhout, J. ter Schegget, C. J. Melief, and W. M. Kast. 1993. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur. J. Immunol. 232242-2249. [DOI] [PubMed] [Google Scholar]
- 19.Forrest, B. D., D. J. Shearman, and J. T. LaBrooy. 1990. Specific immune response in humans following rectal delivery of live typhoid vaccine. Vaccine 8209-212. [DOI] [PubMed] [Google Scholar]
- 20.Fraillery, D., D. Baud, S. Y. Pang, J. Schiller, M. Bobst, N. Zosso, F. Ponci, and D. Nardelli-Haefliger. 2007. Salmonella enterica serovar Typhi Ty21a expressing human papillomavirus type 16 L1 as a potential live vaccine against cervical cancer and typhoid fever. Clin. Vaccine Immunol. 141285-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gewirtz, A. T., T. A. Navas, S. Lyons, P. J. Godowski, and J. L. Madara. 2001. Cutting edge: bacterial flagellin activates basolaterally expressed TLR5 to induce epithelial proinflammatory gene expression. J. Immunol. 1671882-1885. [DOI] [PubMed] [Google Scholar]
- 22.Gewirtz, A. T., P. O. Simon, Jr., C. K. Schmitt, L. J. Taylor, C. H. Hagedorn, A. D. O'Brien, A. S. Neish, and J. L. Madara. 2001. Salmonella typhimurium translocates flagellin across intestinal epithelia, inducing a proinflammatory response. J. Clin. Investig. 10799-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gey, A., P. Kumari, A. Sambandam, F. Lecuru, L. Cassard, C. Badoual, C. Fridman, B. Nagarajan, W. H. Fridman, and E. Tartour. 2003. Identification and characterisation of a group of cervical carcinoma patients with profound downregulation of intratumoral type 1 (IFNγ) and type 2 (IL-4) cytokine mRNA expression. Eur. J. Cancer 39595-603. [DOI] [PubMed] [Google Scholar]
- 24.Giannini, S. L., W. Al-Saleh, H. Piron, N. Jacobs, J. Doyen, J. Boniver, and P. Delvenne. 1998. Cytokine expression in squamous intraepithelial lesions of the uterine cervix: implications for the generation of local immunosuppression. Clin. Exp. Immunol. 113183-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giannini, S. L., P. Hubert, J. Doyen, J. Boniver, and P. Delvenne. 2002. Influence of the mucosal epithelium microenvironment on Langerhans cells: implications for the development of squamous intraepithelial lesions of the cervix. Int. J. Cancer 97654-659. [DOI] [PubMed] [Google Scholar]
- 26.Guo, L., K. B. Lim, J. S. Gunn, B. Bainbridge, R. P. Darveau, M. Hackett, and S. I. Miller. 1997. Regulation of lipid A modifications by Salmonella typhimurium virulence genes phoP-phoQ. Science 276250-253. [DOI] [PubMed] [Google Scholar]
- 27.Haneberg, B., D. Kendall, H. M. Amerongen, F. M. Apter, J. P. Kraehenbuhl, and M. R. Neutra. 1994. Induction of specific immunoglobulin A in the small intestine, colon-rectum, and vagina measured by a new method for collection of secretions from local mucosal surfaces. Infect. Immun. 6215-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hasan, U. A., E. Bates, F. Takeshita, A. Biliato, R. Accardi, V. Bouvard, M. Mansour, I. Vincent, L. Gissmann, T. Iftner, M. Sideri, F. Stubenrauch, and M. Tommasino. 2007. TLR9 expression and function is abolished by the cervical cancer-associated human papillomavirus type 16. J. Immunol. 1783186-3197. [DOI] [PubMed] [Google Scholar]
- 29.Hindle, Z., S. N. Chatfield, J. Phillimore, M. Bentley, J. Johnson, C. A. Cosgrove, M. Ghaem-Maghami, A. Sexton, M. Khan, F. R. Brennan, P. Everest, T. Wu, D. Pickard, D. W. Holden, G. Dougan, G. E. Griffin, D. House, J. D. Santangelo, S. A. Khan, J. E. Shea, R. G. Feldman, and D. J. Lewis. 2002. Characterization of Salmonella enterica derivatives harboring defined aroC and Salmonella pathogenicity island 2 type III secretion system (ssaV) mutations by immunization of healthy volunteers. Infect. Immun. 703457-3467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoiseth, S. K., and B. A. Stocker. 1981. Aromatic-dependent Salmonella typhimurium are non-virulent and effective as live vaccines. Nature 291238-239. [DOI] [PubMed] [Google Scholar]
- 31.Hopkins, S., J. P. Kraehenbuhl, F. Schodel, A. Potts, D. Peterson, P. De Grandi, and D. Nardelli-Haefliger. 1995. A recombinant Salmonella typhimurium vaccine induces local immunity by four different routes of immunization. Infect. Immun. 633279-3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hopkins, S. A., F. Niedergang, I. E. Corthesy-Theulaz, and J. P. Kraehenbuhl. 2000. A recombinant Salmonella typhimurium vaccine strain is taken up and survives within murine Peyer's patch dendritic cells. Cell. Microbiol. 259-68. [DOI] [PubMed] [Google Scholar]
- 33.Kawasaki, K., R. K. Ernst, and S. I. Miller. 2004. 3-O-deacylation of lipid A by PagL, a PhoP/PhoQ-regulated deacylase of Salmonella typhimurium, modulates signaling through Toll-like receptor 4. J. Biol. Chem. 27920044-20048. [DOI] [PubMed] [Google Scholar]
- 34.Kawasaki, K., R. K. Ernst, and S. I. Miller. 2004. Deacylation and palmitoylation of lipid A by Salmonellae outer membrane enzymes modulate host signaling through Toll-like receptor 4. J. Endotoxin Res. 10439-444. [DOI] [PubMed] [Google Scholar]
- 35.Kleine-Lowinski, K., J. G. Rheinwald, R. N. Fichorova, D. J. Anderson, J. Basile, K. Munger, C. M. Daly, F. Rosl, and B. J. Rollins. 2003. Selective suppression of monocyte chemoattractant protein-1 expression by human papillomavirus E6 and E7 oncoproteins in human cervical epithelial and epidermal cells. Int. J. Cancer 107407-415. [DOI] [PubMed] [Google Scholar]
- 36.Kozlowski, P. A., S. Cu-Uvin, M. R. Neutra, and T. P. Flanigan. 1997. Comparison of the oral, rectal, and vaginal immunization routes for induction of antibodies in rectal and genital tract secretions of women. Infect. Immun. 651387-1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kutteh, W. H., A. Kantele, Z. Moldoveanu, P. A. Crowley-Nowick, and J. Mestecky. 2001. Induction of specific immune responses in the genital tract of women after oral or rectal immunization and rectal boosting with Salmonella typhi Ty 21a vaccine. J. Reprod. Immunol. 5261-75. [DOI] [PubMed] [Google Scholar]
- 38.Laga, M., J. P. Icenogle, R. Marsella, A. T. Manoka, N. Nzila, R. W. Ryder, S. H. Vermund, W. L. Heyward, A. Nelson, and W. C. Reeves. 1992. Genital papillomavirus infection and cervical dysplasia—opportunistic complications of HIV infection. Int. J. Cancer 5045-48. [DOI] [PubMed] [Google Scholar]
- 39.Leggatt, G. R., and I. H. Frazer. 2007. HPV vaccines: the beginning of the end for cervical cancer. Curr. Opin. Immunol. 19232-238. [DOI] [PubMed] [Google Scholar]
- 40.Levine, M. M., C. O. Tacket, and M. B. Sztein. 2001. Host-Salmonella interaction: human trials. Microbes Infect. 31271-1279. [DOI] [PubMed] [Google Scholar]
- 41.Li, Q., and B. J. Cherayil. 2003. Role of Toll-like receptor 4 in macrophage activation and tolerance during Salmonella enterica serovar Typhimurium infection. Infect. Immun. 714873-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mastroeni, P. 2002. Immunity to systemic Salmonella infections. Curr. Mol. Med. 2393-406. [DOI] [PubMed] [Google Scholar]
- 43.Maxion, H. K., and K. A. Kelly. 2002. Chemokine expression patterns differ within anatomically distinct regions of the genital tract during Chlamydia trachomatis infection. Infect. Immun. 701538-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.McDermott, M. R., J. R. Smiley, P. Leslie, J. Brais, H. E. Rudzroga, and J. Bienenstock. 1984. Immunity in the female genital tract after intravaginal vaccination of mice with an attenuated strain of herpes simplex virus type 2. J. Virol. 51747-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller, C. J., N. J. Alexander, P. Vogel, J. Anderson, and P. A. Marx. 1992. Mechanism of genital transmission of SIV: a hypothesis based on transmission studies and the location of SIV in the genital tract of chronically infected female rhesus macaques. J. Med. Primatol. 2164-68. [PubMed] [Google Scholar]
- 46.Miller, S. I., A. M. Kukral, and J. J. Mekalanos. 1989. A two-component regulatory system (phoP phoQ) controls Salmonella typhimurium virulence. Proc. Natl. Acad. Sci. USA 865054-5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mittrücker, H. W., and S. H. Kaufmann. 2000. Immune response to infection with Salmonella typhimurium in mice. J. Leukoc. Biol. 67457-463. [DOI] [PubMed] [Google Scholar]
- 48.Morrison, S. G., and R. P. Morrison. 2000. In situ analysis of the evolution of the primary immune response in murine Chlamydia trachomatis genital tract infection. Infect. Immun. 682870-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nardelli-Haefliger, D., J. Benyacoub, R. Lemoine, S. Hopkins-Donaldson, A. Potts, F. Hartman, J. P. Kraehenbuhl, and P. De Grandi. 2001. Nasal vaccination with attenuated Salmonella typhimurium strains expressing the hepatitis B nucleocapsid: dose response analysis. Vaccine 192854-2861. [DOI] [PubMed] [Google Scholar]
- 50.Nardelli-Haefliger, D., J. P. Kraehenbuhl, R. Curtiss III, F. Schodel, A. Potts, S. Kelly, and P. De Grandi. 1996. Oral and rectal immunization of adult female volunteers with a recombinant attenuated Salmonella typhi vaccine strain. Infect. Immun. 645219-5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nardelli-Haefliger, D., R. B. Roden, J. Benyacoub, R. Sahli, J. P. Kraehenbuhl, J. T. Schiller, P. Lachat, A. Potts, and P. De Grandi. 1997. Human papillomavirus type 16 virus-like particles expressed in attenuated Salmonella typhimurium elicit mucosal and systemic neutralizing antibodies in mice. Infect. Immun. 653328-3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.O'Hagan, D. T., D. Rafferty, J. A. McKeating, and L. Illum. 1992. Vaginal immunization of rats with a synthetic peptide from human immunodeficiency virus envelope glycoprotein. J. Gen. Virol. 73(Pt. 8)2141-2145. [DOI] [PubMed] [Google Scholar]
- 53.Paavonen, J., D. Jenkins, F. X. Bosch, P. Naud, J. Salmeron, C. M. Wheeler, S. N. Chow, D. L. Apter, H. C. Kitchener, X. Castellsague, N. S. de Carvalho, S. R. Skinner, D. M. Harper, J. A. Hedrick, U. Jaisamrarn, G. A. Limson, M. Dionne, W. Quint, B. Spiessens, P. Peeters, F. Struyf, S. L. Wieting, M. O. Lehtinen, and G. Dubin. 2007. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet 3692161-2170. [DOI] [PubMed] [Google Scholar]
- 54.Parkin, D. M., and F. Bray. 2006. Chapter 2: the burden of HPV-related cancers. Vaccine 24(Suppl. 3)S11-S25. [DOI] [PubMed] [Google Scholar]
- 55.Parr, E. L., M. B. Parr, and M. Thapar. 1988. A comparison of specific antibody responses in mouse vaginal fluid after immunization by several routes. J. Reprod. Immunol. 14165-176. [DOI] [PubMed] [Google Scholar]
- 56.Pawelek, J. M., K. B. Low, and D. Bermudes. 1997. Tumor-targeted Salmonella as a novel anticancer vector. Cancer Res. 574537-4544. [PubMed] [Google Scholar]
- 57.Revaz, V., J. Benyacoub, W. M. Kast, J. T. Schiller, P. De Grandi, and D. Nardelli-Haefliger. 2001. Mucosal vaccination with a recombinant Salmonella typhimurium expressing human papillomavirus type 16 (HPV16) L1 virus-like particles (VLPs) or HPV16 VLPs purified from insect cells inhibits the growth of HPV16-expressing tumor cells in mice. Virology 279354-360. [DOI] [PubMed] [Google Scholar]
- 58.Rodenburg, W., I. M. J. Bovee-Oudenhoven, E. Kramer, R. van Der Meer, and J. Keijer. 2007. Gene expression response of the rat small intestine following oral Salmonella infection. Physiol. Genomics 30123-133. [DOI] [PubMed] [Google Scholar]
- 59.Rosenberger, C. M., M. G. Scott, M. R. Gold, R. E. Hancock, and B. B. Finlay. 2000. Salmonella typhimurium infection and lipopolysaccharide stimulation induce similar changes in macrophage gene expression. J. Immunol. 1645894-5904. [DOI] [PubMed] [Google Scholar]
- 60.Rossi, D., and A. Zlotnik. 2000. The biology of chemokines and their receptors. Annu. Rev. Immunol. 18217-242. [DOI] [PubMed] [Google Scholar]
- 61.Rüdlinger, R., I. W. Smith, M. H. Bunney, and J. A. Hunter. 1986. Human papillomavirus infections in a group of renal transplant recipients. Br. J. Dermatol. 115681-692. [DOI] [PubMed] [Google Scholar]
- 62.Smith, J. S., L. Lindsay, B. Hoots, J. Keys, S. Franceschi, R. Winer, and G. M. Clifford. 2007. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int. J. Cancer 121621-632. [DOI] [PubMed] [Google Scholar]
- 63.Sun, X. W., L. Kuhn, T. V. Ellerbrock, M. A. Chiasson, T. J. Bush, and T. C. Wright, Jr. 1997. Human papillomavirus infection in women infected with the human immunodeficiency virus. N. Engl. J. Med. 3371343-1349. [DOI] [PubMed] [Google Scholar]
- 64.Taylor, B. N., M. Saavedra, and P. L. Fidel, Jr. 2000. Local Th1/Th2 cytokine production during experimental vaginal candidiasis: potential importance of transforming growth factor-beta. Med. Mycol. 38419-431. [DOI] [PubMed] [Google Scholar]
- 65.Thapar, M. A., E. L. Parr, and M. B. Parr. 1990. The effect of adjuvants on antibody titers in mouse vaginal fluid after intravaginal immunization. J. Reprod. Immunol. 17207-216. [DOI] [PubMed] [Google Scholar]
- 66.Tötemeyer, S., N. Foster, P. Kaiser, D. J. Maskell, and C. E. Bryant. 2003. Toll-like receptor expression in C3H/HeN and C3H/HeJ mice during Salmonella enterica serovar Typhimurium infection. Infect. Immun. 716653-6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van der Burg, S. H., S. J. Piersma, J. A. De, J. M. van der Hulst, K. M. Kwappenberg, M. van den Hende, M. J. Welters, J. J. Van Rood, G. J. Fleuren, C. J. Melief, G. G. Kenter, and R. Offringa. 2007. Association of cervical cancer with the presence of CD4+ regulatory T cells specific for human papillomavirus antigens. Proc. Natl. Acad. Sci. USA 10412087-12092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.van Poelgeest, M. I., M. van Seters, M. van Beurden, K. M. Kwappenberg, C. Heijmans-Antonissen, J. W. Drijfhout, C. J. Melief, G. G. Kenter, T. J. Helmerhorst, R. Offringa, and S. H. van der Burg. 2005. Detection of human papillomavirus (HPV) 16-specific CD4+ T-cell immunity in patients with persistent HPV16-induced vulvar intraepithelial neoplasia in relation to clinical impact of imiquimod treatment. Clin. Cancer Res. 115273-5280. [DOI] [PubMed] [Google Scholar]
- 69.Vazquez-Torres, A., J. Jones-Carson, A. J. Baumler, S. Falkow, R. Valdivia, W. Brown, M. Le, R. Berggren, W. T. Parks, and F. C. Fang. 1999. Extraintestinal dissemination of Salmonella by CD18-expressing phagocytes. Nature 401804-808. [DOI] [PubMed] [Google Scholar]
- 70.Villa, L. L., R. L. Costa, C. A. Petta, R. P. Andrade, J. Paavonen, O.-E. Iversen, S.-E. Olsson, J. Hoye, M. Steinwall, G. Riis-Johannessen, A. Andersson-Ellstrom, K. Elfgren, G. von Krogh, M. Lehtinen, C. Malm, G. M. Tamms, K. Giacoletti, L. Lupinacci, R. Railkar, F. J. Taddeo, J. Bryan, M. T. Esser, H. L. Sings, A. J. Saah, and E. Barr. 2006. High sustained efficacy of a prophylactic quadrivalent human papillomavirus types 6/11/16/18 L1 virus-like particle vaccine through 5 years of follow-up. Br. J. Cancer 951459-1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zippelius, A., P. Batard, V. Rubio-Godoy, G. Bioley, D. Lienard, F. Lejeune, D. Rimoldi, P. Guillaume, N. Meidenbauer, A. Mackensen, N. Rufer, N. Lubenow, D. Speiser, J. C. Cerottini, P. Romero, and M. J. Pittet. 2004. Effector function of human tumor-specific CD8 T cells in melanoma lesions: a state of local functional tolerance. Cancer Res. 642865-2873. [DOI] [PubMed] [Google Scholar]