Abstract
Neisseria gonorrhoeae requires iron for survival in the human host and therefore expresses high-affinity receptors for iron acquisition from host iron-binding proteins. The gonococcal transferrin-iron uptake system is composed of two transferrin binding proteins, TbpA and TbpB. TbpA is a TonB-dependent, outer membrane transporter critical for iron acquisition, while TbpB is a surface-exposed lipoprotein that increases the efficiency of iron uptake. The precise mechanism by which TbpA mediates iron acquisition has not been elucidated; however, the process is distinct from those of characterized siderophore transporters. Similar to these TonB-dependent transporters, TbpA is proposed to have two distinct domains, a β-barrel and a plug domain. We hypothesize that the TbpA plug coordinates iron and therefore potentially functions in multiple steps of transferrin-mediated iron acquisition. To test this hypothesis, we targeted a conserved motif within the TbpA plug domain and generated single, double, and triple alanine substitution mutants. Mutagenized TbpAs were expressed on the gonococcal cell surface and maintained wild-type transferrin binding affinity. Single alanine substitution mutants internalized iron at wild-type levels, while the double and triple mutants showed a significant decrease in iron uptake. Moreover, the triple alanine substitution mutant was unable to grow on transferrin as a sole iron source; however, expression of TbpB compensated for this defect. These data indicate that the conserved motif between residues 120 and 122 of the TbpA plug domain is critical for transferrin-iron utilization, suggesting that this region plays a role in iron acquisition that is shared by both TbpA and TbpB.
Neisseria gonorrhoeae is an obligate human pathogen that causes the sexually transmitted disease gonorrhea. This disease typically presents as urethritis in men and cervicitis or urethritis in women. Serious downstream sequelae can also occur, and these include pelvic inflammatory disease, epididymitis, and disseminated gonococcal infections. Gonococcal infection has been shown to enhance the likelihood of human immunodeficiency virus transmission (16, 41). The lack of protective immunity following infection and the increase in antibiotic resistance (31, 55, 59) point to the need for the development of an effective vaccine against N. gonorrhoeae infection.
The components of the transferrin-iron uptake system, transferrin binding proteins A and B (TbpA and TbpB), are potential vaccine candidates because these proteins are expressed by all gonococcal isolates (43), and expression of the transferrin-iron uptake system is required to initiate infection in male volunteers (21). In the closely related Neisseria meningitidis, antibodies raised against the transferrin binding proteins are cross-reactive and bactericidal and have the ability to block transferrin binding (23, 52, 60). Furthermore, it was recently shown that antibodies raised against recombinant gonococcal transferrin binding proteins are also cross-reactive and bactericidal (48, 49). Taken together, these data suggest that the components of the neisserial transferrin-iron uptake system are promising candidates for an effective vaccine against Neisseria infections.
Iron is an essential macronutrient for the survival of almost all microorganisms (12). The human host represents an extremely iron-limiting environment, and thus, pathogenic microorganisms must have mechanisms for iron acquisition to survive within the host. Many microorganisms produce siderophores that scavenge iron from their microenvironments. These microbes also express TonB-dependent siderophore transporters to internalize these ferric siderophores. Several of the siderophore receptors have been crystallized (13-15, 25, 26, 40), and the mechanisms of siderophore-mediated iron acquisition are well characterized.
Neisseria species do not produce or secrete any known siderophores (2, 42, 62). Instead, they have the ability to utilize human transferrin (2, 8, 38, 42, 43), lactoferrin (2, 8, 38, 42), and hemoglobin (2, 43) via the expression of high-affinity receptors for these host iron-binding proteins. Models of iron acquisition through these high-affinity receptors are based on the well-characterized TonB-dependent siderophore transporters.
All gonococcal clinical isolates are able to utilize human transferrin (43) through expression of the transferrin-iron uptake system, composed of TbpA and TbpB. TbpA is a TonB-dependent outer membrane transporter that is essential for iron uptake, while TbpB is a surface-exposed lipoprotein that discriminates between apo- and holotransferrin (9, 22, 50, 51). Unlike TbpA, TbpB is not required for transferrin-iron utilization but makes the process more efficient (1). Although TbpA has not yet been crystallized, by analogy with characterized TonB-dependent transporters, TbpA is thought to form two distinct domains. The C-terminal β-barrel domain, comprised of 22 transmembrane β-strands, likely functions as a channel for iron transport. The N-terminal plug domain is thought to fold up within the β-barrel domain to occlude the pore.
Characterized TonB-dependent transporters serve as a paradigm for the transferrin-iron uptake system, but the TbpB lipoprotein component and the requirement for iron removal from transferrin are unique to this system. The precise mechanism by which TbpA mediates iron uptake has not been elucidated. However, the mechanism of iron acquisition through TbpA is unique in that the transferrin receptor must bind holotransferrin, remove iron from transferrin, transport iron across the outer membrane, and release apotransferrin. Thus, elucidation of the detailed mechanism of transferrin-iron acquisition through TbpA would represent a significant step toward characterization of a novel system for the acquisition of iron in Neisseria gonorrhoeae.
Previous studies suggested that the TbpA plug domain is important for iron internalization (63); however, the precise mechanism was not defined. We hypothesize that the plug domain of TbpA functions in multiple steps of transferrin-iron uptake. Specifically, we hypothesize that iron coordination by the TbpA plug domain is critical for iron removal from transferrin, iron binding, and iron transport across the outer membrane. The overall goal of this work was to determine the mechanism of iron acquisition through TbpA, specifically with respect to the contribution of the plug domain.
MATERIALS AND METHODS
Strains, plasmids, and media.
Strains and plasmids utilized in this study are described in Table 1. Gonococci were routinely propagated on GC medium base (Difco) with Kellogg's supplement 1 (33) and 12 μM Fe(NO3)3. For selection of gonococcal transformants, gonococci were grown at 37°C with 5% CO2 on GC agar plates, supplemented with 100 μg/ml of streptomycin (Sigma) or 1 μg/ml of chloramphenicol (Sigma). For iron-stressed growth conditions, which promote maximal expression of the transferrin binding proteins, gonococci were cultured from GC plates into liquid chelexed defined medium (CDM) (61) and cultivated at 37°C with 5% CO2. CDM agarose plates were supplemented with 5%, 10%, 30%, 50%, or 80% iron-saturated human transferrin (Sigma) as needed to assess the ability of gonococcal mutants to utilize transferrin-bound iron (20). Gonococci were grown at 37°C with 5% CO2, and growth on CDM-transferrin plates was monitored over 24 h. Plasmids were routinely propagated in TOP10 Escherichia coli (Invitrogen), grown at 37°C in LB (4, 5) supplemented with 50 μg/ml of kanamycin (Sigma).
TABLE 1.
Strains and plasmids
| Strain or plasmid | Relevant characteristic(s) | Reference or source |
|---|---|---|
| Strains | ||
| E. coli TOP10 | F−mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 ΔlacX74 recA1 deoR araD139 Δ(ara-leu)7697 alU galK rpsL(Strr) endA1 nupG | Invitrogen |
| N. gonorrhoeae | ||
| FA19 | Wild type (TbpA+ TbpB+) | 43 |
| FA6905 | TbpA+ TbpB− (ΔtbpB) | 22 |
| FA6747 | TbpA− (tbpA::mTn3cat) TbpB+ | 20 |
| FA6815 | TbpA− TbpB− (tbpB::Ω) | 1 |
| MCV601 | FA19 derivative; LbpB− (lbpB::Ω) | 7 |
| MCV250 | TbpA E118A TbpB+ | This study |
| MCV251 | TbpA E118A TbpB− (ΔtbpB) | This study |
| MCV252 | TbpA E120A TbpB+ | This study |
| MCV253 | TbpA E120A TbpB− (ΔtbpB) | This study |
| MCV254 | TbpA Y121A TbpB+ | This study |
| MCV255 | TbpA Y121A TbpB− (ΔtbpB) | This study |
| MCV256 | TbpA E122A TbpB+ | This study |
| MCV257 | TbpA E122A TbpB− (ΔtbpB) | This study |
| MCV258 | TbpA E120A Y121A TbpB+ | This study |
| MCV259 | TbpA E120A Y121A TbpB− (ΔtbpB) | This study |
| MCV260 | TbpA E120A Y121A E122A TbpB+ | This study |
| MCV261 | TbpA E120A Y121A E122A TbpB− (ΔtbpB) | This study |
| Plasmids | ||
| pCR2.1 TOPO | Kanr Ampr | Invitrogen |
| pHSS6-GCU | Vector containing gonococcal uptake sequence (Kanr) | 24 |
| pUNCH411 | pBS-SK(+) containing entire tbpA gene | 19 |
| pVCU250 | pCR2.1 TOPO containing tbpA gene amplified with oVCU334 and oVCU335; E118A; PvuI restriction site | This study |
| pVCU251 | pHSS6-GCU containing EcoRI fragment from pVCU250 | This study |
| pVCU252 | pCR2.1 TOPO containing tbpA gene amplified with oVCU334 and oVCU335; E120A; NdeI restriction site | This study |
| pVCU253 | pHSS6-GCU containing EcoRI fragment from pVCU252 | This study |
| pVCU254 | pCR2.1 TOPO containing tbpA gene amplified with oVCU334 and oVCU335; Y121A; HaeIII restriction site | This study |
| pVCU255 | pHSS6-GCU containing EcoRI fragment from pVCU254 | This study |
| pVCU256 | pCR2.1 TOPO containing tbpA gene amplified with oVCU334 and oVCU335; E122A; HpyCH4V restriction site | This study |
| pVCU257 | pHSS6-GCU containing EcoRI fragment from pVCU256 | This study |
| pVCU258 | pCR2.1 TOPO containing tbpA gene amplified with oVCU334 and oVCU335; E120A Y121A; PstI restriction site | This study |
| pVCU259 | pHSS6-GCU containing EcoRI fragment from pVCU258 | This study |
| pVCU260 | pCR2.1 TOPO containing tbpA gene amplified with oVCU334 and oVCU335; E120A Y121A E122A; PstI and AluI restriction sites | This study |
| pVCU261 | pHSS6-GCU containing EcoRI fragment from pVCU260 | This study |
Site-directed mutagenesis.
Site-specific, alanine substitution mutagenesis of the tbpA plug-encoding domain was performed using gene splicing by overlap extension (29). Briefly, in the primary PCR step, two reactions were performed to amplify the upstream and downstream portions of the tbpA plug domain. Nonmutagenic and mutagenic primers used in these reactions are listed in Table 2. Each mutagenic primer was designed to encode one or more alanines as well as novel restriction sites. The template used in these reactions was pUNCH411 (19) (Table 1). For the secondary PCR, the two primary amplification products were used as template with nonmutagenic primers. In this reaction, the alanine-encoding sequences annealed to one another and served to prime the next polymerization step. The final PCR product was gel extracted, purified (Qiagen), and cloned into pCR2.1-TOPO (Invitrogen). Plasmids containing mutagenized tbpA fragments (pVCU250 to pVCU261) are listed in Table 1. Mutagenized tbpA fragments were sequenced by the Nucleic Acids Research Facility at Virginia Commonwealth University to verify the expected sequences.
TABLE 2.
Oligonucleotides
| Primer type and name | Oligonucleotide sequence (5′-3′)a | Target(s) |
|---|---|---|
| tbpA specific | ||
| oVCU334 | AAGCTTGTGAAATAAGCACGGCTGCCG | tbpA |
| oVCU335 | GAAGCGGTTGGGGCCCGTGTAGTCTCG | tbpA |
| Alanine encoding | ||
| oVCU336 | GGCGCAATCAATGCGATCGAGTATGAG | E118A |
| oVCU337 | CTCATACTCGATCGCATTGATTGCGCC | E118A |
| oVCU338 | ATCAATGAAATCGCATATGAGAACGTC | E120A |
| oVCU339 | GACGTTCTCATATGCGATTTCATTGAT | E120A |
| oVCU340 | AATGAAATCGAGGCCGAGAACGTCAAG | Y121A |
| oVCU341 | CTTGACGTTCTCGGCCTCGATTTCATT | Y121A |
| oVCU342 | GAAATCGAGTATGCAAACGTCAAGGCT | E122A |
| oVCU343 | AGCCTTGACGTTTGCATACTCGATTTC | E122A |
| oVCU344 | ATCAATGAAATCGCTGCAGAGAACGTCAAG | E120A, Y121A |
| oVCU345 | CTTGACGTTCTCTGCAGCGATTTCATTGAT | E120A, Y121A |
| oVCU346 | GCAATCAATGAAATCGCTGCAGCTAACGTCAAGGCTGTC | E120A, Y121A, E122A |
| oVCU347 | GACAGCCTTGACGTTAGCTGCAGCGATTTCATTGATTGC | E120A, Y121A, E122A |
Underlined nucleotides indicate the alanine-encoding sequences and the novel restriction sites.
Gonococcal transformation.
Alanine-encoding tbpA fragments were subcloned into pHSS6-GCU (24) (Table 1) to incorporate the gonococcal uptake (GCU) sequence necessary for transformation. Resulting plasmids (pVCU250 to pVCU261) were then used to transform gonococcal strains FA19 (TbpB+) and FA6905 (TbpB−) (Table 1). Congression, described below, was used to provide a selectable marker for the transformation event. Chromosomal DNA from MCV601 (7) (Table 1), which contains an Ω cassette inserted into lbpB (encoding lactoferrin binding protein B), was used in conjunction with the linearized plasmid DNA encoding the alanine substitutions. These donor DNAs were combined and used to transform piliated FA19 or FA6905. Transformants were selected on streptomycin, resistance to which was encoded by the Ω cassette. A subsequent PCR and restriction digest identified streptomycin-resistant transformants with the introduced restriction sites. This process yielded strains MCV250 to MCV261 (Table 1), which contained single, double, or triple alanine-encoding mutations within the tbpA plug-encoding domain.
SDS-PAGE and Western blot analysis.
Gonococcal strains were grown in liquid CDM to induce iron stress (61). After 4 hours of growth, aliquots were removed and standardized to culture cell density. Cells were pelleted and lysed with Laemmli solubilizing buffer (37) and stored at −20°C. Before use, 5% β-mercaptoethanol was added to the whole-cell lysates; lysates were heated at 95°C for 3 minutes and then drawn through a 28-gauge needle to decrease viscosity of samples. Whole-cell lysates were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and proteins were subsequently transferred to nitrocellulose membranes (Schleicher and Schuell) in 20 mM Tris base, 150 mM glycine, and 20% methanol (57) within a submerged transfer apparatus (Bio-Rad). For detection of TbpA, membranes were blocked with 5% bovine serum albumin (Roche) in high-salt Tris-buffered saline (TBS) plus 0.05% Tween 20 (Sigma). TbpA blots were then probed with primary anti-TbpA polyclonal antibodies (22), washed with high-salt TBS plus 0.05% Tween, and probed with a secondary goat anti-rabbit alkaline phosphatase antibody (Bio-Rad). For detection of TbpB, membranes were blocked with 5% skim milk in low-salt TBS (LS TBS). TbpB blots were then probed with primary anti-TbpB polyclonal antibodies (56), washed with LS TBS, and also probed with a secondary goat anti-rabbit alkaline phosphatase antibody. Blots were developed using nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate developing (Sigma).
Solid-phase transferrin binding assay.
Gonococcal strains were grown in liquid CDM for 4 hours to induce iron stress (61). Aliquots were removed and standardized to culture cell density and then spotted onto nitrocellulose membranes (Schleicher and Schuell). Membranes were dried and then blocked with 5% skim milk in LS TBS. To assess solid-phase transferrin binding by gonococcal cells, membranes were incubated with horseradish peroxidase-conjugated transferrin (Jackson ImmunoResearch), washed with LS TBS, and then developed with Opti-4CN (Bio-Rad).
Equilibrium-phase transferrin binding assay.
Equilibrium-phase transferrin binding assays were performed as previously described (22). Briefly, human transferrin (Calbiochem) was iodinated with 125I (GE Biosciences) and specific activity was determined by gamma counting. Both iodinated transferrin and unlabeled, competitor transferrin were quantitated using the bicinchoninic acid (BCA) assay (Pierce). Gonococcal strains were grown in liquid CDM for 3 hours to induce iron stress (61). Following growth, 100 μl of each culture was added to a Millipore multiscreen microtiter plate and incubated with various concentrations of 125I-transferrin (0 to 100 nM) to determine total transferrin binding. In parallel, cultures were incubated with 125I-transferrin plus excess unlabeled transferrin to determine nonspecific transferrin binding. Specific transferrin binding was determined by subtracting nonspecific binding from total binding. Specific transferrin bound (ng) was standardized to micrograms of total cell protein, as determined by BCA assays (Pierce). Each graph represents data from at least three separate assays, each of which was performed in triplicate. Kd (dissociation constant) and capacity values as well as standard errors were calculated using Grafit software (Erithacus Software) and are listed in Table 3.
TABLE 3.
Affinity and capacity measurements generated from equilibrium-phase transferrin binding assays
| Strain | Phenotype | Kda (nM) | Capacity (no. of receptors/ μg TCP)b |
|---|---|---|---|
| FA6905 | TbpA+ TbpB− | 10.7 ± 2.9 | 1.67 × 108 |
| MCV251 | TbpA E118A TbpB− | 9.2 ± 1.8 | 1.53 × 108 |
| MCV253 | TbpA E120A TbpB− | 9.4 ± 1.4 | 2.37 × 108 |
| MCV255 | TbpA E121A TbpB− | 6.8 ± 1.1 | 1.65 × 108 |
| MCV257 | TbpA E122A TbpB− | 9.2 ± 2.1 | 1.32 × 108 |
| MCV259 | TbpA E120A/Y121A TbpB− | 11.2 ± 1.9 | 1.19 × 108 |
| MCV261 | TbpA E120A/Y121A/E122A TbpB− | 6.0 ± 1.2 | 1.10 × 108 |
Kd, capacity, and standard error values were calculated with Grafit software.
Total cell protein (TCP) was determined by bicinchoninic acid assay.
Transferrin-iron uptake assay.
Transferrin-iron uptake assays were performed as previously described (1, 6, 18). Briefly, human transferrin (Calbiochem) was ferrated with 55Fe (Perkin-Elmer) to achieve 20% saturation. Gonococcal strains were grown in liquid CDM for 3 hours to induce iron stress (61). Following growth, 100 μl of each culture was added in quadruplicate sets to two Millipore multiscreen microtiter plates. To one set of cultures, 40 μM potassium cyanide (KCN) (Sigma) was added to determine nonspecific iron binding. Approximately 0.9 μM, 20% iron-saturated transferrin was added to each well and incubated at 37°C with 5% CO2 for 30 min to allow for iron internalization. Following incubation, plates were filtered to remove transferrin, washed, dried, and counted using a Beckman LS6500 beta counter. All counts were averaged, and nonspecific counts (KCN plate) were subtracted from total counts to obtain specific iron internalization. Iron internalization, reported in picomoles, was standardized to micrograms of total cellular protein in 100 μl of culture, as determined by BCA assays (Pierce). Each graph represents the data from at least six separate assays, each of which was performed in quadruplicate.
Statistical analysis.
Statistical significance of equilibrium transferrin binding and transferrin-iron uptake data was determined using a two-tailed, equal-variance Student t test. Statistical significance is noted when P ≤ 0.01.
RESULTS
Site-directed mutagenesis.
TbpA is highly conserved among bacterial pathogens (17). The amino acid sequences of the TbpA plug domains from a variety of bacterial pathogens are shown in Fig. 1. The plug domain of TbpA contains several conserved sequence motifs, some of which have potential iron-coordinating residues similar to those that coordinate iron in human transferrin (32) and bacterial ferric binding protein A (FbpA) (54). We hypothesize that the TbpA plug domain coordinates iron and that potential iron-coordinating residues function to remove iron from transferrin and transport iron across the outer membrane. To test this hypothesis, site-directed, alanine substitution mutagenesis of conserved, potential iron-coordinating residues within the TbpA plug domain was performed. We selected the EIEYE conserved sequence motif, shown in Fig. 1, for analysis because of the abundance of potential iron-coordinating residues but, more importantly, because the region immediately upstream was shown to be critical for transferrin-iron utilization (63). We successfully generated six alanine substitution mutants: four single mutants at amino acids 118 (E), 120 (E), 121 (Y), and 122 (E); one double mutant at amino acids 120 and 121; and one triple mutant at amino acids 120 to 122. These alanine substitution mutants are identified by their strain names (MCV250 to MCV261) but, for clarity, are also identified by the amino acid substitutions (Table 1).
FIG. 1.
Sequence alignment of TbpA plug domains from bacterial pathogens. The first two letters preceding the amino acid sequences represent the genus and species name of the bacterial pathogen: Ng, Neisseria gonorrhoeae; Nm, Neisseria meningitidis; Hi, Haemophilus influenzae; Ap, Actinobacillus pleuropneumoniae; Mc, Moraxella catarrhalis. The subsequent numbers or letters represent the bacterial strain selected for analysis. The sequences represent mature TbpA plug domains, and the amino acids are numbered accordingly to the right of the sequence. Dots indicate identical amino acids, dashes represent positions in which gaps were introduced in the alignment, and letters indicate the specific amino acid changes. Amino acids in bold type and underlined represent potential iron-coordinating residues. These amino acids coordinate iron in human transferrin (YHD) (32) and bacterial ferric binding protein A (YHE) (54). The box indicates the conserved sequence motif selected for site-directed, alanine substitution mutagenesis at amino acids 118 (E), 120 (E), 121 (Y), and 122 (E).
Alanine substitution mutants expressed full-length TbpA and TbpB and bound transferrin to the cell surface.
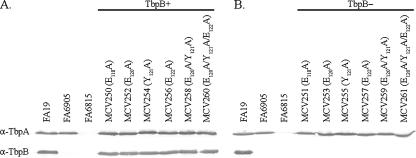
To determine if alanine mutagenesis of the TbpA plug domain disrupted TbpA protein expression and to ensure that TbpB expression was similar to the parental strain, Western blot analyses were carried out (Fig. 2). In Fig. 2A, expression of TbpA and TbpB by the alanine substitution mutants in the FA19 (TbpB+) background was evaluated. All alanine substitution mutants in this background expressed wild-type levels of both TbpA and TbpB. In Fig. 2B, expression of TbpA and TbpB by the alanine mutants in the FA6905 (TbpB−) background was evaluated. Alanine substitution mutants in this background expressed wild-type levels of TbpA and did not express TbpB, as was detected in the FA6905 parental control. We conclude that site-directed, alanine substitution mutagenesis did not disrupt TbpA protein expression in any of the mutants.
FIG. 2.
Alanine substitution mutants expressed wild-type levels of TbpA and TbpB. Iron-stressed gonococci were lysed and standardized to a constant cell density. Whole-cell lysates were separated by SDS-PAGE and then transferred to nitrocellulose membranes. Blots were probed with anti-TbpA (α-TbpA) or anti-TbpB (α-TbpB) polyclonal antibodies as labeled on the left. Each lane is labeled according to the strain name with amino acid substitutions in parentheses. (A) Alanine substitution mutants in the FA19 (TbpB+) background; (B) alanine substitution mutants in the FA6905 (TbpB−) background. Controls include FA19 (positive control, TbpA+ TbpB+), FA6905 (TbpA+ TbpB−), and FA6815 (negative control, TbpA− TbpB−).
In addition to protein expression, we sought to determine if mutagenized TbpA localized to the cell surface and retained the ability to bind transferrin. To evaluate surface exposure and transferrin binding, we carried out solid-phase transferrin binding assays, in which whole gonococcal cells were spotted to a nitrocellulose membrane and probed with horseradish peroxidase-labeled transferrin. Cell surface transferrin binding was evaluated for alanine substitution mutants in the FA19 (TbpB+) and FA6905 (TbpB−) backgrounds. Although not quantitative, this qualitative assay allowed us to conclude that all alanine substitution mutants bound transferrin on the cell surface compared to the positive and negative controls (data not shown). Overall, these results show that site-directed, alanine substitution mutagenesis did not affect TbpA expression levels, surface exposure, or the ability of TbpA to bind human transferrin to the cell surface.
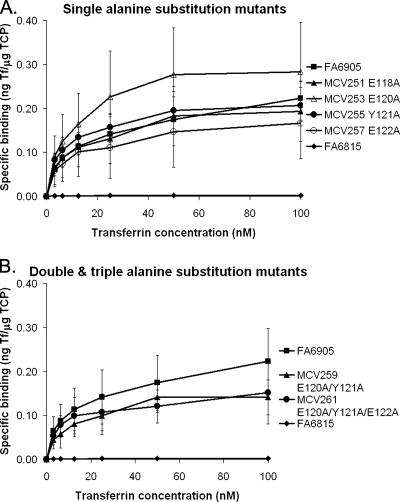
Alanine substitution mutants bound transferrin with wild-type affinity.
Solid-phase transferrin binding assays are not quantitative; therefore, to determine the transferrin binding affinity of the mutated TbpA proteins, we carried out whole-cell, equilibrium-phase transferrin binding assays (Fig. 3). Since both TbpA and TbpB function in transferrin binding, these assays were performed with only alanine substitution mutants in the FA6905 (TbpB−) background. In this manner, transferrin binding by TbpA alone could be assessed and quantitated. Fig. 3A shows transferrin binding by the single alanine substitution mutants in the FA6905 (TbpB−) background, while Fig. 3B shows binding by the double and triple alanine substitution mutants in the FA6905 (TbpB−) background. All alanine substitution mutants bound levels of transferrin similar to those of the parental control FA6905. Affinity and capacity measurements were calculated from the equilibrium-phase transferrin binding data. These values, shown in Table 3, revealed that all alanine substitution mutants have transferrin binding affinities (Kd) and capacities similar to those of the parental control FA6905. Overall these data support the solid-phase transferrin binding data but specifically show that mutagenesis did not have a quantitative effect on transferrin binding affinity or on receptor capacity.
FIG. 3.
Alanine substitution mutants bound transferrin at wild-type levels in equilibrium-phase transferrin binding assays. Whole, iron-stressed gonococci were mixed with various concentrations of 125I-labeled human transferrin (0 to 100 nM). Specific binding of transferrin was determined by subtracting nonspecific binding (with excess competing unlabeled human transferrin) from total binding. Specific transferrin binding is reported on the y axis as nanograms of transferrin bound per microgram of total cell protein (ng Tf/μg TCP). Only strains in the FA6905 (TbpB−) background are shown in order to evaluate specific transferrin binding attributable to TbpA. Each curve is labeled according to the strain name with amino acid substitutions shown. Each point represents an average of at least three independent experiments. (A) Single alanine substitution mutants; (B) double and triple alanine substitution mutants in comparison to the appropriate controls. Controls include FA6905 (positive control, TbpA+ TbpB−) and FA6815 (negative control, TbpA− TbpB−). Standard deviations are represented by error bars.
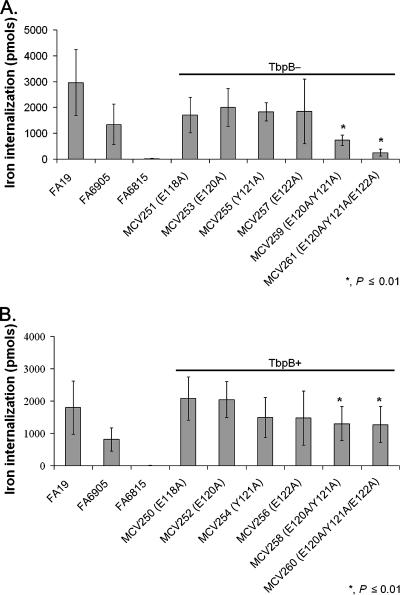
Double and triple alanine substitution mutants demonstrated decreased transferrin-iron internalization.
TbpA plays two major roles in the process of iron acquisition by the gonococcal cell: transferrin binding and iron internalization. Transferrin binding was evaluated in the alanine substitution mutants and found to be similar to that of the wild type. Iron acquisition, the other major step in transferrin-mediated iron uptake, was also evaluated. This assay was carried out by incubating gonococci with 20% iron-saturated human transferrin, and iron internalization was measured in picomoles (Fig. 4). Strain FA6815 (TbpA− TbpB−) was unable to internalize iron due to the absence of TbpA and therefore served as the negative control in this assay. Although TbpB is not required for transferrin-iron uptake, it has been shown to make the process of iron uptake more efficient (1), and here we showed that FA6905 (TbpB−) internalized iron at 50% of wild-type levels. Alanine substitution mutants in the FA6905 (TbpB−) background were tested to determine the specific contribution of TbpA in the process of iron internalization (Fig. 4A). All single alanine substitution mutants in the FA6905 (TbpB−) background internalized amounts of iron similar to those of the FA6905 parental control. However, the double and triple alanine substitution mutants had significantly decreased iron internalization (P ≤ 0.01) compared to the FA6905 parental control. Specifically, the double alanine substitution mutant (MCV259) internalized approximately 50% less iron than FA6905 did, while the triple alanine substitution mutant (MCV261) internalized approximately 80% less iron than FA6905 did. These data show that the single alanine substitution mutants bound transferrin with a wild-type affinity and internalized iron at a rate similar to that of the wild type. While the double and triple alanine substitution mutants had wild-type transferrin binding affinities, they demonstrated a significant decrease in iron internalization. Alanine substitution mutants in the FA19 (TbpB+) background were evaluated in Fig. 4B. All single alanine substitution mutants in the FA19 (TbpB+) background internalized amounts of iron similar to those of the FA19 parental control. However, the double and triple alanine substitution mutants still demonstrated a significant decrease in iron internalization (P ≤ 0.01) compared to the FA19 parental control, but the presence of TbpB resulted in an iron internalization increase of about 50% in both mutants. These results suggest that the wild-type TbpA sequence at residues 120 to 122 facilitates efficient iron internalization.
FIG. 4.
Double and triple alanine substitution mutants internalized less iron in transferrin-iron uptake assays. Iron-stressed gonococci were incubated with 55Fe-labeled human transferrin. 55Fe internalization was measured as picomoles of iron internalized after 30 min. Each bar represents the mean of at least six independent experiments and is labeled according to the strain name with amino acid substitutions in parentheses. (A) Alanine substitution mutants in the FA6905 (TbpB−) background; (B) alanine substitution mutants in the FA19 (TbpB+) background. Controls include FA19 (positive control, TbpA+ TbpB+), FA6905 (TbpA+ TbpB−), and FA6815 (negative control, TbpA− TbpB−). Standard deviations are represented by error bars. The asterisk indicates P ≤ 0.01 in comparison to both FA19 and FA6905.
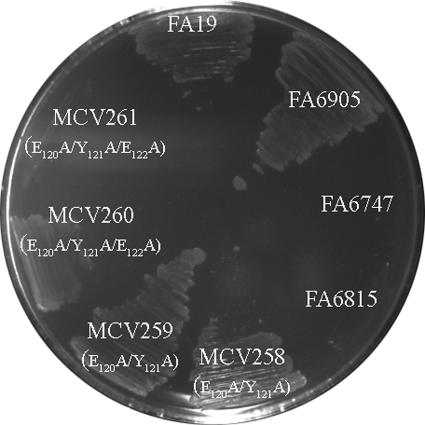
The triple alanine substitution mutant was unable to utilize transferrin as a sole iron source in the absence of TbpB.
Since the double and triple alanine substitution mutants demonstrated a significant decrease in iron internalization, we sought to determine whether these decreased levels of iron internalization were sufficient for growth when transferrin was provided as a sole iron source. To test this, we carried out transferrin-iron utilization assays, in which gonococci were grown on CDM plates supplemented with 30% iron-saturated human transferrin as the sole iron source (Fig. 5). Alanine substitution mutants in both the FA19 (TbpB+) and FA6905 (TbpB−) backgrounds were tested. The single alanine substitution mutants in both backgrounds maintained the ability to utilize transferrin as a sole source of iron (data not shown). In addition, although the double alanine substitution mutants (MCV258 and MCV259) had significantly decreased iron internalization, these mutants were able to utilize transferrin-bound iron for growth in both the presence and the absence of TbpB. The triple alanine substitution mutant in the FA19 (TbpB+) background (MCV260), despite its decreased iron internalization, maintained the ability to utilize transferrin as a sole iron source. However, consistent with the significant decrease in iron internalization, the triple alanine substitution mutant in the FA6905 (TbpB−) background (MCV261) was unable to utilize transferrin, as shown in Fig. 5. These results suggest that TbpB has the ability to compensate for the defect observed in the triple alanine substitution mutant. Overall, these data suggest that residues 120 to 122 of the TbpA plug domain are critical for optimal iron internalization from transferrin, but the function provided by this conserved plug domain motif can be compensated for by the presence of TbpB.
FIG. 5.
The triple alanine substitution mutant was unable to utilize transferrin as a sole iron source in the absence of TbpB in transferrin-iron utilization growth assays. Gonococcal strains were grown on CDM plates containing 30% iron-saturated human transferrin as a sole iron source. The ability of mutants to utilize transferrin as a sole source of iron was evaluated by growth at 37°C with 5% CO2 for 24 h. Strains are labeled according to the strain name with specific amino acid substitutions in parentheses. Controls include FA19 (positive control, TbpA+ TbpB+), FA6905 (TbpA+ TbpB−), FA6747 (negative control, TbpA− TbpB+), and FA6815 (negative control, TbpA− TbpB−).
Since these assays were carried out using 30% iron-saturated transferrin, we sought to assess the growth phenotypes of the alanine substitution mutants using a variety of iron saturations, ranging from 5% to 80%. The single and double alanine substitution mutants in both backgrounds maintained the ability to utilize transferrin at all the transferrin-iron saturations tested (data not shown). Similar to the results in Fig. 5, the triple alanine substitution mutant in the FA19 (TbpB+) background (MCV260) maintained the ability to utilize transferrin at all the transferrin-iron saturation levels. However, the triple alanine substitution mutant in the FA6905 (TbpB−) background (MCV261) was unable to utilize transferrin regardless of the level of iron saturation (data not shown). These data suggest that TbpA with alanine substitutions at residues 120 to 122 is unable to function in transferrin-iron utilization and that the defect in transferrin-iron utilization is not due to inefficiency at low iron saturation levels. Furthermore, these data imply that the compensatory function provided by TbpB is not due solely to TbpB's ability to specifically bind the ferrated form of transferrin.
DISCUSSION
In order for gonococci to utilize transferrin-bound iron, TbpA must carry out two major functions: iron removal from transferrin and iron transport across the outer membrane. This study was designed to analyze the role of the TbpA plug domain in this process and to begin to address the hypothesis that the plug functions in iron coordination and subsequent transferrin-mediated iron acquisition. Single, double, and triple alanine substitution mutagenesis of putative iron-coordinating residues in the TbpA plug domain resulted in mutants that expressed wild-type levels of TbpA on the gonococcal cell surface and bound transferrin with wild-type affinities. Furthermore, no growth abnormalities were observed with any of the alanine substitution mutants, suggesting that the alanine mutagenesis had relatively little impact on the structure or function of gonococcal TbpA. Previous studies in which deletion and insertion mutagenesis of TbpA were performed (10, 63) are in agreement with these data and support the theory that gonococcal TbpA, like other TonB-dependent receptors (3, 11, 35, 36, 39, 44, 45), is resilient to various types of mutagenesis.
Analysis of the double and triple alanine substitution mutants in transferrin-iron uptake assays revealed a significant defect in iron internalization. Furthermore, analysis of the triple alanine substitution mutant in transferrin-iron utilization growth assays revealed that this mutant was unable to utilize transferrin-bound iron in the absence of TbpB. This suggests that the defect in the TbpA plug domain can be overcome by the presence of TbpB. This phenomenon has been previously observed with insertion mutations in putative loops 2, 9, and 11 of TbpA (63), suggesting that TbpA and TbpB have some redundant functions. Similar to the triple alanine substitution mutant, the loop 2, 9, and 11 TbpA insertion mutants showed no defects in transferrin binding but a defect in transferrin-iron utilization in the absence of TbpB (63). Therefore, the function of TbpB is not limited to transferrin binding and holo-/apotransferrin discrimination (9, 22, 50, 51) but also appears to play an additional role in the mechanism of transferrin-mediated iron uptake. The compensatory function provided by TbpB suggests that TbpA and TbpB work together to accomplish transferrin-iron uptake. Although not much is known about specific interactions between TbpA and TbpB, it has been shown that both gonococcal and meningococcal TbpB associates with TbpA (27, 28, 34, 53). The defect in transferrin-iron uptake and utilization seen in the TbpA triple alanine substitution mutant could be attributed to either a defect in iron removal from transferrin, iron binding by the plug, and/or iron transport through the β-barrel. Therefore, TbpB may provide one or more of these functions in wild-type gonococci. Since TbpB is a surface-exposed lipoprotein, it most likely does not compensate for iron transport through the TbpA β-barrel, but it may function in iron removal from transferrin by an unknown mechanism.
As mentioned previously, gonococcal TbpB is not typically required for transferrin-iron utilization, but in the case of the TbpA triple alanine substitution mutant, TbpB is required for iron acquisition from transferrin. This dependence on TbpB function is also observed in Neisseria meningitidis strain B16B6, which expresses low-molecular-weight classes of the transferrin binding proteins. In B16B6, iron acquisition from human transferrin requires TbpB (30), which is most likely due to the lack of critical regions in the TbpA from this strain. Meningococcal TbpA from B16B6 shares only 75% identity to gonococcal TbpAs (17) and shows sequence and length diversity within the proposed exposed loop regions (17, 47). These data suggest that there are multiple domains within TbpA and TbpB that are important in transferrin-mediated iron acquisition. Cumulatively, our data (63) demonstrate that disruption of one or more epitopes prevents TbpA function in the absence of TbpB. These domains may similarly be lacking or nonfunctional in meningococcal strain B16B6.
Our data demonstrate that residues 120 to 122 of the TbpA plug domain are critical for transferrin-iron utilization. This may be due to alteration of an iron binding site, which may promote iron removal from transferrin, subsequent iron binding by the plug, and transport across the outer membrane. It has been shown that the plug domain of the E. coli TonB-dependent transporter FepA binds directly to its ligand, ferric enterobactin, in the absence of the β-barrel domain (58), which suggests the importance of the plug in ligand binding and possibly transport. Oke et al. found that the neisserial TbpA plug domain was unable to bind human transferrin (46). However, the TbpA plug domain most likely has a ligand specificity for iron rather than transferrin. Although TbpA is a characterized transferrin receptor, it is also an iron transporter. Therefore, TbpA has two ligand specificities during the process of transferrin-mediated iron internalization. It is important to note that, although residues 120 to 122 of the TbpA plug domain are clearly involved in transferrin-mediated iron acquisition, other, perhaps distant, residues may be involved as well.
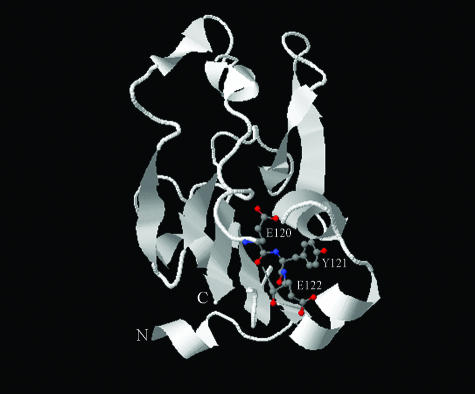
Figure 6 shows a homology model for the gonococcal TbpA plug domain, which was derived from comparison with the known crystal structure of the homologous plug domain of the E. coli ferric-enterobactin transporter FepA. The positions of amino acids 120 (E), 121 (Y), and 122 (E), mutated in the current study, are shown as ball-and-stick representations. Interestingly, this conserved motif is located near the bottom of the plug in this model. The position of this critical domain relative to the surface-exposed opening of the barrel (top) is consistent with the hypothesis that the plug coordinates iron via ligands from distant amino acid residues within the plug. Alternatively, iron may be coordinated at multiple, different sites within the plug domain during transport through the β-barrel. It is also possible that these substitution mutations resulted in indirect impacts within the plug domain, leading to the plug's inability to bind or transport iron. Thus, while the results of this study support the hypothesis that the plug domain coordinates iron during transport, no direct evidence is yet available that demonstrates direct interaction between iron and specific plug residues.
FIG. 6.
Predicted structural model of N. gonorrhoeae TbpA plug domain. The mature N. gonorrhoeae TbpA plug domain (amino acids 1 to 162) was aligned with the homologous sequence of the E. coli FepA plug domain using the 3D-Jigsaw comparative modeling program. The modeled region of TbpA spans from Thr25 (N terminus) to Thr151 (C terminus). The resulting output file was visualized with First Glance in Jmol. The image shown was captured and imported into Adobe Photoshop. The amino acids 120 (E), 121 (Y), and 122 (E) are represented by balls and sticks. Red indicates hydrophobic atoms, while blue indicates hydrophilic atoms. Alpha helices and beta strands are shown as ribbons, with arrowheads pointing toward the C terminus. Random coils are shown as smooth backbone traces.
Overall, these studies provide insight into the mechanism of TbpA-mediated iron acquisition. Further studies are necessary to precisely define the function of the TbpA plug domain with respect to the multiple steps of transferrin-iron uptake: iron removal from transferrin, iron binding and coordination, and iron transport across the outer membrane. A better understanding of this mechanism may reveal new sites within TbpA to target for vaccine development or to exploit for treatment of gonococcal infection.
Acknowledgments
Funding for this work was provided by Public Health Service grant R01 AI047141 from the National Institute of Allergy and Infectious Diseases, National Institutes of Health. J. M. Noto was supported by the Training in Molecular Pathogenesis grant (T32 AI07617) from the National Institutes of Health.
We gratefully acknowledge William Barton at Virginia Commonwealth University for his assistance with TbpA homology modeling and Susan Buchanan at NIH for critical reading of the manuscript. We thank Cara Olsen at Uniformed Services University for useful discussions regarding appropriate statistical analysis. Finally, we also acknowledge Lori Walsh for assistance in construction of some mutant strains in this study.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 17 March 2008.
REFERENCES
- 1.Anderson, J. E., P. F. Sparling, and C. N. Cornelissen. 1994. Gonococcal transferrin-binding protein 2 facilitates but is not essential for transferrin utilization. J. Bacteriol. 1763162-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archibald, F. S., and I. W. DeVoe. 1980. Iron acquisition by Neisseria meningitidis in vitro. Infect. Immun. 27322-334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Armstrong, S. K., and M. A. McIntosh. 1995. Epitope insertions define functional and topological features of the Escherichia coli ferric enterobactin receptor. J. Biol. Chem. 2702483-2488. [DOI] [PubMed] [Google Scholar]
- 4.Bertani, G. 2004. Lysogeny at mid-twentieth century: P1, P2, and other experimental systems. J. Bacteriol. 186595-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bertani, G. 1951. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J. Bacteriol. 62293-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beucher, M., and P. F. Sparling. 1995. Cloning, sequencing, and characterization of the gene encoding FrpB, a major iron-regulated, outer membrane protein of Neisseria gonorrhoeae. J. Bacteriol. 1772041-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biswas, G. D., J. E. Anderson, C.-J. Chen, C. N. Cornelissen, and P. F. Sparling. 1999. Identification and functional characterization of the Neisseria gonorrhoeae lbpB gene product. J. Bacteriol. 67455-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blanton, K. J., G. D. Biswas, J. Tsai, J. Adams, D. W. Dyer, S. M. Davis, G. G. Koch, P. K. Sen, and P. F. Sparling. 1990. Genetic evidence that Neisseria gonorrhoeae produces specific receptors for transferrin and lactoferrin. J. Bacteriol. 1725225-5235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boulton, I. C., A. R. Gorringe, N. Allison, A. Robinson, B. Gorinsky, C. L. Joannou, and R. W. Evans. 1998. Transferrin-binding protein B isolated from Neisseria meningitidis discriminates between apo and diferric human transferrin. Biochem. J. 334269-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boulton, I. C., M. K. Yost, J. E. Anderson, and C. N. Cornelissen. 2000. Identification of discrete domains within gonococcal transferrin-binding protein A that are necessary for ligand binding and iron uptake functions. Infect. Immun. 686988-6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braun, V., H. Killmann, and R. Benz. 1994. Energy-coupled transport through the outer membrane of Escherichia coli small deletions in the gating loop convert the FhuA transport protein into a diffusion channel. FEBS Lett. 34659-64. [DOI] [PubMed] [Google Scholar]
- 12.Briat, J. F. 1992. Iron assimilation and storage in prokaryotes. J. Gen. Microbiol. 1382475-2483. [DOI] [PubMed] [Google Scholar]
- 13.Buchanan, S. K., B. S. Smith, L. Venkatramani, D. Xia, L. Esser, M. Palnitkar, R. Chakraborty, D. van der Helm, and J. Deisenhofer. 1999. Crystal structure of the outer membrane active transporter FepA from Escherichia coli. Nat. Struct. Biol. 656-63. [DOI] [PubMed] [Google Scholar]
- 14.Cobessi, D., H. Celia, N. Folschweiller, I. J. Schalk, M. A. Abdallah, and F. Pattus. 2005. The crystal structure of the pyoverdine outer membrane receptor FpvA from Pseudomonas aeruginosa at 3.6 A resolution. J. Mol. Biol. 347121-134. [DOI] [PubMed] [Google Scholar]
- 15.Cobessi, D., H. Celia, and F. Pattus. 2005. Crystal structure at high resolution of ferric-pyochelin and its membrane receptor FptA from Pseudomonas aeruginosa. J. Mol. Biol. 352893-904. [DOI] [PubMed] [Google Scholar]
- 16.Cohen, M. S., I. F. Hoffman, R. A. Royce, P. Kazembe, J. R. Dyer, C. C. Daly, D. Zimba, P. L. Vernazza, M. Maida, S. A. Fiscus, J. J. Eron, Jr., et al. 1997. Reduction of concentration of HIV-1 in semen after treatment of urethritis: implications for prevention of sexual transmission of HIV-1. Lancet 3491868-1873. [DOI] [PubMed] [Google Scholar]
- 17.Cornelissen, C. N., J. E. Anderson, I. C. Boulton, and P. F. Sparling. 2000. Antigenic and sequence diversity in gonococcal transferrin-binding protein A. Infect. Immun. 684725-4735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cornelissen, C. N., J. E. Anderson, and P. F. Sparling. 1997. Energy-dependent changes in the gonococcal transferrin receptor. Mol. Microbiol. 2625-35. [DOI] [PubMed] [Google Scholar]
- 19.Cornelissen, C. N., G. D. Biswas, and P. F. Sparling. 1993. Expression of gonococcal transferrin-binding protein 1 causes Escherichia coli to bind human transferrin. J. Bacteriol. 1752448-2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cornelissen, C. N., G. D. Biswas, J. Tsai, D. K. Paruchuri, S. A. Thompson, and P. F. Sparling. 1992. Gonococcal transferrin-binding protein 1 is required for transferrin utilization and is homologous to TonB-dependent outer membrane receptors. J. Bacteriol. 1745788-5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cornelissen, C. N., M. Kelley, M. M. Hobbs, J. E. Anderson, J. G. Cannon, M. S. Cohen, and P. F. Sparling. 1998. The transferrin receptor expressed by gonococcal strain FA1090 is required for the experimental infection of human male volunteers. Mol. Microbiol. 27611-616. [DOI] [PubMed] [Google Scholar]
- 22.Cornelissen, C. N., and P. F. Sparling. 1996. Binding and surface exposure characteristics of the gonococcal transferrin receptor are dependent on both transferrin-binding proteins. J. Bacteriol. 1781437-1444.8631722 [Google Scholar]
- 23.Danve, B., L. Lissolo, M. Mignon, P. Dumas, S. Colombani, A. B. Schryvers, and M. J. Quentin-Millet. 1993. Transferrin-binding proteins isolated from Neisseria meningitidis elicit protective and bactericidal antibodies in laboratory animals. Vaccine 111214-1220. [DOI] [PubMed] [Google Scholar]
- 24.Elkins, C., C. E. Thomas, H. S. Seifert, and P. F. Sparling. 1991. Species-specific uptake of DNA by gonococci is mediated by a 10-base-pair sequence. J. Bacteriol. 1733911-3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferguson, A. D., R. Chakraborty, B. S. Smith, L. Esser, D. van der Helm, and J. Deisenhofer. 2002. Structural basis of gating by the outer membrane transporter FecA. Science 2951715-1719. [DOI] [PubMed] [Google Scholar]
- 26.Ferguson, A. D., E. Hofmann, J. W. Coulton, K. Diederichs, and W. Welte. 1998. Siderophore-mediated iron transport: crystal structure of FhuA with bound lipopolysaccharide. Science 2822215-2220. [DOI] [PubMed] [Google Scholar]
- 27.Fuller, C. A., R. Yu, S. W. Irwin, and A. B. Schryvers. 1998. Biochemical evidence for a conserved interaction between bacterial transferrin binding protein A and transferrin binding protein B. Microb. Pathog. 2475-87. [DOI] [PubMed] [Google Scholar]
- 28.Gomez, J. A., M. T. Criado, and C. M. Ferreiros. 1998. Cooperation between the components of the meningococcal transferrin receptor, TbpA and TbpB, in the uptake of transferrin iron by the 37-kDa ferric-binding protein (FbpA). Res. Microbiol. 149381-387. [DOI] [PubMed] [Google Scholar]
- 29.Horton, R. M. 1995. PCR-mediated recombination and mutagenesis. SOEing together tailor-made genes. Mol. Biotechnol. 393-99. [DOI] [PubMed] [Google Scholar]
- 30.Irwin, S. W., N. Averil, C. Y. Cheng, and A. B. Schryvers. 1993. Preparation and analysis of isogenic mutants in the transferrin receptor protein genes, tbpA and tbpB, from Neisseria meningitidis. Mol. Microbiol. 81125-1133. [DOI] [PubMed] [Google Scholar]
- 31.Ison, C. A., J. A. Dillon, and J. W. Tapsall. 1998. The epidemiology of global antibiotic resistance among Neisseria gonorrhoeae and Haemophilus ducreyi. Lancet 351(Suppl. 3)8-11. [DOI] [PubMed] [Google Scholar]
- 32.Jeffrey, P. D., M. C. Bewley, R. T. A. MacGillivray, A. B. Mason, R. C. Woodworth, and E. N. Baker. 1998. Ligand-induced conformational change in transferrins: crystal structure of the open form of the N-terminal half-molecule of human transferrin. Biochemistry 3713978-13986. [DOI] [PubMed] [Google Scholar]
- 33.Kellogg, D. S., Jr., W. L. Peacock, Jr., W. E. Deacon, L. Brown, and C. I. Pirkle. 1963. Neisseria gonorrhoeae. I. Virulence genetically linked to clonal variation. J. Bacteriol. 851274-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kenney, C. D., and C. N. Cornelissen. 2002. Demonstration and characterization of a specific interaction between gonococcal transferrin binding protein A and TonB. J. Bacteriol. 1846138-6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Killmann, H., R. Benz, and V. Braun. 1996. Properties of the FhuA channel in the Escherichia coli outer membrane after deletion of FhuA portions within and outside the predicted gating loop. J. Bacteriol. 1786913-6920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koebnik, R., and V. Braun. 1993. Insertion derivatives containing segments of up to 16 amino acids identify surface- and periplasm-exposed regions of the FhuA outer membrane receptor of Escherichia coli K-12. J. Bacteriol. 175826-839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227680-685. [DOI] [PubMed] [Google Scholar]
- 38.Lee, B. C., and A. B. Schryvers. 1988. Specificity of the lactoferrin and transferrin receptors in Neisseria gonorrhoeae. Mol. Microbiol. 2827-829. [DOI] [PubMed] [Google Scholar]
- 39.Liu, J., J. M. Rutz, J. B. Feix, and P. E. Klebba. 1993. Permeability properties of a large gated channel within the ferric enterobactin receptor, FepA. Proc. Natl. Acad. Sci. USA 9010653-10657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Locher, K. P., B. Rees, R. Koebnik, A. Mitschler, L. Moulinier, J. P. Rosenbusch, and D. Moras. 1998. Transmembrane signaling across the ligand-gated FhuA receptor: crystal structures of free and ferrichrome-bound states reveal allosteric changes. Cell 95771-778. [DOI] [PubMed] [Google Scholar]
- 41.McClelland, R. S., C. C. Wang, K. Mandaliya, J. Overbaugh, M. T. Reiner, D. D. Panteleeff, L. Lavreys, J. Ndinya-Achola, J. J. Bwayo, and J. K. Kreiss. 2001. Treatment of cervicitis is associated with decreased cervical shedding of HIV-1. AIDS 15105-110. [DOI] [PubMed] [Google Scholar]
- 42.McKenna, W. R., P. A. Mickelsen, P. F. Sparling, and D. W. Dyer. 1988. Iron uptake from lactoferrin and transferrin by Neisseria gonorrhoeae. Infect. Immun. 56785-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mickelsen, P. A., and P. F. Sparling. 1981. Ability of Neisseria gonorrhoeae, Neisseria meningitidis, and commensal Neisseria species to obtain iron from transferrin and iron compounds. Infect. Immun. 33555-564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moeck, G. S., B. S. Bazzaz, M. F. Gras, T. S. Ravi, M. J. Ratcliffe, and J. W. Coulton. 1994. Genetic insertion and exposure of a reporter epitope in the ferrichrome-iron receptor of Escherichia coli K-12. J. Bacteriol. 1764250-4259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Newton, S. M. C., J. D. Igo, D. C. Scott, and P. E. Klebba. 1999. Effect of loop deletions on the binding and transport of ferric enterobactin by FepA. Mol. Microbiol. 321153-1165. [DOI] [PubMed] [Google Scholar]
- 46.Oke, M., R. Sarra, R. Ghirlando, S. Farnaud, A. R. Gorringe, R. W. Evans, and S. K. Buchanan. 2004. The plug domain of a neisserial TonB-dependent transporter retains structural integrity in the absence of its transmembrane beta-barrel. FEBS Lett. 564294-300. [DOI] [PubMed] [Google Scholar]
- 47.Pajon, R., G. Chinea, E. Marrero, D. Gonzalez, and G. Guillen. 1997. Sequence analysis of the structural tbpA gene: protein topology and variable regions within neisserial receptors for transferrin iron acquisition. Microb. Pathog. 2371-84. [DOI] [PubMed] [Google Scholar]
- 48.Price, G. A., H. P. Masri, A. M. Hollander, M. W. Russell, and C. N. Cornelissen. 2007. Gonococcal transferrin binding protein chimeras induce bactericidal and growth inhibitory antibodies in mice. Vaccine 257247-7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Price, G. A., M. W. Russell, and C. N. Cornelissen. 2005. Intranasal administration of recombinant Neisseria gonorrhoeae transferrin binding proteins A and B conjugated to the cholera toxin B subunit induces systemic and vaginal antibodies in mice. Infect. Immun. 733945-3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Renauld-Mongenie, G., M. Latour, D. Poncet, S. Naville, and M.-J. Quentin-Millet. 1998. Both the full-length and the N-terminal domain of the meningococcal transferrin-binding protein B discriminate between human iron-loaded and apo-transferrin. FEMS Microbiol. Lett. 169171-177. [DOI] [PubMed] [Google Scholar]
- 51.Retzer, M. D., R.-H. Yu, Y. Zhang, G. C. Gonzalez, and A. B. Schryvers. 1998. Discrimination between apo and iron-loaded forms of transferrin by transferrin binding protein B and its N-terminal subfragment. Microb. Pathog. 25175-180. [DOI] [PubMed] [Google Scholar]
- 52.Rokbi, B., M. Mignon, G. Maitre-Wilmotte, L. Lissolo, B. Danve, D. A. Caugant, and M. J. Quentin-Millet. 1997. Evaluation of recombinant transferrin-binding protein B variants from Neisseria meningitidis for their ability to induce cross-reactive and bactericidal antibodies against a genetically diverse collection of serogroup B strains. Infect. Immun. 6555-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stokes, R. H., J. S. Oakhill, C. L. Joannou, A. R. Gorringe, and R. W. Evans. 2005. Meningococcal transferrin-binding proteins A and B show cooperation in their binding kinetics for human transferrin. Infect. Immun. 73944-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taboy, C. H., K. G. Vaughan, T. A. Mietzner, P. Aisen, and A. L. Crumbliss. 2001. Fe3+ coordination and redox properties of a bacterial transferrin. J. Biol. Chem. 2762719-2724. [DOI] [PubMed] [Google Scholar]
- 55.Tapsall, J. 2001. Antimicrobial resistance in Neisseria gonorrhoeae. World Health Organization, Geneva, Switzerland.
- 56.Thomas, C. E., W. Zhu, C. N. Van Dam, N. L. Davis, R. E. Johnston, and P. F. Sparling. 2006. Vaccination of mice with gonococcal TbpB expressed in vivo from Venezuelan equine encephalitis viral replicon particles. Infect. Immun. 741612-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 764350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Usher, K. C., E. Ozkan, K. H. Gardner, and J. Deisenhofer. 2001. The plug domain of FepA, a TonB-dependent transport protein from Escherichia coli, binds its siderophore in the absence of the transmembrane barrel domain. Proc. Natl. Acad. Sci. USA 9810676-10681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wang, S. A., A. B. Harvey, S. M. Conner, A. A. Zaidi, J. S. Knapp, W. L. H. Whittington, C. del Rio, F. N. Judson, and K. K. Holmes. 2007. Antimicrobial resistance for Neisseria gonorrhoeae in the United States, 1988 to 2003: the spread of fluoroquinolone resistance. Ann. Intern. Med. 14781-88. [DOI] [PubMed] [Google Scholar]
- 60.West, D., K. Reddin, M. Matheson, R. Heath, S. Funnell, M. Hudson, A. Robinson, and A. Gorringe. 2001. Recombinant Neisseria meningitidis transferrin binding protein A protects against experimental meningococcal infection. Infect. Immun. 691561-1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.West, S. E., and P. F. Sparling. 1987. Aerobactin utilization by Neisseria gonorrhoeae and cloning of a genomic DNA fragment that complements Escherichia coli fhuB mutations. J. Bacteriol. 1693414-3421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.West, S. E. H., and P. F. Sparling. 1985. Response of Neisseria gonorrhoeae to iron limitation: alterations in expression of membrane proteins without apparent siderophore production. Infect. Immun. 47388-394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yost-Daljev, M. K., and C. N. Cornelissen. 2004. Determination of surface-exposed, functional domains of gonococcal transferrin-binding protein A. Infect. Immun. 721775-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]