Abstract
The 10-kDa culture filtrate protein (CFP-10) and 6-kDa early secretory antigen of T cells (ESAT-6) are secreted in abundance by Mycobacterium tuberculosis and are frequently recognized by T cells from infected people. The genes encoding these proteins have been deleted from the genome of the vaccine strain Mycobacterium bovis bacillus Calmette-Guérin (BCG), and it is hypothesized that these proteins are important targets of protective immunity. Indeed, vaccination with ESAT-6 elicits protective CD4+ T cells in C57BL/6 mice. We have previously shown that M. tuberculosis infection of C3H mice elicits CFP-10-specific CD8+ and CD4+ T cells. Here we demonstrate that immunization with a CFP-10 DNA vaccine stimulates a specific T-cell response only to the H-2Kk-restricted epitope CFP-1032-39. These CFP-1032-39-specific CD8+ cells undergo a rapid expansion and accumulate in the lung following challenge of immunized mice with aerosolized M. tuberculosis. Protective immunity is induced by CFP-10 DNA vaccination as measured by a CFU reduction in the lung and spleen 4 and 8 weeks after challenge with M. tuberculosis. These data demonstrate that CFP-10 is a protective antigen and that CFP-1032-39-specific CD8+ T cells elicited by vaccination are sufficient to mediate protection against tuberculosis.
Cellular immune responses are critical for the control of Mycobacterium tuberculosis infection. The contribution of CD4+ T cells has been extensively documented, and the increased susceptibility of human immunodeficiency virus-infected people to tuberculosis underlines the importance of CD4+ T cells in preventing the disease caused by M. tuberculosis (22). On the other hand, the role of CD8+ T cells in immunity to M. tuberculosis is still being elucidated. Several animal models, including normal mice depleted of CD8+ T cells, immunodeficient mice reconstituted with immune CD8+ T cells, and knockout (KO) mice that lack CD8+ T cells, have clearly shown that CD8+ T cells are required for optimum control of bacterial replication and host resistance to M. tuberculosis infection (reviewed in reference 30). However, it remains to be determined how CD8+ T cells mediate their protective effect and whether they can mediate vaccine-induced protection against tuberculosis.
The paucity of mycobacterial antigens known to be recognized by CD8+ T cells has been an impediment to the study of CD8+ T cells during M. tuberculosis infection. The 10-kDa culture filtrate protein (CFP-10) is a well-characterized, secreted mycobacterial protein that elicits CD8+ T cells in both people and mice following M. tuberculosis infection (14, 18, 26). CFP-10 is so commonly recognized by T cells from M. tuberculosis-infected people that it is one of the antigens now being used for the immunodiagnosis of tuberculosis (17). The cfp-10 gene is in the same operon as the esat-6 gene. The two genes share 40% sequence homology and both encode members of the 6-kDa early secretory antigen of T cells (ESAT-6)-related family of small proteins. These two genes are located within the ESX-1 locus of the M. tuberculosis genome, a region of DNA that is present in M. tuberculosis and pathogenic Mycobacterium bovis strains but is deleted from all M. bovis Bacille Calmette-Guerin (BCG) strains. Complementation of BCG with the ESX-1 locus restores the virulence of BCG, and deleting this locus from M. tuberculosis leads to attenuation; thus, CFP-10 and ESAT-6 are putative virulence factors. Since CFP-10 and ESAT-6 are absent from BCG and BCG does not provide significant protection against pulmonary tuberculosis, it is hypothesized that CFP-10 and ESAT-6 are important targets of protective immunity. Indeed, recombinant BCG engineered to express ESAT-6 improves on the protection normally afforded by BCG in animal models (25). We wished to determine whether CFP-10 vaccination would similarly stimulate protective T cells. Specifically, as we had previously shown that the CD8+ T-cell response in C3H mice is dominated by CFP-10-specific CD8+ T cells following M. tuberculosis infection, we sought to establish whether or not CFP-10-specific CD8+ T cells mediate protection against M. tuberculosis (14).
In this study, we evaluate the immune responses generated by a DNA vaccine encoding CFP-10 and determine the epitope specificity of the elicited T cells. We find that the CFP-10 DNA vaccine elicits class I major histocompatibility complex (MHC-I)-restricted CFP-1032-39-specific CD8+ T cells in C3H mice. The challenge of immunized mice with aerosolized M. tuberculosis led to memory response characterized by a specific increase in CFP-1032-39-specific CD8+ T cells. Mice vaccinated with the CFP-10 DNA vaccine had a lower bacterial load 4 and 8 weeks after M. tuberculosis challenge than mice immunized with the “empty” DNA vector. Therefore, we demonstrate that CFP-10 is a target of protective immunity. Furthermore, by determining the epitope specificity of the T cells elicited by vaccination, we demonstrate that CFP-1032-39-specific CD8+ T cells are sufficient to provide protection. These data provide important proof for the principle that vaccine strategies that induce stimulation of CD8+ T cells can provide protection against tuberculosis.
MATERIALS AND METHODS
Mice.
Female C3H/HeJ (C3H) mice 6 to 8 weeks old were purchased from Jackson Laboratories (Bar Harbor, ME). Mice were housed in a biosafety level 3 facility under specific-pathogen-free conditions at the Animal Biohazard Containment Suite (Dana Farber Cancer Institute, Boston, MA) and were used in a protocol approved by the institution.
DNA vaccination.
The CFP-10 gene was cloned into the vector pVAX (Invitrogen). Endotoxin-free plasmids were prepared using an EndoFree plasmid purification mega prep kit (Qiagen). Mice were immunized intramuscularly with 100 μg DNA per mouse (50 μg per quadriceps). As a control, some groups of mice were immunized with 100 μg pVAX without any insert (i.e., “empty vector”) or phosphate-buffered saline (PBS). Mice were immunized three times at 3-week intervals. Four weeks after the last vaccination, mice were challenged with M. tuberculosis by the aerosol route.
Bacteria and aerosol infections.
All infections were performed using virulent M. tuberculosis (Erdman strain). For each infection, a bacterial aliquot was thawed, sonicated twice for 10 s in a cup horn sonicator, and then diluted in 0.9% NaCl-0.02% Tween 80. A 15-ml suspension of M. tuberculosis was loaded into a nebulizer (Mini Heart nebulizer; Vortran Medical Technologies). Mice were infected via the aerosol route using a nose-only exposure unit (Intox Products) and received approximately 100 to 200 CFU/mouse (3, 13, 14).
CFU determination.
After euthanasia by CO2 inhalation, the left lungs and spleens were aseptically removed and individually homogenized in 0.9% NaCl-0.02% Tween 80 with a Mini-Bead-Beater-8 (BioSpec Products, Bartlesville, OK). Viable bacteria were enumerated by plating 10-fold serial dilutions of organ homogenates onto 7H11 agar plates (Remel). Colonies were counted after 3 weeks of incubation at 37°C.
Peptides.
Overlapping peptides spanning the CFP-10 protein sequence of M. tuberculosis have previously been described (13). In addition, CFP-1032-39, CFP-1011-25, and ESAT-653-75 were commercially synthesized (Biosource International). The peptides were dissolved in dimethyl sulfoxide and stored at −20°C until used. Peptides used for immunological assays were not purified. The purity of peptides used for tetramer production was >95%.
Flow cytometry.
Cells were resuspended in staining buffer (2% fetal bovine serum and 0.02% NaN3 in PBS) containing 50 μg/ml anti-FcR Ab (clone 2.4G2; American Type Culture Collection). Fluorescein isothiocyanate-, phycoerythrin-, or peridinin chlorophyll protein-conjugated antibodies specific for mouse CD3, CD4, CD8, and appropriate isotype control antibodies were purchased from BD Pharmingen. Tetramers were produced using CFP-1032-39-loaded H-2Kk complexed with phycoerythrin (National Institute of Allergy and Infectious Diseases tetramer facility). Cells from individual mice were stained with the antibodies and tetramer at an optimum concentration for 20 min at 4°C and then washed and fixed overnight at 4°C in 1% paraformaldehyde in PBS. Cells were analyzed using a FACSCanto flow cytometer (BD Biosciences), and the data analyzed using FlowJo software (Tree Star).
In vitro restimulation assays.
CD4+ or CD8+ T cells were purified from spleens of immunized mice by a two-step procedure. Using a Pan T-cell isolation kit, total T cells were purified by negative selection and then positively enriched using either anti-CD4 or anti-CD8 immunomagnetic beads (Miltenyi Biotec). In all experiments, the purity of the cells was 90 to 95% as determined by flow cytometry. Purified CD8+ T cells (105 cells/well) were stimulated with mycobacterial sonicate in the presence of irradiated naive C57BL/6 splenocytes (4 × 105 cells/well) for 48 h in vitro. Culture supernatants were assayed for gamma interferon (IFN-γ) by enzyme-linked immunosorbent assay.
ELISPOT assay for IFN-γ.
An enzyme-linked immunospot assay (ELISPOT) was done using the BD ELISPOT kit (BD Biosciences) (13, 14). Briefly, ELISPOT plates were coated with the capture anti-IFN-γ monoclonal antibody (MAb) overnight at 4°C. The capture MAb was discarded, and the plates were washed and blocked with complete media for 2 h at room temperature. Lung tissue was pooled from groups of three to five mice, and single-cell suspensions were prepared as previously described (13). Total pulmonary T cells were purified by depleting MHC-II-positive cells using anti-MHC-II immunomagnetic beads (Miltenyi Biotec) and then positively selected using CD90 microbeads. Flow cytometric analysis was performed to assess the cell purity and determine the percentage of CD4+ and CD8+ T cells. The numbers of purified T cells were varied, and the T cells were cultured with 10 μM CFP-1032-39, CFP-1011-25, or ESAT-653-75 and irradiated naive splenocytes (4 × 105 cells/well) and cultured for 18 h at 37°C. All conditions were repeated in triplicate. The cells were discarded, and after washing the plates with deionized water and PBS/Tween 20, the biotinylated anti-IFN-γ MAb was added for 2 h at room temperature. The plate was washed, supplemented with streptavidin-alkaline phosphatase for 1 h, and washed again, and the color reaction was developed using 5-bromo-4-chloro-3-indolylphosphate/nitroblue tetrazolium (BCIP/NBT) tablets from Sigma. The reaction was stopped when the spots developed by running the plate under water. An immunospot analyzer (Series 3A; Cellular Technology) was used to enumerate the spots. Based on the flow cytometric analysis, the number of spots per 100,000 CD4+ or CD8+ T cells was calculated.
Statistics.
The Prism software program was used to perform all statistical analyses (GraphPad, San Diego, CA). The number of CFU was log10 transformed before analysis. The statistical significance of two-way comparisons was tested using a two-tailed t test. Three-way comparisons were analyzed using one-way analysis of variance using Dunnett's multiple comparison posttest.
RESULTS
Priming a CFP-10-specific CD8+ T-cell response in C3H mice.
Two distinct CFP-10 epitopes are recognized by T cells elicited following M. tuberculosis infection of C3H mice. One, CFP-1032-39, is recognized by CD8+ T cells, and the other, CFP-1011-25, is recognized by CD4+ T cells (14). In order to determine whether CFP-10-specific CD8+ T cells can mediate host resistance to M. tuberculosis, we sought to develop a vaccination strategy that elicits only CFP-1032-39-specific CD8+ T cells.
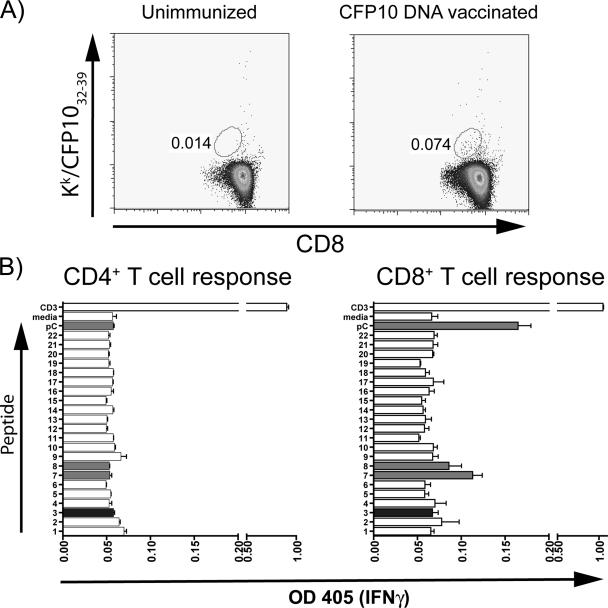
DNA vaccines have been successfully used to elicit CD8+ T-cell responses. To determine whether a DNA vaccine encoding the full-length CFP-10 gene elicits CD8+ T cells specific for CFP-1032-39, C3H mice were immunized intramuscularly three times. After the third immunization, splenocytes were analyzed by flow cytometry using CFP-1032-39-loaded H-2Kk tetramers. A discrete population of CD8+ T cells was identified in the spleens of vaccinated mice by staining with CFP-1032-39-loaded H-2Kk tetramers (Fig. 1A). Since this population was nearly at our limit of detection by flow cytometry, we sought to independently confirm this result and assess whether T cells were elicited that recognized other CFP-10 epitopes. Purified CD4+ and CD8+ T cells from DNA-vaccinated mice were tested for their recognition of overlapping peptides that spanned the length of the CFP-10 protein (14). Corroborating the tetramer staining results, CD8+ T cells from vaccinated mice produced IFN-γ when stimulated with CFP-1032-39 (peptide pC in Fig. 1B) and peptides 7 and 8 from the CFP-10 peptide library, which contain the CFP-1032-39 epitope (Fig. 1B) (14). In contrast, none of the other peptides, including CFP-1011-25, which contains the MHC-II-restricted epitope, stimulated IFN-γ production by CD4+ or CD8+ T cells (Fig. 1B). Thus, immunization with the CFP-10 DNA vaccine specifically primes CFP-1032-39-specific CD8+ T cells in C3H mice.
FIG. 1.
The CFP-10 DNA vaccine elicits CFP-1032-39-specific CD8+ T-cell responses in C3H mice. Unimmunized C3H mice and vaccinated mice that received three doses of the CFP-10 DNA vaccine were analyzed 3 weeks after their last vaccination. (A) Splenocytes were analyzed using CFP-1032-39-loaded H-2Kk tetramers. CD8+ cells were identified by their forward and side scatter and staining with anti-CD8 MAb. The percentage of CD8+ cells stained by CFP-1032-39-loaded H-2Kk tetramers is indicated. (B) CD4+ and CD8+ T cells were purified from the spleens of mice vaccinated with the CFP-10 DNA vaccine. The T cells were cultured with irradiated antigen-presenting cells and overlapping peptides spanning the CFP-10 sequence (peptides are numbered as in reference 14). IFN-γ production was measured by enzyme-linked immunosorbent assay 48 h later. Peptides containing the CFP-1032-39 epitope (indicated by light gray shading) stimulate CD8+ T cells from C3H mice vaccinated with the CFP-10 DNA. Peptides p7 and p8 both contain the CFP-1032-39 epitope, and this minimal epitope is represented by peptide pC. In contrast, no response to the CFP-1011-25 epitope (dark gray shading) was detected. OD 405, optical density at 405 nm.
Primed CFP-10-specific CD8+ T cells expand when C3H mice are infected with M. tuberculosis.
An important feature of T cells elicited by vaccination is their capacity to generate long-lived memory T cells. To determine whether CFP-10-specific CD8+ T cells elicited by DNA vaccination undergo expansion when challenged with M. tuberculosis, C3H mice primed with the CFP-10 DNA vaccine or mock vaccinated were challenged with M. tuberculosis by the aerosol route. C3H mice have a delay in the initiation of their T-cell response to M. tuberculosis compared to C57BL/6 mice (3). Therefore, 2 weeks after infection was chosen as an early time point before a T-cell response to CFP-10 is observed in unvaccinated M. tuberculosis-infected mice (14); this time point would be suitable to compare CD8+ T-cell responses between the two experimental groups.
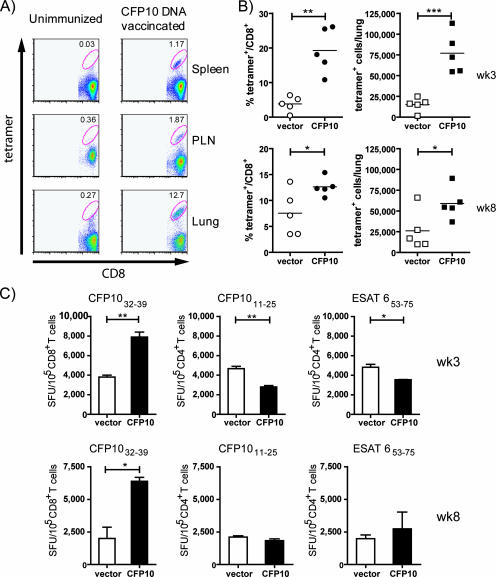
Two weeks after M. tuberculosis challenge, cells from the lung, spleen, and draining lymph nodes (LN) were obtained and stained with anti-CD8 and CFP-1032-39-loaded H-2Kk tetramer. The sham-vaccinated mice had a low level of tetramer-positive CD8+ cells from the lung, spleen, and pulmonary LN, consistent with our previous results at this early time point (Fig. 2A). In contrast, the CFP-10 DNA-vaccinated mice had a significant increase in the frequency of tetramer-positive CD8+ T cells, particularly from the lung, where 12% of the CD8+ T cells were specific for CFP-1032-39 (Fig. 2A).
FIG. 2.
CFP-10 DNA vaccination primes CFP-1032-39 CD8+ T cells that expand following challenge with aerosolized M. tuberculosis. (A) CFP-1032-39-specific CD8+ T cells rapidly increase in the tissues of vaccinated mice following M. tuberculosis challenge. Mice were either vaccinated with CFP-10 DNA vaccine or injected with PBS, as described in Materials and Methods. Four weeks after the last vaccination, the mice were infected with aerosolized M. tuberculosis. Two weeks after infection, the spleen, pulmonary LN (PLN), and lung cells of the vaccinated mice were analyzed by flow cytometry using tetramers. The number in each dot plot is the percentage of CD8+ T cells that stained with CFP-1032-39-loaded H-2Kk tetramers. (B) Mice (n = 5 per group) were vaccinated with the CFP-10 DNA vaccine or the empty vector, and the frequency (left) and absolute number (right) of CD8+ T cells specific for CFP-1032-39 were determined by flow cytometry 3 and 8 weeks after challenge with M. tuberculosis. Each point represents an individual mouse. Bar, the mean; *, P < 0.05; **, P < 0.01; ***, P < 0.001. (C) An IFN-γ ELISPOT was used to determine the frequency of lung CD8+ T cells specific for CFP-1032-39 or CD4+ T cells specific for CFP-1011-25 or for ESAT-653-75 in the lungs of vaccinated mice 3 and 8 weeks after challenge with M. tuberculosis. The frequency of antigen-specific T cells per 100,000 CD4+ or CD8+ T cells is shown. Bars represent the means ± standard deviations of triplicate wells. *, P < 0.05; **, P < 0.01. SFU, spot-forming units.
To determine the specificity, kinetics, and durability of the CFP-1032-39-specific CD8+ T-cell response, this analysis was expanded to other time points. In two independent experiments, the immune responses of mice that were vaccinated either with the “empty” DNA vaccine or the CFP-10 DNA vaccine were characterized 3 and 8 weeks after M. tuberculosis challenge. Similar to the results at week 2, an increase in tetramer-positive CD8+ T cells was observed in mice immunized with the CFP-10 DNA vaccine. Both the frequency of CFP-10-specific CD8+ T cells and their absolute number were increased fivefold in the lungs of CFP-10 DNA-vaccinated mice compared to those of the “empty” vector-vaccinated controls (P = 0.001 and P = 0.0007, respectively) at 3 and 8 weeks postchallenge (Fig. 2B). Concurrently, the frequency of CFP-1032-39-specific CD8+ T cells, CFP-1011-25-specific CD4+ T cells, and ESAT-653-75-specific CD4+ T cells in the lungs of M. tuberculosis-challenged mice was determined using an IFN-γ ELISPOT. The frequency of IFN-γ-producing CFP-1032-39-specific CD8+ T cells was elevated twofold in the CFP-10 DNA vaccine group compared to that of the controls (P = 0.0016) at week 3 (Fig. 2B). As expected, the frequencies of CFP-1011-25- and ESAT-653-75-specific CD4+ T cells were not increased in the CFP-10 DNA-vaccinated mice, confirming that immunization with a CFP-10 DNA vaccine specifically elicits CFP-10-specific CD8+ T cells. Finally, in two independent experiments, we observed that a significant increase in CFP-10-specific CD8+ T cells, detected using tetramers and an ELISPOT, persisted through week 8, the latest time point examined (Fig. 2C).
These results demonstrate that the CFP-10 DNA vaccine specifically primes CFP-10-specific CD8+ T cells, which were detected at a frequency of ∼1 in 2,000 splenic CD8+ T cells. Importantly, these CD8+ T cells function as memory T cells, since they undergo significant expansion following in vivo challenge with M. tuberculosis and their frequency increases to ∼1 in 5 CD8+ T cells in the lungs of infected mice.
Vaccination with CFP-10 DNA protects C3H mice from aerosol challenge with M. tuberculosis.
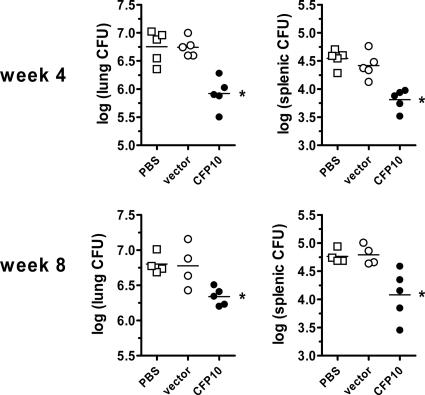
To determine whether the CFP-10-specific CD8+ T cells elicited by CFP-10 DNA vaccination can contribute to protective immunity, we determined whether there was a difference in the bacterial burdens in the lungs and spleens of vaccinated and control C3H mice challenged with virulent M. tuberculosis. The bacterial loads in the lung and spleen 4 weeks after aerosol M. tuberculosis challenge were reduced in CFP-10 DNA-vaccinated mice compared to control mice that received the “empty” DNA vector or PBS alone. The bacterial burdens in the lungs and spleen of the “empty” vector and PBS control groups were similar, indicating that the vector did not have any nonspecific effect on protection. The log10 reduction compared to the vector control was 0.82 and 0.61, in the lung and spleen, respectively (P < 0.05) (Fig. 3). A similar reduction in the bacterial burdens in the lungs and spleens of CFP-10 DNA-vaccinated mice was observed 8 weeks after M. tuberculosis challenge (0.46 and 0.71 log10 reduction, respectively; P < 0.05). These results demonstrate that CFP-1032-39-specific CD8+ T cells are sufficient to mediate protection against M. tuberculosis.
FIG. 3.
CFP-10 DNA vaccination protects C3H mice against M. tuberculosis. Mice (n = 5 per group) were vaccinated with a DNA vaccine containing the CFP-10 gene (CFP-10), the vector alone (vector), or a vehicle (PBS). Vaccinations were done a total of three times, 3 weeks apart, as described in Materials and Methods. Four weeks after the last vaccination, all mice were challenged with M. tuberculosis by the aerosol route, and the numbers of CFU were determined in the lung and spleen cells 4 and 8 weeks after infection. *, P < 0.05 versus vector by one-way analysis of variance (Bonferroni's posttest). The experiment was repeated twice with similar results.
DISCUSSION
T cells make an important contribution to host control of M. tuberculosis replication, and following infection, MHC-II-restricted CD4+ T cells and MHC-I-restricted CD8+ T cells specific for many protein antigens are primed and expand and accumulate at sites of disease, particularly in the lung. Many of the mycobacterial epitopes that are recognized by CD4+ and CD8+ T cells have been defined, although only a few of these have been shown to be protective in challenge models of tuberculosis. We have previously defined CFP-10 as an immunodominant antigen that is recognized by T cells elicited following M. tuberculosis infection of C3H mice.
In this study, we evaluate the immune responses generated by a DNA vaccine encoding CFP-10 and determine the epitope specificity of the elicited T cells. We find that the CFP-10 DNA vaccine selectively elicits CFP-1032-39-specific CD8+ T cells in C3H mice. Challenge of immunized mice with aerosolized M. tuberculosis leads to a specific increase in CFP-1032-39-specific CD8+ T cells that can be detected as early as 2 weeks after infection and persists for at least 8 weeks. Mice vaccinated with the CFP-10 DNA vaccine had fewer pulmonary CFU 4 and 8 weeks after M. tuberculosis challenge than mice immunized with the “empty” DNA vector. Thus, CFP-10 is a target of protective immunity.
While the ability of vaccine-induced CD4+ T cells to provide protection has been demonstrated, it has been more difficult to demonstrate that CD8+ T cells elicited after immunization augment host defense against tuberculosis. Most protective vaccines elicit a mixture of epitope-specific CD4+ and CD8+ T-cell responses (2, 10, 27), and we are not aware of vaccines that uniquely elicit CD8+ T cells and are protective. In fact, only a few MHC-II-restricted epitopes and no MHC-I-restricted epitopes have been identified that are sufficient to elicit protective immunity (23). The induction of significant protection by DNA vaccines has been achieved only when a cocktail of DNA vaccines is administered (4, 5, 9, 21) or genes such as Ag85A, which is known to induce both CD4+ and CD8+ T cells recognizing multiple epitopes, are used (12, 15, 16, 19). In contrast, vaccination of C3H mice with the CFP-10 DNA vaccine elicits a specific CD8+ T-cell response to a single epitope without any evidence of CFP-10-specific CD4+ T-cell priming as part of the vaccine-induced immune response. Our vaccination strategy may not be optimized, given the relatively weak response following DNA vaccination. Nevertheless, the T cells that are primed develop into functional memory T cells, since a dramatic increase in the frequency and absolute number of CFP-1032-39-specific CD8+ T cells is observed following challenge with M. tuberculosis. By defining the epitope specificity of the T cells elicited by vaccination, we demonstrate that CFP-1032-39-specific CD8+ T cells are sufficient to mediate protection against tuberculosis.
Several vaccine strategies are designed to elicit antigen-specific CD8+ T cells. Vaccination with plasmid DNA encoding microbial antigens is used to induce T-cell immunity against intracellular pathogens such as M. tuberculosis. This approach elicits CD8+ cytotoxic T lymphocyte and Th1 responses, and several mycobacterial DNA vaccines can protect mice against M. tuberculosis challenge, although translation into the clinical arena has been frustrating. Similarly, recombinant viruses and intracellular bacteria, including BCG, can be used to induce CD8+ T-cell responses that recognize M. tuberculosis. Recently, Hinchey et al. found that M. tuberculosis mutants that induce greater macrophage apoptosis were more effective in priming CD8+ T cells and, when used as a vaccine, provided greater protection against tuberculosis than BCG (11). However, even when a vaccine is effective in an animal model, the reason for its success is often unknown. The lack of defined epitopes recognized by CD8+ T cells makes it challenging to assess the success of vaccines designed to elicit CD8+ T-cell responses. Assessment is also complicated, since nearly all of these vaccine strategies elicit complex immune responses, which make it difficult to know how the various components of the immune response benefit the host.
There have been several reports that have tried to show that CD8+ T cells elicited by vaccination are sufficient to protect mice from M. tuberculosis challenge. Several of these studies have used the CD4 KO mouse (7, 29); however, these studies suffer from the confounder that despite the lack of CD4+ T cells, these mice still have CD4− CD8− and CD8+ MHC-II-restricted T cells that can mediate many of the functions of conventional CD4+ T cells (24, 28). Derrick et al. found that their DNA vaccine cocktail protects CD4 KO mice against M. tuberculosis challenge and that protection under these conditions is dependent upon CD8+ T cells, since in vivo depletion of CD8+ T cells using MAb abolishes the vaccine-induced protection (8); however, the MHC restriction of the protective CD8+ T-cell subset was not determined. Our data extend these results by showing that vaccine-induced MHC-I-restricted CD8+ T cells can mediate protection in normal mice with intact immune systems. These findings provide an important rationale for further evaluating whether strategies that induce both M. tuberculosis-specific CD4+ and CD8+ T cells would provide greater protection than vaccines that elicit a predominantly CD4+ T-cell response.
A previous study found that a similar CFP-10 DNA vaccine provided no protection to BALB/c mice at 2, 4, or 8 weeks after challenge with M. tuberculosis (20). However, no immunological evaluation was performed, and we suspect that the CFP-10 DNA vaccine did not elicit CFP-10-specific T cells. Since CFP-10-specific T cells are not detected following M. tuberculosis infection of BALB/c mice, CFP-10 peptides may not prime T cells in mice of the H-2d haplotype (14). In contrast, CFP-10 DNA vaccination of C3H mice induces a CD8+ T-cell response to CFP-1032-39, an epitope that is immunodominant following M. tuberculosis infection, and we show in this report that this T-cell response is protective. Thus, the capacity of the CFP-10 antigen to induce a protective response depends on the host's immunogenetic background. This is an important consideration, as the inability of the CFP-10 DNA vaccine to protect BALB/c mice from M. tuberculosis challenge should not eliminate it from consideration as a vaccine candidate. CFP-10 is clearly an important target of the human T-cell response, even across multiple MHC haplotypes and ethnic populations (17, 26). Our results indicate that CFP-10 should be considered a vaccine candidate.
These issues highlight the importance of understanding the basis for the success or failure of different vaccine strategies. An immunization strategy may fail because it does not elicit antigen-specific T cells, in which case it is a vaccine failure, or it may fail because antigen-specific T cells are elicited that are unable to mediate protection—in other words, an immunological failure. An important reason why T cells elicited by a vaccine may fail to protect the host against M. tuberculosis is that the epitope recognized by the vaccine-induced T cells is not presented or presented inefficiently by M. tuberculosis-infected cells. Vaccine strategies affect the selection of epitopes that prime CD4+ and CD8+ T cells in ways that are not yet understood. We now recognize that the epitopes that stimulate T cells after vaccination can differ from the epitopes that stimulate T cells after M. tuberculosis infection (1, 6). This scenario can result in antigen-specific T cells induced by vaccination that are unable to recognize infected cells. Identification of mycobacterial epitopes recognized by CD4+ and CD8+ T cells will enable careful immunological monitoring of T-cell responses following vaccination and following infection. Such a careful epitope-based analysis will be required to understand how vaccine-induced immunity correlates with host protection.
Acknowledgments
This work was supported by grant R01 AI67731 from the National Institute of Allergy and Infectious Disease.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 10 March 2008.
REFERENCES
- 1.Bennekov, T., J. Dietrich, I. Rosenkrands, A. Stryhn, T. M. Doherty, and P. Andersen. 2006. Alteration of epitope recognition pattern in Ag85B and ESAT-6 has a profound influence on vaccine-induced protection against Mycobacterium tuberculosis. Eur. J. Immunol. 363346-3355. [DOI] [PubMed] [Google Scholar]
- 2.Brookes, R. H., A. A. Pathan, H. McShane, M. Hensmann, D. A. Price, and A. V. Hill. 2003. CD8+ T cell-mediated suppression of intracellular Mycobacterium tuberculosis growth in activated human macrophages. Eur. J. Immunol. 333293-3302. [DOI] [PubMed] [Google Scholar]
- 3.Chackerian, A. A., J. M. Alt, T. V. Perera, C. C. Dascher, and S. M. Behar. 2002. Dissemination of Mycobacterium tuberculosis is influenced by host factors and precedes the initiation of T-cell immunity. Infect. Immun. 704501-4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delogu, G., A. Howard, F. M. Collins, and S. L. Morris. 2000. DNA vaccination against tuberculosis: expression of a ubiquitin-conjugated tuberculosis protein enhances antimycobacterial immunity. Infect. Immun. 683097-3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delogu, G., A. Li, C. Repique, F. Collins, and S. L. Morris. 2002. DNA vaccine combinations expressing either tissue plasminogen activator signal sequence fusion proteins or ubiquitin-conjugated antigens induce sustained protective immunity in a mouse model of pulmonary tuberculosis. Infect. Immun. 70292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denis, O., A. Tanghe, K. Palfliet, F. Jurion, T.-P. van den Berg, A. Vanonckelen, J. Ooms, E. Saman, J. B. Ulmer, J. Content, and K. Huygen. 1998. Vaccination with plasmid DNA encoding mycobacterial antigen 85A stimulates a CD4+ and CD8+ T-cell epitopic repertoire broader than that stimulated by Mycobacterium tuberculosis H37Rv infection. Infect. Immun. 661527-1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derrick, S. C., T. H. Evering, V. K. Sambandamurthy, K. V. Jalapathy, T. Hsu, B. Chen, M. Chen, R. G. Russell, A. P. Junqueira-Kipnis, I. M. Orme, S. A. Porcelli, W. R. Jacobs, Jr., and S. L. Morris. 2007. Characterization of the protective T-cell response generated in CD4-deficient mice by a live attenuated Mycobacterium tuberculosis vaccine. Immunology 120196-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Derrick, S. C., C. Repique, P. Snoy, A. L. Yang, and S. Morris. 2004. Immunization with a DNA vaccine cocktail protects mice lacking CD4 cells against an aerogenic infection with Mycobacterium tuberculosis. Infect. Immun. 721685-1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derrick, S. C., A. L. Yang, and S. L. Morris. 2004. A polyvalent DNA vaccine expressing an ESAT6-Ag85B fusion protein protects mice against a primary infection with Mycobacterium tuberculosis and boosts BCG-induced protective immunity. Vaccine 23780-788. [DOI] [PubMed] [Google Scholar]
- 10.Goonetilleke, N. P., H. McShane, C. M. Hannan, R. J. Anderson, R. H. Brookes, and A. V. Hill. 2003. Enhanced immunogenicity and protective efficacy against Mycobacterium tuberculosis of bacille Calmette-Guerin vaccine using mucosal administration and boosting with a recombinant modified vaccinia virus Ankara. J. Immunol. 1711602-1609. [DOI] [PubMed] [Google Scholar]
- 11.Hinchey, J., S. Lee, B. Y. Jeon, R. J. Basaraba, M. M. Venkataswamy, B. Chen, J. Chan, M. Braunstein, I. M. Orme, S. C. Derrick, S. L. Morris, W. R. Jacobs, Jr., and S. A. Porcelli. 2007. Enhanced priming of adaptive immunity by a proapoptotic mutant of Mycobacterium tuberculosis. J. Clin. Investig. 1172279-2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huygen, K., J. Content, O. Denis, D. L. Montgomery, A. M. Yawman, R. R. Deck, C. M. DeWitt, I. M. Orme, S. Baldwin, C. D'Souza, A. Drowart, E. Lozes, P. Vandenbussche, J. P. Van Vooren, M. A. Liu, and J. B. Ulmer. 1996. Immunogenicity and protective efficacy of a tuberculosis DNA vaccine. Nat. Med. 2893-898. [DOI] [PubMed] [Google Scholar]
- 13.Kamath, A., J. S. Woodworth, and S. M. Behar. 2006. Antigen-specific CD8+ T cells and the development of central memory during Mycobacterium tuberculosis infection. J. Immunol. 1776361-6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kamath, A. B., J. Woodworth, X. Xiong, C. Taylor, Y. Weng, and S. M. Behar. 2004. Cytolytic CD8+ T cells recognizing CFP10 are recruited to the lung after Mycobacterium tuberculosis infection. J. Exp. Med. 2001479-1489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kamath, A. T., C. G. Feng, M. Macdonald, H. Briscoe, and W. J. Britton. 1999. Differential protective efficacy of DNA vaccines expressing secreted proteins of Mycobacterium tuberculosis. Infect. Immun. 671702-1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirman, J. R., T. Turon, H. Su, A. Li, C. Kraus, J. M. Polo, J. Belisle, S. Morris, and R. A. Seder. 2003. Enhanced immunogenicity to Mycobacterium tuberculosis by vaccination with an alphavirus plasmid replicon expressing antigen 85A. Infect. Immun. 71575-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lalvani, A. 2007. Diagnosing tuberculosis infection in the 21st century: new tools to tackle an old enemy. Chest 1311898-1906. [DOI] [PubMed] [Google Scholar]
- 18.Lewinsohn, D. M., L. Zhu, V. J. Madison, D. C. Dillon, S. P. Fling, S. G. Reed, K. H. Grabstein, and M. R. Alderson. 2001. Classically restricted human CD8+ T lymphocytes derived from Mycobacterium tuberculosis-infected cells: definition of antigenic specificity. J. Immunol. 166439-446. [DOI] [PubMed] [Google Scholar]
- 19.McShane, H., R. Brookes, S. C. Gilbert, and A. V. Hill. 2001. Enhanced immunogenicity of CD4+ T-cell responses and protective efficacy of a DNA-modified vaccinia virus Ankara prime-boost vaccination regimen for murine tuberculosis. Infect. Immun. 69681-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mollenkopf, H. J., L. Grode, J. Mattow, M. Stein, P. Mann, B. Knapp, J. Ulmer, and S. H. E. Kaufmann. 2004. Application of mycobacterial proteomics to vaccine design: improved protection by Mycobacterium bovis BCG prime-Rv3407 DNA boost vaccination against tuberculosis. Infect. Immun. 726471-6479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morris, S., C. Kelley, A. Howard, Z. Li, and F. Collins. 2000. The immunogenicity of single and combination DNA vaccines against tuberculosis. Vaccine 182155-2163. [DOI] [PubMed] [Google Scholar]
- 22.Murray, J. F. 1998. Tuberculosis and HIV infection: a global perspective. Respiration 65335-342. [DOI] [PubMed] [Google Scholar]
- 23.Olsen, A. W., P. R. Hansen, A. Holm, and P. Andersen. 2000. Efficient protection against Mycobacterium tuberculosis by vaccination with a single subdominant epitope from the ESAT-6 antigen. Eur. J. Immunol. 301724-1732. [DOI] [PubMed] [Google Scholar]
- 24.Pearce, E. L., D. J. Shedlock, and H. Shen. 2004. Functional characterization of MHC class II-restricted CD8+CD4- and CD8-CD4- T cell responses to infection in CD4-/- mice. J. Immunol. 1732494-2499. [DOI] [PubMed] [Google Scholar]
- 25.Pym, A. S., P. Brodin, L. Majlessi, R. Brosch, C. Demangel, A. Williams, K. E. Griffiths, G. Marchal, C. Leclerc, and S. T. Cole. 2003. Recombinant BCG exporting ESAT-6 confers enhanced protection against tuberculosis. Nat. Med. 9533-539. [DOI] [PubMed] [Google Scholar]
- 26.Shams, H., P. Klucar, S. E. Weis, A. Lalvani, P. K. Moonan, H. Safi, B. Wizel, K. Ewer, G. T. Nepom, D. M. Lewinsohn, P. Andersen, and P. F. Barnes. 2004. Characterization of a Mycobacterium tuberculosis peptide that is recognized by human CD4+ and CD8+ T cells in the context of multiple HLA alleles. J. Immunol. 1731966-1977. [DOI] [PubMed] [Google Scholar]
- 27.Skeiky, Y. A., M. R. Alderson, P. J. Ovendale, J. A. Guderian, L. Brandt, D. C. Dillon, A. Campos-Neto, Y. Lobet, W. Dalemans, I. M. Orme, and S. G. Reed. 2004. Differential immune responses and protective efficacy induced by components of a tuberculosis polyprotein vaccine, Mtb72F, delivered as naked DNA or recombinant protein. J. Immunol. 1727618-7628. [DOI] [PubMed] [Google Scholar]
- 28.Tyznik, A. J., J. C. Sun, and M. J. Bevan. 2004. The CD8 population in CD4-deficient mice is heavily contaminated with MHC class II-restricted T cells. J. Exp. Med. 199559-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang, J., L. Thorson, R. W. Stokes, M. Santosuosso, K. Huygen, A. Zganiacz, M. Hitt, and Z. Xing. 2004. Single mucosal, but not parenteral, immunization with recombinant adenoviral-based vaccine provides potent protection from pulmonary tuberculosis. J. Immunol. 1736357-6365. [DOI] [PubMed] [Google Scholar]
- 30.Woodworth, J. S., and S. M. Behar. 2006. Mycobacterium tuberculosis-specific CD8+ T cells and their role in immunity. Crit. Rev. Immunol. 26317-352. [DOI] [PMC free article] [PubMed] [Google Scholar]