Abstract
Brucellosis is still a widespread zoonotic disease. Very little is known about the interaction between Brucella abortus and trophoblastic cells, which is essential for better understanding the pathogenesis of the Brucella-induced placentitis and abortion, a key event for transmission of the disease. The goal of this study was to evaluate the profile of gene expression by bovine trophoblastic cells during infection with B. abortus. Explants of chorioallantoic membranes were inoculated with B. abortus strain 2308. Microarray analysis was performed at 4 h after infection, and expression of cytokines and chemokines by trophoblastic cells was assessed by real-time reverse transcription-PCR at 6 and 12 h after inoculation. In addition, cytokine and chemokine expression in placentomes from experimentally infected cows was evaluated. Expression of proinflammatory genes by trophoblastic cells was suppressed at 4 h after inoculation, whereas a significant upregulation of CXC chemokines, namely, CXCL6 (GCP-2) and CXCL8 (interleukin 8), was observed at 12 but not at 6 h after inoculation. Placentomes of experimentally infected cows had a similar profile of chemokine expression, with upregulation of CXCL6 and CXCL8. Our data indicate that B. abortus modulates the innate immune response by trophoblastic cells, suppressing the expression of proinflammatory mediators during the early stages of infection that is followed by a delayed and mild expression of proinflammatory chemokines, which is similar to the profile of chemokine expression in the placentomes of experimentally infected cows. This trophoblastic response is likely to contribute to the pathogenesis of B. abortus-induced placentitis.
Brucella abortus is a facultative intracellular gram-negative bacterium that causes abortion and temporary infertility in cattle (13, 19, 29). It is also a zoonotic pathogen causing fever, weakness, endocarditis, arthritis, osteomyelitis, and meningitis in humans (51). In cattle, the infection tends to be chronic with tropism for the reproductive system of pregnant cows. Abortion is the most significant clinical sign, but an infected cow may be completely asymptomatic. Transmission of the disease occurs mainly after abortion or parturition of infected cows via contaminated fetus, fetal membranes, and uterine secretions (8, 37, 38). During the early stages of infection, B. abortus is found mostly in lymph nodes. The infection may progress to bacteremia and colonization of the uterus, where the organism replicates preferentially within trophoblasts in the rough endoplasmic reticulum (9, 10, 29). As a result, the cow develops placentitis, fetal death, and abortion, particularly during the last third of the gestation (2). B. abortus grows primarily in the extracotyledonary trophoblasts and then spreads to the cotyledonary (placental) trophoblasts (3). Therefore, proliferation of B. abortus within trophoblastic cells is a key event in the mechanism of abortion. Trophoblasts favor bacterial growth by producing erythritol and progesterone, which stimulate in vitro growth of B. abortus (15, 42). In acute infection of pregnant cows, more than 85% of the bacteria are located in the placenta and allantoic fluid (43). In spite of the importance of intracellular multiplication of B. abortus within trophoblastic cells and consequent placentitis, which are key events in the pathogenesis of B. abortus-induced abortion, information regarding the interaction between B. abortus and bovine trophoblast is extremely scarce.
Innate immunity against B. abortus involves a system of pattern recognition receptors which recognizes conserved sequences known as pathogen-associated molecular patterns such as Brucella lipopolysaccharide (LPS), which is recognized by Toll-like receptor 4 (TLR4). TLRs signal through an adapter molecule, MyD88, to activate the NF-κB pathway, resulting in cytokine induction and regulation of costimulatory molecules (30, 44). However, Brucella LPS is not a strong agonist of TLR4, which favors evasion of the host innate immunity (4, 11, 18, 26, 45). Conversely, Brucella lipoproteins, which are TLR2 ligands, have a proinflammatory effect (17). In spite of the knowledge accumulated over the last few years on the interaction of the host with Brucella, little is known about the interaction of this organism with trophoblastic cells, particularly in regard to the role played by the trophoblastic cells in eliciting the placental inflammatory response. This is a biologically relevant theme, since in contrast to silent chronic infections, infection of placenta (primarily trophoblastic cells) with Brucella results in an acute inflammatory response (2, 3, 31). Due to the lack of bovine trophoblastic cell lines, phagocytic cells or poorly differentiated cell lines are often used for studying the pathogenesis of B. abortus. However, Brucella-elicited proinflammatory trophoblastic response may play a fundamental role in the pathogenesis of placentitis in bovine brucellosis.
Trophoblastic cells have been intensively studied in the context of implantation, maternal recognition of pregnancy, and placental development, including gene expression profiling, which resulted in identification of expression of several genes such as interferon tau (5), matrix metalloproteinases (50), proteoglycans, adhesion molecules, integrins, and growth factors (46-48). However, none of these studies have focused on the response of the trophoblast to intracellular pathogens, particularly Brucella. Considering the upregulation of proinflammatory genes in Brucella-infected mouse macrophage RAW 264.7 (14) as well as in vivo in the mouse (36), an evaluation of gene expression profile of bovine trophoblasts during infection can contribute significantly to our understanding of the pathogenesis of B. abortus-induced abortion.
In this study, we employed a previously developed model of infection of explants of the bovine chorioallantoic membranes (CAM) with B. abortus (37, 39) in association with microarray technology and quantitative real-time reverse transcription-PCR (RT-PCR). Therefore, considering our hypothesis that the interaction of B. abortus with trophoblasts results in a change in the profile of gene expression with upregulation of proinflammatory genes, the aim of this study was to identify differentially expressed genes in bovine trophoblastic cells during infection with B. abortus.
MATERIALS AND METHODS
Bacterial strain and growth conditions.
Brucella abortus strain 2308 was used in all experimental inoculations in this study. Frozen stocks of B. abortus strain 2308 were prepared by growing the bacterium on tryptose agar (Difco) for 48 h at 37°C in 5% CO2. Prior to experimental inoculation, B. abortus was grown in 5 ml of Brucella broth (Difco) for 24 h at 37°C under agitation (200 rpm).
CAM explant.
CAM were obtained at local slaughterhouses from bovine pregnant uteruses with gestational ages ranging from 180 to 240 days as assessed by measuring the fetal crown-rump length as previously described (16). All tissues were obtained from cows serologically negative for brucellosis and were processed according to a previously described method (37, 39) with modifications as detailed below. CAM were aseptically removed from the uterus and immediately placed into RPMI 1640 (Invitrogen; Carlsbad, CA) with 50 U/ml of penicillin and 50 μg/ml of streptomycin (Invitrogen) for 20 min. The CAM were then washed two times with RPMI 1640 to remove antibiotic residues, mounted onto a support (Fig. 1), and placed into six-well cell culture plates with supplemented medium (RPMI 1640 with 10% fetal calf serum and 4 mM l-glutamine) in contact with the trophoblastic and allantoic or amniotic surfaces, which were completely separated from each other (Fig. 1).
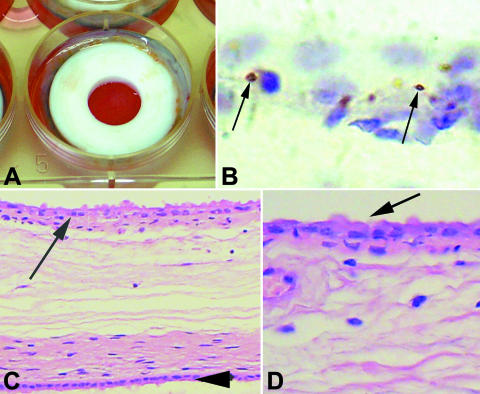
FIG. 1.
Infection of cultured bovine CAM explants with Brucella abortus. (A) CAM explants in a six-well plate with the trophoblastic side toward the well in the center of the ring. (B) Immunohistochemical localization of B. abortus (arrows) within trophoblastic cells in a cultured CAM explant. (C and D) CAM cultured for 48 h. Trophoblastic cells are indicated by arrows, and the amnion is indicated by the arrowhead. H&E staining.
CAM experimental infection.
CAM were divided into two experimental groups (infected and control). CAM were inoculated on their trophoblastic surface with a suspension of 2.0 × 107 CFU of B. abortus in supplemented RPMI per well, corresponding to a multiplicity of infection of approximately 1:1,000, considering an average of 20,000 trophoblasts per explant, as determined morphometrically (described below). An equal volume of sterile medium was added to the trophoblastic surface of control CAM. After inoculation, CAM were cultured for 4, 6, or 12 h in RPMI 1640 supplemented with 10% fetal calf serum and 4 mM l-glutamine at 37°C in a humidified atmosphere with 5% CO2 followed by washing with the same medium and culture with medium containing 50 μg/ml of gentamicin for 1 h to inactivate extracellular bacteria. Additional CAM inoculated in parallel under the same conditions were processed for CFU counting as described below. At different time points after inoculation, the media were removed and RNA extraction was performed by adding Trizol (Invitrogen; Carlsbad, CA) directly onto the trophoblastic surface of the CAM explants.
In vivo experimental infection.
The experimental protocol for in vivo infection used in this study has been previously described (31, 34). All heifers were serologically negative for brucellosis before challenge, as assessed by the Rose Bengal plate agglutination test. The heifers underwent estrous synchronization followed by artificial insemination. Pregnancy was confirmed by ultrasonography at 35 days after insemination. Between 6 and 7 months of pregnancy, the heifers were challenged by conjunctival administration of the virulent B. abortus strain 2308. Each heifer was inoculated with 50 μl in each eye (total of 100 μl per heifer) with a bacterial suspension containing a total of 3.0 × 107 CFU. The heifers were then kept under observation until abortion or calving. This experimental protocol was approved by the local Committee for Ethical Use of Experimental Animals (CETEA-UFMG, protocol 028/05). The heifers were divided in three groups: (i) heifers with negative bacteriology and term gestation (n = 6), (ii) heifers with positive bacteriology and term gestation (n = 5), and (iii) heifers with positive bacteriology and abortion (n = 5). Samples of placentomes were collected immediately frozen in liquid nitrogen and stored at −80°C until RNA extraction. Additional samples were fixed and processed for histopathology.
Bacteriological analysis.
Following treatment with gentamicin for inactivation of extracellular bacteria (as described above), trophoblastic cells from infected CAM explants were lysed with a solution of 0.1% sterile Triton X-100 (Roche; Mannheim, Germany). The lysate was serially diluted, plated on tryptose agar plates (Difco), and incubated for 48 h at 37°C with 5% CO2 for CFU counting.
Histopathology and immunohistochemistry.
Morphological integrity of CAM was assessed by histological analysis of CAM explants from each placenta. CAM explants were fixed for 24 h in 10% neutral-buffered formalin and further processed for paraffin embedding, sectioning, and staining with hematoxylin and eosin stain (H&E stain). Immunohistochemistry was performed as previously described (40) for confirmation of the intracellular localization of B. abortus in trophoblasts. Briefly, sections (4 μm) were deparaffinized, hydrated, and incubated in 4% hydrogen peroxide in phosphate-buffered saline (PBS) (0.01 M, pH 7.2) for 30 min, incubated with skim milk (1:10 dilution) as a blocking solution for 30 min, and then incubated with a polyclonal anti-B. abortus antibody (1:100 dilution) as the primary antibody for 30 min in a humidified chamber at room temperature. After being washed in PBS, the slides were incubated with biotinylated secondary antibody for 20 min at room temperature, washed in PBS again, and then incubated with streptavidin-peroxidase complex (LSAB1 kit; DAKO Corporation, Carpinteria, CA) for 20 min at room temperature. The reaction was developed with a 0.024% diaminobenzidine solution (Dako), and sections were counterstained with Mayer's hematoxylin. H&E-stained sections of CAM explants were morphometrically analyzed with a micrometric ocular to estimate the number of trophoblastic cells per explant.
Oligonucleotide microarray analysis.
A high-density microarray containing 13,249 70-mer oligonucleotides (NCBI Gene Expression Omnibus number, GPL2853) was used in this study (28). Total RNA used for microarray analysis was obtained from six placentas with 12 infected and 12 control explants from each placenta. At 4 h after inoculation, total RNA was isolated from the trophoblastic surface of CAM by use of Trizol (Invitrogen) according to the manufacturer's instructions. Total RNA was further purified with the RNeasy mini kit (Qiagen, Valencia, CA). RNA samples were analyzed by spectrophotometry and agarose gel electrophoresis. Three RNA pools from two placentas each were generated prior to cDNA synthesis. cDNA from infected and control CAM explants was synthesized and labeled using random hexamers and amino allyl dUTP. cDNAs from infected CAM explants were labeled with Alexa Fluor reactive dye 647 (Molecular Probes/Invitrogen) and cDNAs from control CAM explants were labeled with Alexa Fluor reactive dye 555 (Molecular Probes/Invitrogen). Labeled cDNAs were hybridized with the spotted oligonucleotides for 48 h in a hybridization station (Perkin Elmer HybArray). After being washed, the slides were scanned using the ProScanArray Microarray (Perkin Elmer). The images were analyzed using the GenePix Pro (Molecular Devices) and GeneSpring (Agilent Technologies) software.
Real-time RT-PCR analysis.
Quantitative real-time RT-PCR was used for validation of the microarray results and to assess expression of cytokines and chemokines in cultured CAM explants and in placentomes of experimentally infected cows. The same pools of RNA used for the microarray analysis were also processed for validation of the microarray data by RT-PCR. For assessing cytokine and chemokine expression in CAM explants, another five placentas were collected and total RNA was extracted from infected and control CAM explants at 6 and 12 h after inoculation. Cytokine and chemokine expression in placentomes from experimentally infected cows was performed using total RNA isolated from placental tissues. Total RNA from CAM explants or tissue samples was extracted using the Trizol reagent (Invitrogen) according to the manufacturer's instructions. One microgram of total RNA from each sample was retrotranscribed in a total volume of 50 μl (TaqMan RT reagent; Applied Biosystems, Foster City, CA). Each real-time PCR was performed using 2 μl of cDNA, 600 nM of sense and antisense primers (Table 1), and 17 μl of SYBR green mix (Applied Biosystems). Cycling parameters for real-time PCR were 50°C for 2 min, 95°C for 10 min, and 40 cycles of 95°C for 15 s and 60°C for 1 min, using the 7900HT fast real-time PCR system (Applied Biosystems). The data were analyzed using the comparative threshold cycle (CT) method (27). Severalfold changes in expression of target genes in infected CAM explants were calculated relative to the average level of the respective gene in control CAM explants from the corresponding time point after inoculation. CT values were normalized based on GAPDH expression. The primers used in this study are listed in Table 1.
TABLE 1.
List of genes and primers for real-time RT-PCR
| Gene | Primers | Product size (nt) |
|---|---|---|
| CXCL1 (GROα) | 5′-CTATTTTTGGGGAGAGGGTATTCC-3′ | 94 |
| 5′-CGTGACCTATCTGTTTGCTTGAACC-3′ | ||
| CXCL3 (GROγ) | 5′-GGATGGCTGTTCCAGAAGTAGACC-3′ | 64 |
| 5′-GGTGATTCCTCTTTTCCCTCTTTG-3′ | ||
| CXCL6 (GCP-2) | 5′-ATTCATCCCAAAACGGTCAGTG-3′ | 101 |
| 5′-CAGACTTCCCTTCCATTCTTCAAG-3′ | ||
| CXCL8 (IL-8) | 5′-ACACATTCCACACCTTTCCACC-3′ | 117 |
| 5′-GCAGACCTCGTTTCCATTGGTAAG-3′ | ||
| IL-1 beta | 5′-TCCTCCGACGAGTTTCTGTGTG-3′ | 76 |
| 5′-GGGATTTTTGCTCTCTGTCCTGG-3′ | ||
| IL-18 | 5′-GCTCTCCAATGCTTTCAGCG-3′ | 147 |
| 5′-AGCCATCTTTATGCCTGTGCTC-3′ | ||
| GAPDH | 5′-ATGGTGAAGGTCGGAGTGAACG-3′ | 121 |
| 5′-TGTAGTGAAGGTCAATGAAGGGGTC-3′ | ||
| Complement component 9 | 5′-ACAGCAGGCTATGGGATCAA-3′ | 58 |
| 5′-TGTCAAAAGGTGTGCTTAGGG-3′ | ||
| IL-4 | 5′-TGTCACTGCAAATCGACACC-3′ | 74 |
| 5′-ATGCCAGCAGGAAGAACAGA-3′ | ||
| Lymphotoxin β | 5′-CAGGAGCCACTTCTCTGGTG-3′ | 96 |
| 5′-TTACCAGTCCTCCCTGATCCT-3′ | ||
| Serum amyloid A4 | 5′-CTTCCTGCTCCTCTGCTCTC-3′ | 77 |
| 5′-GTGACCCTGTGTCCCTGTCT-3′ | ||
| TNF (ligand) superfamily member 8 | 5′-GAGGAGGTTGACACAGCACA-3′ | 84 |
| 5′-GGATGAGAGGGACTTGAGAGAA-3′ | ||
| CXCL11 | 5′-GTTCAAGGCTTCCCCATGTT-3′ | 77 |
| 5′-TCTGCCACTTTCACTGCTTTT-3′ |
Statistical analysis.
The microarray results were analyzed using the statistical tools of the GeneSpring software. The statistical significance of change in real-time RT-PCR assays was verified by the Student Newman Keuls test after logarithmic transformation of the raw data with Graphpad Instat software, version 3.05 (Graphpad Software, Inc., CA).
Microarray data accession number.
The entire microarray data set has been submitted to the Gene Expression Omnibus database at NCBI (http://www.ncbi.nlm.nih.gov/geo/) and has been assigned accession number GSE10342.
RESULTS
Invasion of bovine trophoblastic cells by Brucella abortus.
In order to evaluate the suitability of the CAM explant model for studying the early stages of infection, the invasion of trophoblastic cells by B. abortus in cultured CAM explants was evaluated by bacteriological and immunohistochemical methods. Morphometric analysis indicated an estimated number of trophoblasts ranging from 13,225 to 31,651 per well (Fig. 1). Based on an approximate average of 20,000 trophoblasts per CAM explant, the number of B. abortus per cell was estimated over the time course of infection between 2 and 12 h after inoculation (Fig. 2). A marked increase in the intracellular numbers of B. abortus during the first 4 h after inoculation reflects invasion rather than intracellular multiplication of the organism. Considering the number of intracellular organisms at 6 and 12 h after inoculation, the intracellular doubling time in trophoblasts was estimated to be approximately 8.7 h. Furthermore, immunohistochemistry confirmed that the organisms were indeed localized intracellularly in trophoblastic cells (Fig. 1). After 12 h in culture, marked losses of trophoblastic cells in CAM explants were observed. Morphologically, this loss was characterized by a reduction in the number and size of trophoblastic cells, which were flattened and vacuolated, with detachment of trophoblasts from the basal membrane. Therefore, all experiments with CAM explants in this study were limited to 12 h.
FIG. 2.
Time course of invasion of bovine trophoblastic cells in CAM explants by Brucella abortus. Explants were inoculated with an MOI of 1,000 of B. abortus strain 2308, treated with gentamicin to kill extracellular bacteria, lysed, serially diluted, and plated for CFU counting. Data points represent means and standard errors of the mean for five independent experiments performed in triplicate.
Profile of trophoblastic gene expression during the early stages of Brucella abortus infection.
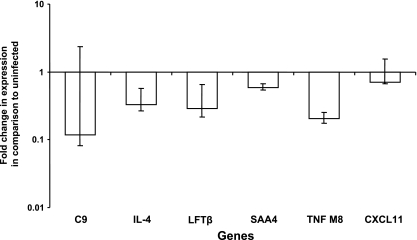
Changes in the profile of gene expression in trophoblastic cells of CAM explants during the early stages of infection with B. abortus were determined by microarray analysis at 4 h after inoculation. This time point was selected based on the curve of invasion of trophoblastic cells by B. abortus (Fig. 2) and a previous microarray study with Brucella-infected macrophages (14). RNAs from six independent experiments were pulled to generate three pools from two experiments each. Thus, the microarray analysis reflects the results of three independent hybridizations, which were analyzed by filtering based on severalfold change, considering up- or downregulated genes as those with at least a twofold increase or decrease from the level seen for the uninfected controls. The data were further filtered based on statistical significance (P < 0.05), thus eliminating genes whose increase or decrease in the amount of mRNA was not consistent in all of the three RNA pools. This approach resulted in the identification of 25 genes that had a statistically significant increase in expression (Table 2) and 37 downregulated genes (Table 3) at 4 h postinfection. Surprisingly, a significant downregulation of several proinflammatory genes was observed at 4 h after inoculation of CAM explants with B. abortus (Table 3). Considering that this study was focused on evaluating innate immunity and inflammatory response, reduced expression of genes associated with inflammation and immune response was confirmed by real-time RT-PCR analysis (Fig. 3), including complement component 9, interleukin 4 (IL-4), lymphotoxin beta, serum amyloid A4, member 8 of the tumor necrosis factor (TNF) superfamily, and chemokine CXC ligand 11 (CXCL11). Levels of expression of selected genes involved in inflammation were similar when analyzed by microarray and real-time RT-PCR, which also demonstrated a trend of decreased expression of these genes (Table 3 and Fig. 3), thus validating the microarray analysis.
TABLE 2.
Upregulated genes in cultured explants of bovine chorioallantoic membranes infected with Brucella abortus at 4 h after inoculation
| Function and GenBank no. | Fold upregulation | P value | Gene or gene product/function |
|---|---|---|---|
| Inflammation and immune response | |||
| BQ940635 | 2.95 | 0.00873 | DnaJ (Hsp40) homolog, subfamily C, member 14 |
| AL833759 | 10.0 | 0.00637 | Hypothetical protein FLJ39827 |
| Transcription | |||
| BX537915 | 2.008 | 0.000012 | Transcription elongation factor A (SII), 3 |
| BQ575777 | 2.793 | 0.0126 | TAF12 RNA polymerase II, TATA box binding protein-associated factor, 20 kDa |
| AF353674 | 2.849 | 0.0226 | BTB (POZ) domain containing 6 |
| Cell cycle | |||
| AK090488 | 2.242 | 0.0239 | Protein phosphatase 2 (formerly 2A), regulatory subunit A (PR 65), alpha isoform |
| NM_057749 | 3.194 | 0.0464 | Cyclin E2 |
| AY376439 | 2.04 | 0.0381 | Epithelial cell transforming sequence 2 oncogene |
| Transport | |||
| NM_021614 | 10.0 | 0.00637 | Potassium intermediate/small conductance calcium-activated channel, subfamily N, member 2 |
| Signaling | |||
| AK074259 | 4.184 | 0.00621 | Pleckstrin homology domain containing family B (evectins) member 2 |
| CN434563 | 2.439 | 0.028 | HSPC054 protein |
| Regulation | |||
| NM_024321 | 2.066 | 0.046 | Hypothetical protein MGC10433 |
| BM729023 | 2.77 | 0.0443 | CUG triplet repeat, RNA binding protein 2 |
| Adhesion | |||
| BQ716387 | 2.061 | 0.00518 | Cadherin 5, type 2, vascular epithelium cadherin |
| Metabolism | |||
| BX647562 | 2.008 | 0.000808 | Hypothetical protein MGC4767 |
| CR627424 | 4.184 | 0.026 | Transcribed locus, moderately similar to XP_526970.1 (Pan troglodytes) |
| NM_020773 | 2.415 | 0.0187 | TBC1 domain family, member 14 |
| Membrane | |||
| AK091890 | 2.024 | 0.0482 | CD34 antigen |
| Other | |||
| BM563524 | 2.053 | 0.011 | PWWP domain containing 1 |
| BU729749 | 2.096 | 0.00666 | Similar to CG14894-PA |
| NM_024067 | 2.169 | 0.024 | Chromosome 7 open reading frame 26 |
| NG010008A20C05 | 2.61 | 0.0485 | Unknown |
| BM363643 | 2.688 | 0.00594 | Unknown |
| W31247 | 3.69 | 0.0335 | Phosphoprotein enriched in astrocytes 15 |
| CN434037 | 2.564 | 0.0444 | Unknown |
TABLE 3.
Downregulated genes in cultured explants of bovine chorioallantoic membranes infected with Brucella abortus at 4 h after inoculation
| Function and GenBank no. | Fold downregulation | P value | Gene or gene product/function |
|---|---|---|---|
| Inflammation and immune response | |||
| NM_001737 | 14.09 | 0.000306 | Complement component 9 |
| CD640452 | 7.379 | 0.0125 | IL-4 |
| BQ064345 | 3.321 | 0.00472 | Lymphotoxin beta (TNF superfamily member 3) |
| BG618424 | 2.977 | 0.00241 | Serum amyloid A4, constitutive |
| AW452023 | 2.831 | 0.0138 | TNF (ligand) superfamily member 8 |
| U66096 | 2.162 | 0.000687 | CXCL11 |
| Transcription | |||
| NM_016276 | 3.451 | 0.0384 | Serum/glucocorticoid-regulated kinase 2 |
| AW266874 | 3.288 | 0.01 | Unknown |
| Cell cycle | |||
| D15049 | 2.124 | 0.00767 | Protein tyrosine phosphatase, receptor type H |
| Stress | |||
| AK098671 | 4.016 | 0.0199 | Dimethylarginine dimethylaminohydrolase 2 |
| CR455999 | 2.243 | 0.000287 | Glutathione synthetase |
| Transport | |||
| BC006404 | 2.753 | 0.00853 | Suppressor of potassium transport defect 3 |
| NM_170736 | 2.524 | 0.0103 | Potassium inwardly rectifying channel, subfamily J, member 15 |
| Signaling | |||
| AK092516 | 2.657 | 0.0238 | Hypothetical protein LOC150084 |
| NM_145803 | 2.222 | 0.0169 | TNF receptor-associated factor 6 |
| Regulatory | |||
| CN432381 | 24.54 | 0.0258 | Unknown |
| NM_000035 | 5.368 | 0.0255 | Aldolase B, fructose-bisphosphate |
| BG117301 | 3.564 | 0.0223 | Pleiotropic regulator 1 (PRL1homolog, Arabidopsis) |
| BX648731 | 2.555 | 0.0426 | Junction-mediating and regulatory protein |
| AI971356 | 2.482 | 0.0197 | Transcribed locus, strongly similar to NP_000446.1 serine/threonine kinase 11 |
| BE294317 | 2.142 | 0.0444 | p53 and DNA damage regulated 1 |
| Metabolism | |||
| AA035275 | 2.886 | 0.00485 | Transcribed locus, moderately similar to XP_126676.1 RIKEN cDNA 1810057P16 gene [Mus musculus] |
| NM_031438 | 2.884 | 0.045 | Nudix (nucleoside diphosphate linked moiety X)-type motif 12 |
| BC047129 | 2.519 | 0.0446 | Flavin-containing monooxygenase 1 |
| BM808152 | 2.255 | 0.0355 | Adenine phosphoribosyltransferase |
| Y14385 | 2.235 | 0.0465 | Inositol polyphosphate phosphatase-like 1 |
| Membrane | |||
| DN547823 | 3.502 | 0.0323 | Unknown |
| Other | |||
| CR456122 | 3.452 | 0.0454 | Unknown |
| AK094654 | 3.094 | 0.00241 | Chromosome 14 open reading frame 129 |
| DN642227 | 3.082 | 0.00241 | Unknown |
| NM_005382 | 2.912 | 0.0418 | Neurofilament 3 (150-kDa medium) |
| CN438371 | 2.824 | 0.0337 | Unknown |
| AK125351 | 2.805 | 0.0136 | RNA binding motif protein 21 |
| NM_182684 | 2.789 | 0.0297 | Uroplakin IIIb |
| BC015335 | 2.721 | 0.00418 | Immature colon carcinoma transcript 1 |
| BF440457 | 2.649 | 0.0195 | Transcribed locus |
FIG. 3.
Validation of the microarray results by real-time RT-PCR, indicating downregulated expression of proinflammatory genes in CAM explants infected with Brucella abortus in comparison to the uninfected group at 4 h postinoculation. Bars represent geometric means and standard errors of the mean for three pools of two placentas each, totaling six independent experiments. Abbreviations: C9, complement component 9; LFTβ, lymphotoxin beta; SAA4, serum amyloid A4; TNF M8, TNF (ligand) superfamily member 8.
Expression of proinflammatory chemokines and cytokines by bovine trophoblastic cells during infection with Brucella abortus.
Considering the acute inflammatory response in the placentas of cattle infected with B. abortus and the lack of proinflammatory response by trophoblastic cells at 4 h after inoculation, as demonstrated by microarray analysis, we evaluated expression of CXC chemokines (CXCL8 [IL-8], CXCL1 [GROα], CXCL3 [GROγ], and CXCL6 [GCP-2]) as well as proinflammatory cytokines (IL-1β and IL-18) at later time points during infection (6 and 12 h). Epithelial cells are known to express CXC chemokines (12), and although the two proinflammatory cytokines selected for this study are primarily secreted by professional phagocytes, they were measured, since in addition to trophoblastic cells the trophoblastic surface of the CAM explants contain other resident cells, which may include small but significant populations of lymphocytes and phagocytic cells. The limitation of using transcripts to measure IL-1β and IL-18 is noteworthy, since these cytokines require caspase 1 cleavage for activation (6, 25).
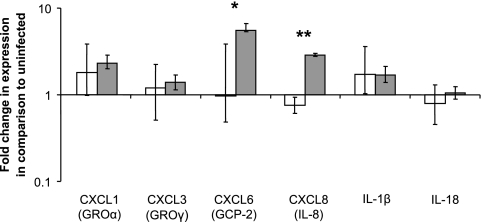
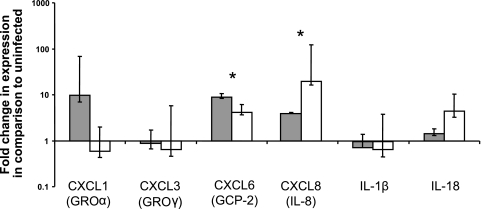
Infected CAM explants had a significant increase in expression of CXCL8 and CXCL6 at 12 h but not at 6 h postinfection, compared to uninfected controls (Fig. 4). Consistent with the microarray results at 4 h after inoculation, no significant increases in expression of the selected chemokines and proinflammatory cytokines were detected at 6 h after inoculation.
FIG. 4.
Expression of proinflammatory chemokines and cytokines by CAM explants infected with Brucella abortus at 6 (white bars) and 12 (gray bars) h after inoculation. Normalized CT values for CXCL6 (GCP-2) and CXCL8 (IL-8) are significantly different between 6 and 12 h after inoculation (*, P = 0.0172; **, P = 0.0184). Data points represent geometric means and standard errors for five independent experiments.
Placentomes from experimentally infected cows have a profile of proinflammatory chemokine expression that is similar to that of infected trophoblastic cells in CAM explants.
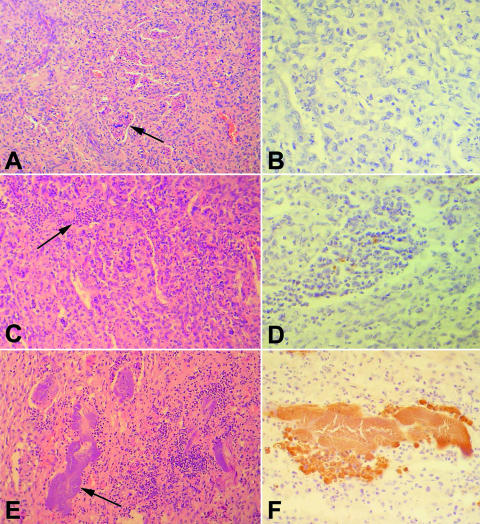
To evaluate the correlation of proinflammatory gene expression in vivo and in vitro, expression of the same selected cytokines and chemokines that were analyzed for CAM explants was also assessed for placentomes from experimentally infected cows. Severalfold changes in expression of proinflammatory genes in placentomes from cows with positive bacteriology with or without abortion were calculated in comparison to what was seen for placentomes from cows with negative bacteriology and normal parturition. No significant differences were observed between the groups of bacteriologically positive cows with or without abortion (Fig. 5), in spite of differences in histopathology between these two groups (Fig. 6). Therefore, when severalfold changes in expression of these two groups with positive bacteriology were grouped together, there was a statistically significant increase in the expression of CXCL8 and CXCL6 (Fig. 5). Thus, the profile of chemokine expression in the placentomes of experimentally infected cows, with an approximately 10-fold increase in expression of CXCL8 and CXCL6, was similar to the profile observed for B. abortus-infected CAM explants (Fig. 4). Increased expression of these proinflammatory genes was associated with histopathological changes that were more severe than those seen for bacteriologically negative placentomes (Fig. 6).
FIG. 5.
Expression of proinflammatory chemokines and cytokines by placental tissue of cows experimentally infected with Brucella abortus divided in two groups: (i) positive bacteriology and term parturition (gray bars) and (ii) positive bacteriology with abortion (white bars). Severalfold change of expression was calculated in comparison to the control group level (bacteriologically negative and normal parturition). *, Normalized CT values for CXCL6 (GCP-2) and CXCL8 (IL-8) are significantly different between controls and both positive bacteriology groups combined (P < 0.05; means were compared by Student Newman Keuls test after logarithmic transformation of the raw data). Columns represent geometric means and standard errors.
FIG. 6.
Bovine placentomes from cows experimentally infected with Brucella abortus. (A) Histologically normal placentome from a bacteriologically negative cow. Chorionic villi within caruncular crypts are indicated by the arrow; H&E staining. (B) Same tissue sample as shown in panel A, with a negative result on immunohistochemistry; streptavidin-peroxidase staining. (C) Suppurative placentitis with a moderated and multifocal inflammatory infiltrate (arrow) in a cow with positive bacteriology and normal parturition. (D) Same tissue sample as shown in panel C, with immunohistochemical labeling of cell-associated B. abortus antigens; streptavidin-peroxidase staining. (E) Severe necrotizing placentitis with intense and multifocal inflammatory infiltrate associated with a myriad of intralesional bacterial colonies (arrow) in a cow with positive bacteriology and abortion; H&E staining. (F) Same tissue sample as shown in panel E, with a large colony of immunohistochemically labeled B. abortus; streptavidin-peroxidase staining.
DISCUSSION
Infection of pregnant cows with B. abortus results in placentitis and abortion (29). Although there is evidence to support the contention that intracellular multiplication of B. abortus within trophoblastic cells is important in the pathogenesis of B. abortus-induced abortion and placentitis (3), very little is known about the interaction between B. abortus and bovine trophoblastic cells, particularly about the role played by trophoblastic cells in the inflammatory response in placenta. Here we describe a thorough study aimed to identify changes in the profile of gene expression during the early stages of infection of trophoblastic cells with B. abortus, with emphasis on proinflammatory mediators. Our data can be summarized as follows. (i) There was a suppression of proinflammatory response by trophoblastic cells during the early stages of infection (i.e., 4 h after inoculation). (ii) Expression of proinflammatory chemokines was detected at later stages of infection (i.e., 12 h after inoculation). (iii) A pattern of proinflammatory response similar to that observed for infected CAM explants was also detected in vivo for placentomes from experimentally infected cows. Therefore, these data support the notion of a biphasic response of trophoblastic cells to B. abortus infection, with an initial suppression of proinflammatory genes that is followed by a mild proinflammatory response at later stages of infection. Implications of these findings are discussed in detail below.
The model of infection based on explants of CAM proved suitable for studying the early events of the interaction between B. abortus and bovine trophoblastic cells. However, the explants are fully viable for only 12 h in culture (although some explants remain viable for up to 48 h in culture), so this model does not allow evaluation of later events such as the kinetics of intracellular bacterial multiplication and trophoblastic responses at later time points. Brucella sp. is able to infect phagocytes and nonphagocytic cells in vivo and in vitro (9, 10, 32, 33). However, Brucella sp. requires a long period of time to interact, invade, and replicate in nonphagocytic cells. Only 40 to 50% of Vero cells inoculated with B. abortus 2308 contain the organism at 8 h after inoculation (9, 10), which is pretty similar to the kinetics observed for trophoblastic cells in this study.
It has been shown that Brucella induces expression of proinflammatory cytokines such as TNF-α and IL-12 both in vivo and in vitro (7, 21, 49). Surprisingly, our microarray data demonstrated a suppression of proinflammatory response from trophoblastic cells in response to B. abortus infection during the first 4 h of infection. This response included downregulation of some inflammatory markers and CXCL11 (Table 3). Interestingly, our data are in good agreement with a recent study that demonstrated low levels of cytokine response during the early stages of B. abortus infection in mice (4). In the mouse model of Brucella infection, expression of CXC chemokines occurs in the spleen during the first days of infection, which correlates with an early neutrophilic response in the liver, which progresses toward a granulomatous inflammation by 1 week postinfection (36). These in vivo observations correlate well with previous microarray analysis of macrophages infected with B. abortus, which displayed a marked proinflammatory response at 4 h postinoculation, with upregulation of TNF and IL-1α and -β (14). Therefore, suppression of proinflammatory response in nonphagocytic cells may be an important mechanism for the evasion of innate immunity by B. abortus. This mechanism seems particularly appealing during the course of invasion of epithelial cells in the gastrointestinal tract during the initial steps of infection in vivo. It is noteworthy that the internalization of B. abortus by phagocytic cells occurs much faster than that by nonphagocytic cells (14, 24), which may account for some of the differences in the responses of these cells to infection with B. abortus.
Interestingly, the interaction of B. abortus with epithelial cells during the early stages of infection in vivo does not result in lesions or inflammatory response in the gastrointestinal tract, which is one of the primary sites of entry for B. abortus, the respiratory tract being the other. Experimental infections demonstrated that B. abortus is endocytosed by lymphoepithelial cells present in the intestinal tract. Brucella localizes within phagocytic cells in the lamina propria of domed villi from 2 to 4 h after infection. Invasion in this location does not trigger any significant inflammatory reaction (1). Conversely, intracellular localization of B. abortus in trophoblastic cells is associated with an intense inflammatory response in infected pregnant cows (29, 31). Importantly, the interaction of Brucella with intestinal epithelial cells is transient, whereas the interaction with trophoblasts occurs for prolonged periods of time. Therefore, the suppression of epithelial proinflammatory response is likely to be the default response triggered by B. abortus, which is eventually overcome by a proinflammatory response later on during infection, as demonstrated in this study.
Upregulation of neutrophil chemoattractants such as CXCL8 and CXCL6 in trophoblastic cells from CAM explants at 12 h after inoculation supports the hypothesis that trophoblastic cells may play an active role eliciting neutrophil influx to the placentome during infection with B. abortus. This trophoblastic response is likely to contribute to the pathogenesis of the neutrophilic and necrotizing placentitis observed in vivo as a result of B. abortus infection of pregnant cows. Importantly, Brucella-induced placentitis is thought to be a major factor causing abortion in bovine brucellosis, which is a key event for transmission of the disease. We also demonstrated that the profile of proinflammatory chemokine expression in placentomes infected by B. abortus in vivo was very similar to that observed for infected trophoblastic cells in vitro, which indicates that the CAM explant model reflects the response of the whole placental tissue during infection with B. abortus. Upregulation of these chemokines in vivo was associated with more-severe histopathological changes in vivo.
Our data indicate that following an initial suppression of proinflammatory genes, induction of CXC chemokines was observed at a later time point during infection of trophoblasts (i.e., at 12 h). However, this induction of proinflammatory mediators is delayed and mild compared to epithelial responses to other gram-negative organisms. Although a direct comparison between different tissues and animal models of infection has several limitations, Salmonella enterica induces a very strong and rapid proinflammatory response in intestinal tissues (35, 41). Supporting this notion, a recent study demonstrated that Salmonella enterica serotype Typhimurium induces higher levels of cytokines during the early stages of infection in mice; this is in contrast to B. abortus infection in the same model, which results in the induction of low levels of cytokines (4). This mild inflammatory response triggered by B. abortus in comparison to those seen for other gram-negative bacteria is at least in part due to the structure of its LPS, which is impaired for signaling through the TLR4 (11, 18, 26, 45).
Another interesting finding in this study was the suppression of expression of four genes belonging to the TNF superfamily in trophoblastic cells during the early stages of infection (i.e., 4 h after inoculation), namely, lymphotoxin beta, member 8 of TNF superfamily, CXCL11, and TNF receptor-associated factor 6. Although the majority of members of the TNF superfamily are expressed by immune cells, the expression of these genes has also been described for epithelial cells (23). These genes are thought to play a role favoring protective immunity in infectious diseases (20). There are several experimental evidences indicating that TNF-α plays a role in immunity to B. abortus in the mouse (7, 21, 49), although Brucella suis is able to suppress TNF-α production by human macrophages (22). Indeed, Brucella spp. have been recognized as stealthy invaders, with very low impact on the immune cells (4). TNF acts as a cofactor for gamma interferon stimulation by lymphocytes, and it is also known to contribute to resistance through mechanisms independent of gamma interferon (52). The early activation of TNF after the infection of mice with B. abortus requires NF-κB activation through a TLR2 receptor MyD88-dependent pathway (26, 52). During the infection of mice, TNF depletion favors infection of macrophages by B. abortus in the spleen, resulting in the accumulation of cells and splenomegaly (52). Thus, the downregulation of TNF superfamily genes observed during the early stages of infection of trophoblasts with B. abortus further supports the hypothesis that the organism somehow suppresses a proinflammatory response by epithelial host cells.
In conclusion, taken together our data indicate that B. abortus modulates innate immune response by trophoblastic cells, suppressing the expression of proinflammatory mediators during the early stages of infection that is followed by a delayed and mild expression of proinflammatory chemokines by trophoblastic cells, which is similar to the profile of chemokine expression detected for the placentome of experimentally infected cows.
Acknowledgments
This work was supported by International Foundation for Science (Stockholm, Sweden) grant number B/3524-1 for R.L.S. A.V.C.N., A.P.L., A.P.R.S., K.L.M., T.A.P., and R.L.S. are supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico, Brasília, Brazil). Publication costs were covered by FAPEMIG (Fundacao de Amparo a Pesquisa do Estado de Minas Gerais, Belo Horizonte, Brazil).
We are grateful to Almir de Sousa Martins, Virginia Mara Pereira, and Luis Ernesto Samartino for technical assistance and Edel F. Barbosa-Stacioli for critically reviewing the manuscript.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 3 March 2008.
REFERENCES
- 1.Ackermann, M. R., N. F. Cheville, and B. L. Deyoe. 1988. Bovine ileal dome lymphoepithelial cell: endocytosis and transport of Brucella abortus strain 19. Vet. Pathol. 2528-35. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, T. D., V. P. Meador, and N. F. Cheville. 1986. Pathogenesis of placentitis in the goat inoculated with Brucella abortus. I. Gross and histologic lesions. Vet. Pathol. 23219-226. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, T. D., V. P. Meador, and N. F. Cheville. 1986. Pathogenesis of placentitis in the goat inoculated with Brucella abortus. II. Ultrastructural studies. Vet. Pathol. 23227-239. [DOI] [PubMed] [Google Scholar]
- 4.Barqueiro-Calvo, E., E. Chaves-Olarte, D. S. Weiss, C. Guzmán-Verri, C. Chacon-Diaz, A. Rucavado, I. Moriyon, and E. Moreno. 2007. Brucella abortus uses a stealthy strategy to avoid activation of the innate immune system during the onset of infection. PLoS ONE 7e631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bazer, F. W., T. E. Spencer, and T. L. Ott. 1997. Interferon tau: a novel pregnancy recognition signal. Am. J. Reprod. Immunol. 37412-420. [DOI] [PubMed] [Google Scholar]
- 6.Black, R. A., S. R. Kronheim, M. Cantrell, M. C. Deeley, C. J. March, K. S. Prickett, J. Wignall, P. J. Conlon, D. Cosman, T. P. Hopp, and D. Y. Mochizuki. 1988. Generation of biologically active interleukin-18 by proteolytic cleavage of the inactive precursor. J. Biol. Chem. 2639437-9944. [PubMed] [Google Scholar]
- 7.Campos, M. A., G. M. S. Rosinha, I. C. Almeida, X. S. Salgueiro, B. W. Jarvis, G. A Splitter, N. Qureshi, O. Bruna-Romero, R. T. Gazinelli, and S. C. Oliveira. 2004. Role of Toll-like receptor 4 in induction of cell-mediated immunity and resistance to Brucella abortus infection in mice. Infect. Immun. 72176-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crawford, R. P., J. D. Huber, and B. S. Adams. 1990. Epidemiology and surveillance, pp. 131-151. In K. Nielsen and J. R. Duncan, Animal brucellosis. CRC Press, Boca Raton, FL.
- 9.Detilleux, P. G., B. L. Deyoe, and N. F. Cheville. 1990. Entry and intracellular localization of Brucella spp. in vero cells: fluorescence and electron microscopy. Vet. Pathol. 27317-328. [DOI] [PubMed] [Google Scholar]
- 10.Detilleux, P. G., B. L. Deyoe, and N. F. Cheville. 1990. Penetration and intracellular growth of Brucella abortus in nonphagocytic cells in vitro. Infect. Immun. 582320-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duenas, A. I., A. Orduna, M. S. Crespo, and C. Garcia-Rodriguez. 2004. Interaction of endotoxins with Toll-like receptor 4 correlates with their endotoxic potential and may explain the proinflammatory effect of Brucella spp. LPS. Int. Immunol. 161467-1475. [DOI] [PubMed] [Google Scholar]
- 12.Eckmann, L., M. F. Kagnoff, and J. Fierer. 1993. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect. Immun. 614569-4574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enright, F. M., J. V. Walker, G. Jeffers, and B. L. Deyoe. 1984. Cellular and humoral responses of Brucella abortus-infected bovine fetuses. Am. J. Vet. Res. 45424-430. [PubMed] [Google Scholar]
- 14.Eskra, L., A. Mathison, and G. Splitter. 2003. Microarray analysis of mRNA levels from raw264.7 macrophages infected with Brucella abortus. Infect. Immun. 711125-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Essenberg, R. C., R. Seshadri, K. Nelson, and I. Paulsen. 2002. Sugar metabolism by Brucellae. Vet. Microbiol. 90249-261. [DOI] [PubMed] [Google Scholar]
- 16.Evans, H. E., and W. O. Sack. 1973. Prenatal development of domestic and laboratory mammals: growth curves, external features and selected references. Anat. Histol. Embryol. 211-15. [DOI] [PubMed] [Google Scholar]
- 17.Giambartolomei, G. H., A. Zwerdling, J. Cassataro, L. Bruno, C. A. Fossati, and M. T. Philipp. 2004. Lipoproteins, not lipopolysaccharide, are the key mediators of the proinflammatory response elicited by heat-killed Brucella abortus. J. Immunol. 1734635-4642. [DOI] [PubMed] [Google Scholar]
- 18.Godstein, J., T. Hoffman, C. Frasch, E. F. Lizzio, P. R. Beining, D. Hochstein, Y. L. Lee, R. D. Angus, and B. Golding. 1992. Lipopolysaccharide (LPS) from Brucella abortus is less toxic than that from Escherichia coli, suggesting the possible use of B. abortus or LPS from B. abortus as carrier in vaccines. Infect. Immun. 601385-1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gorham, S. L., F. M. Enright, T. G. Snider III, and E. D. Roberts. 1986. Morphologic lesions in Brucella abortus infected ovine fetuses. Vet. Pathol. 23331-332. [DOI] [PubMed] [Google Scholar]
- 20.Hehlgans, T., and K. Pfeffer. 2005. The intriguing biology of tumor necrosis factor/tumor necrosis factor receptor superfamily: players, rules and the games. Immunology 1151-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang, L. Y., J. Aliberti, C. A. Leifer, D. M. Segal, A. Sher, D. T. Golenbock, and B. Golding. 2003. Heat killed Brucella abortus induces TNF and IL-12p40 by distinct MyD88-dependent pathways: TNF, unlike IL-12p40 secretion, is toll-like receptor 2 dependent. J. Immunol. 1711441-1446. [DOI] [PubMed] [Google Scholar]
- 22.Jubier-Maurin, V., R. A. Boigegrain, A. Cloeckaert, A. Gross, M. T. Alvarez-Martinez, A. Terraza, J. Liautard, S. Köhler, B. Rouot, J. Dornand, and J. P. Liautard. 2001. Major outer membrane protein Omp25 of Brucella suis is involved in inhibition of tumor necrosis factor alpha production during infection of human macrophages. Infect. Immun. 694823-4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jung, H. C., L. Eckmann, S. K. Yang, A. Panja, J. Fierer, E. Morzycka-Wroblewska, and M. F. Kagnoff. 1995. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J. Clin. Investig. 9555-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ko, J., and G. A. Splitter. 2003. Molecular host-pathogen interaction in brucellosis: current understanding and future approaches to vaccine development for mice and humans. Clin. Microbiol. Rev. 1665-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kostura, M. J., M. J. Tocci, G. Limjuco, J. Chin, P. Cameron, A. G. Hillman, N. A. Chartrain, and J. A. Schmidt. 1989. Identification of a monocyte specific pre-interleukin 1β convertase activity. Proc. Natl. Acad. Sci. USA 865227-5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lapaque, N., O. Takeuchi, F. Corrales, S. Akira, I. Moriyon, J. C. Howard, and J. P. Gorvel. 2006. Differential inductions of TNF-alpha and IGTP, IIGP by structurally diverse classic and non-classic lipopolysaccharides. Cell. Microbiol. 8401-413. [DOI] [PubMed] [Google Scholar]
- 27.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 25402-408. [DOI] [PubMed] [Google Scholar]
- 28.Loor, J. J., R. E. Everts, M. Bionaz, H. M. Dann, D. Morin, R. Oliveira, S. L. Rodriguez-Zas, J. K. Drackley, and H. A. Lewin. 2007. Nutrition-induced ketosis alters metabolic and signaling gene networks in liver of periparturient dairy cows. Physiol. Genomics 32105-116. [DOI] [PubMed] [Google Scholar]
- 29.Meador, V. P., and B. L. Deyoe. 1989. Intracellular localization of Brucella abortus in bovine placenta. Vet. Pathol. 26513-515. [DOI] [PubMed] [Google Scholar]
- 30.Medzhitov, Jr., R., and C. A. Janeway. 1997. Innate immunity: the virtues of a monoclonal system of recognition. Cell 91295-298. [DOI] [PubMed] [Google Scholar]
- 31.Paixão, T. A., F. P. Poester, A. V. Carvalho Neta, A. M. Borges, A. P. Lage, and R. L. Santos. 2007. NRAMP1 3′ untranslated region polymorphisms are not associated with natural resistance to Brucella abortus in cattle. Infect. Immun. 752493-2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pizarro-Cerdá, J., E. Moreno, V. Sanguedolce, J. L. Mege, and J. P. Gorvel. 1998. Virulent Brucella abortus prevents lysosome fusion and is distributed within autophagosome-like compartments. Infect. Immun. 662387-2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pizarro-Cerdá, J., S. Méresse, R. G. Parton, G. Van Der Goot, A. Sola-Landa, I. Lopez-Goni, E. Moreno, and J. P. Gorvel. 1998. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect. Immun. 665711-5724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poester, F. P., V. S. P. Gonçalves, T. A. Paixão, R. L. Santos, S. O. Olsen, G. G. Schurig, and A. P. Lage. 2006. Efficacy of strain RB51 vaccine in heifers against experimental brucellosis. Vaccine 245327-5334. [DOI] [PubMed] [Google Scholar]
- 35.Raffatellu, M., R. L. Santos, D. Chessa, R. P. Wilson, S. Winter, C. L. Bevins, L. G. Adams, and A. J. Bäumler. 2007. The capsule encoding the viaB locus reduces interleukin-17 expression and mucosal innate responses in the bovine intestinal mucosa during infection with Salmonella enterica serotype Typhi. Infect. Immun. 754342-4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roux, C. M., H. G. Rolán, R. L. Santos, P. D. Beremand, T. L. Thomas, L. G. Adams, and R. M. Tsolis. 2007. Brucella requires a functional type IV secretion system to elicit innate immune responses in mice. Cell. Microbiol. 91851-1869. [DOI] [PubMed] [Google Scholar]
- 37.Samartino, L. E., and F. M. Enright. 1992. Interaction of bovine chorioallantoic membrane explants with three strains of Brucella abortus. Am. J. Vet. Res. 53359-363. [PubMed] [Google Scholar]
- 38.Samartino, L. E., and F. M. Enright. 1993. Pathogenesis of abortion of bovine brucellosis. Comp. Immunol. Microbiol. Infect. Dis. 1695-101. [DOI] [PubMed] [Google Scholar]
- 39.Samartino, L. E., and F. M. Enright. 1996. Brucella abortus differs in the multiplication within bovine chorioallantoic membrane explants from early and late gestation. Comp. Immunol. Microbiol. Infect. Dis. 1955-63. [DOI] [PubMed] [Google Scholar]
- 40.Santos, R. L., M. T. D. Peixoto, R. Serakides, G. M. Costa, and N. E. Martins. 1998. Detección de Brucella abortus (muestra B19) por el complejo inmunoenzimático avidina-biotina-peroxidasa en el testículo y en el epidídimo de bovinos inoculados experimentalmente. Arch. Reprod. Anim. 634-41. [Google Scholar]
- 41.Santos, R. L., S. Zhang, R. M. Tsolis, A. J. Baumler, and L. G. Adams. 2002. Morphologic and molecular characterization of Salmonella typhimurium in neonatal calves. Vet. Pathol. 39200-215. [DOI] [PubMed] [Google Scholar]
- 42.Smith, H., A. E. Williams, J. H. Pearce, J. Keppie, P. W. Harris-Smith, R. B. Futzgeorge, and K. Witt. 1962. Foetal erythritol: a cause of the localization of Brucella abortus in bovine contagious abortion. Nature 19347-49. [DOI] [PubMed] [Google Scholar]
- 43.Smith, H., J. Keppie, J. H. Pearce, R. Fuller, and A. E. Williams. 1961. The chemical basis of the virulence of Brucella abortus. I. Isolation of Brucella abortus from bovine foetal tissue. Br. J. Exp. Pathol. 42631. [PMC free article] [PubMed] [Google Scholar]
- 44.Takeda, K., T. Kaisho, and S. Akira. 2003. Toll like receptors. Annu. Rev. Immunol. 21335-376. [DOI] [PubMed] [Google Scholar]
- 45.Tumurkhuu, G., N. Koide, K. Takahashi, F. Hassan, H. Islam, I. Mori, T. Yoshida, and T. Yokochi. 2006. Characterization of biological activities of Brucella melitensis lipopolysaccharide. Microbiol. Immunol. 50421-427. [DOI] [PubMed] [Google Scholar]
- 46.Ushizawa, K., C. B. Herath, K. Kaneyama, S. Shiojima, A. Hirasawa, T. Takahashi, K. Imai, K. Ochiai, T. Tokunaga, Y. Tsunoda, G. Tsujimoto, and K. Hashizume. 2004. cDNA microarray analysis of bovine embryo gene expression profiles during the pre-implantation period. Reprod. Biol. Endocrinol. 277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ushizawa, K., T. Takahashi, K. Kaneyama, T. Tokunaga, Y. Tsunoda, and K. Hashizume. 2005. Gene expression profiles of bovine trophoblastic cell line (BT-1) analysed by a custom cDNA microarray. J. Reprod. Dev. 51211-220. [DOI] [PubMed] [Google Scholar]
- 48.Ushizawa, K., T. Takahashi, M. Hosoe, H. Ishiwata, K. Kaneyama, K. Kizaki, and K. Hashizume. 2007. Global gene expression analysis and regulation of the principal genes expressed in bovine placenta in relation to transcription factor AP-2 family. Reprod. Biol. Endocrinol. 517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Weiss, D. S., K. Takeda, S. Akira, A. Zychlinsky, and E. Moreno. 2005. MyD88, but not Toll-like receptors 4 and 2, is required for efficient clearance of Brucella abortus. Infect. Immun. 735137-5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whiteside, E. J., M. Kan, M. M. Jackson, J. G. Thompson, C. Mcnaughton, A. C. Herington, and M. B. Harvey. 2001. Urokinase-type plasminogen activator (uPA) and matrix metalloproteinase-9 (MMP-9) expression and activity during early embryo development in the cow. Anat. Embryol. 204477-483. [DOI] [PubMed] [Google Scholar]
- 51.Young, E. J. 1995. An overview of human brucellosis. Clin. Infect. Dis. 21283-289. [DOI] [PubMed] [Google Scholar]
- 52.Zhan, Y., Z. Liu, and C. Cheers. 1996. Tumor necrosis factor alpha and interleukin 12 contribute to resistance to the intracellular bacterium Brucella abortus by different mechanisms. Infect. Immun. 642782-2786. [DOI] [PMC free article] [PubMed] [Google Scholar]