Abstract
Enterohemorrhagic Escherichia coli (EHEC) is a noninvasive food-borne pathogen that colonizes the distal ileum and colon. Proteins encoded in the EHEC locus of enterocyte effacement (LEE) pathogenicity island are known to contribute to this pathogen's adherence to epithelial cells and intestinal colonization. The role of non-LEE-encoded proteins in these processes is not as clear. We found that the Z2053 gene (designated adfO here), a gene located in a cryptic EHEC prophage, exhibits similarity to adherence and/or colonization factor genes found in several other enteric pathogens. An EHEC adfO mutant exhibited marked reductions in adherence to HeLa cells and in the secretion of several proteins into the supernatant. YodA, one of these secreted proteins, was found to be a substrate of the EHEC pO157-encoded type 2 secretion system (T2SS). Both the T2SS and YodA proved to be essential for EHEC adherence to cultured HeLa cell monolayers. Using an infant rabbit model of infection, we found that the adfO mutation did not affect colonization but that the colonization of an etpC (T2SS) mutant was reduced ∼5-fold. A strain deficient in YodA had a more severe colonization defect; however, this strain also exhibited a growth defect in vitro. Overall, our findings indicate that the pO157-encoded T2SS contributes to EHEC adherence and intestinal colonization and thus show that EHEC pathogenicity depends on type 2 secretion as well as type 3 secretion.
Enterohemorrhagic Escherichia coli (EHEC) is an important cause of food-borne illness in developed nations around the world. Several EHEC serotypes cause disease, but the O157:H7 serotype is the most common cause of EHEC-related disease in North America, the United Kingdom, and Japan (22). After ingestion of food or beverages contaminated with this pathogen, humans can develop a variety of symptoms ranging from mild diarrhea to the severe and sometimes life-threatening hemolytic-uremic syndrome (35). Besides causing significant human disease, EHEC is a major economic burden due to the high cost associated with the recall of contaminated food (www.fsis.usda.gov/FSIS_Recalls/index.asp).
EHEC is a noninvasive pathogen that, following ingestion and passage through the human stomach and proximal small bowel, colonizes the distal ileum and colon (54). While growing in the intestine, EHEC produces Shiga toxins; systemic absorption of these prophage-encoded toxins is thought to account for the severe clinical manifestations of EHEC infection, such as hemolytic-uremic syndrome (22). The genes and mechanisms that enable EHEC to colonize the human intestine are incompletely understood. However, it is clear that the EHEC locus of enterocyte effacement (LEE) pathogenicity island is essential for tight adherence of EHEC to tissue culture cells and for intestinal colonization in experimental animals (9, 36, 43, 62). The LEE pathogenicity island encodes a type 3 secretion system (T3SS), several effector proteins that are translocated directly into host cells by the T3SS, intimin (an outer membrane protein important for adherence), and transcriptional regulators (for a review, see reference 14).
EHEC tightly adheres to epithelial cells by subverting host cell cytoskeletal processes, yielding “attaching and effacing” lesions (27). These histologic lesions are defined by the intimate attachment of bacteria to the epithelial cell surface along with the localized loss (effacement) of microvilli and assembly of electron-dense fibrillar structures underneath attached bacteria, forming a “pedestal-like” protrusion from the cell (33). Interactions between two LEE-encoded proteins, intimin and Tir (a T3SS effector that inserts into the host plasma membrane), are thought to mediate “intimate” adherence (8, 18, 23). Interactions between translocated Tir, EspFU (a T3SS effector protein encoded by the cryptic prophage CP-933U; also known as TccP), and host proteins are thought to promote actin polymerization in host cells, resulting in pedestal formation (3, 15, 31).
A variety of models have been used to investigate EHEC adherence to host cells. These models include assays of adherence to cultured epithelial cell lines (e.g., HeLa, HEp-2, HT-29, Caco-2, and T84) (57, 61) and to intestinal explants from pigs and cows (1, 16). In general, these assays measure the number of adherent bacteria rather than actin rearrangements that result from translocation of type 3 effector proteins. Several EHEC proteins that promote EHEC adherence, including ToxB/Efa-1′ (51, 56), OmpA (60), and hemorrhagic coli pilus (64), have been identified using these assays; it is likely that these proteins contribute to EHEC adherence independent of T3SS-mediated adherence. One possibility is that T3SS-independent factors mediate initial interactions between bacteria and epithelial cells, thereby facilitating the protein transfer that enables tight adherence and pedestal formation. To date, none of these accessory adherence proteins has been shown to contribute to E. coli O157 intestinal colonization in animal models.
Most O157:H7 strains possess pO157, an F-related plasmid that encodes a number of proteins that may influence EHEC pathogenicity. Loss of pO157 has been associated with a reduction in O157:H7 virulence (30). Among the putative virulence factors encoded on pO157 is the secreted mucin protease StcE. Although deletion of stcE does not significantly alter EHEC adherence, addition of purified StcE protein has been shown to enhance EHEC intimate attachment to cultured monolayers (17). StcE is secreted by the O157 Etp type 2 secretion system (T2SS) (28), which is also encoded by pO157 (47). The T2SSs of several gram-negative pathogens secrete numerous proteins that influence pathogenesis (46). T2SS substrates are usually first released into the periplasmic space by the general secretory apparatus after cleavage of a signal sequence. These substrates are then recognized by the T2SS in an as-yet-undetermined fashion and exported past the outer membrane. StcE is the only EHEC T2SS substrate identified to date. The role of this secretion system in EHEC pathogenesis has not been defined.
Besides the ∼90-kb pO157 plasmid, the sequenced O157:H7 genomes contain more than 1 Mb of DNA that is not present in the genome of nonpathogenic E. coli K-12 (19, 38). This additional DNA is interspersed as “O-islands” along the backbone of DNA that is shared with E. coli K-12. In the prototype O157:H7 strain EDL933, 18 of these O-islands are thought to correspond to prophages that in most cases are believed to be defective (38). Since genes implicated in virulence are often located in prophages or other mobile elements (58, 63), we looked for potential virulence-associated genes located in the EDL933 prophages. We noticed that the cryptic prophage CP-933O (O-island 57) contains a gene, the Z2053 gene, whose predicted product exhibits similarity to virulence-associated proteins in other enteric pathogens. Batisson et al. previously noted that the sequence of the Z2053 gene was nearly identical to the sequence of paa, a gene of porcine enteropathogenic E. coli that was implicated in this pathogen's colonization of the pig ileum (2). Here we studied the role of the Z2053 gene (designated adfO here) in EHEC pathogenicity and determined that adfO promotes adherence of EHEC to HeLa cells. AdfO also appears to facilitate the secretion of YodA, a protein encoded by DNA that is also present in the E. coli K-12 chromosome and (as we show here) is secreted by the pO157-encoded T2SS. Both the T2SS and YodA proved to be essential for adherence of EHEC to cultured HeLa cell monolayers and to promote colonization of the infant rabbit intestine. Thus, the EHEC T2SS and at least one of its substrates promote EHEC virulence.
MATERIALS AND METHODS
Bacterial strains and growth.
The strains used in this study are shown in Table 1. Unless otherwise noted, strains were grown in LB broth or on LB agar plates. For antibiotic selection, agar plates were supplemented with ampicillin (100 μg/ml), spectinomycin (100 μg/ml), or chloramphenicol (10 μg/ml). EDL933, a sequenced clinical O157:H7 isolate (38), was used as the wild-type strain in our experiments. We used a two-step approach to generate deletions in adfO (Z2053), yodA (Z3065), and etpC in the EDL933 background. Initially, deletion-insertion mutations in each of these genes were constructed in TEA023 (EDL933 Δgal::aad-7) or TEA028 (EDL933 Δgal::tetA) (20) using the lambda red recombinase-based gene replacement system (5). The primers used to generate these mutations are shown in Table 2. Then, for each mutation, the antibiotic resistance gene-marked deletion was transduced into EDL933 using phage P1 as described previously (20). In each case, the deletion in EDL933 was confirmed by PCR.
TABLE 1.
Strains and plasmids used in this study
| Strain or plasmid | Genotype and/or description | Primers used in construction |
|---|---|---|
| Strains | ||
| EDL933 | O157:H7 wild type (clinical isolate) | |
| TEA009 | EDL933 ΔadfO::FRT-cat-FRT (deletes AdfO15-238) | TE030, TE031 |
| TEA017 | EDL933 Δtir::FRT-cat-FRT (deletes Tir13-545) | JRW016, JRW017 |
| TEA022 | EDL933 ΔetpC::FRT-aad-7-FRT (deletes EtpC11-280) | TE083, TE084 |
| TEA053 | EDL933 ΔyodA::FRT-cat-FRT (deletes YodA13-212) | TE150, TE151 |
| Plasmidsa | ||
| pTHE012 | Para-PadfO-adfO-V5-His6 (Ampr) | TE046, TE050 |
| pAdfO (= pTHE073) | PadfO-adfO-V5-His6 (Ampr) | |
| pEspFu (= pTHE016) | PespFU-espFU-V5-His6 (Ampr) | TE112, TE074 |
| pTir (= pTHE034) | Para-Ptir-tir-V5-His6 (Ampr) | TE113, TE068 |
| pYodA (= pTHE065) | Para-yodA-V5-His6 (Ampr) | TE160, TE161 |
| pStcE (= pTHE066) | Para-stcE-V5-His6 (Ampr) | TE162, TE163 |
All plasmids are derivatives of pBAD-TOPO.
TABLE 2.
Oligonucleotide primers used in this study
| Primer | Sequence (5′-3′) |
|---|---|
| JRW016 | GACGAAACGATGGGATCCCGCGCTGGTGGGTTATTGTGTAGGCTGGAGCTGCTTCG |
| JRW017 | ATGCCTATTGGTAATCTTGGTCATAATCCCAATGTGCATATGAATATCCTCCTTA |
| TE030 | GAGGAACATAATGGCAGGTTTTTTAATATTCCTGTCTTCTGTG TAGGCTGGAGCTGCTTC |
| TE031 | AATGCAGGCGCCGCTTCATCGGTTTGTAGCCATTCTGCAAACAATGAATATCCTCCTTA |
| TE046 | AACAACATATGCACGAGGTAAA |
| TE050 | AGTGCCTTTCCTGGTCCAGCC |
| TE068 | GACGAAACGATGGGATCCCGG |
| TE074 | TTTTTTGAGAGGATATATGTCAACATCGAC |
| TE083 | ATGTTGTTCTTTCTATCATTCCGGGGTGACGTGTAGGCTGGAGCTGCTTC |
| TE084 | GTTTTCTCCTACAGCAATAAATGCATCATAATTCCGGGGATCCGTCGACC |
| TE112 | TCGGAGGTCATACCATTAATCAA |
| TE113 | TGGTCGATGTCATGTTCAGC |
| TE150 | ATGACTCTGGAGGAAACTGTTTTGGCGATTCGTCTTGTGTAGGCTGGAGCTGCTTC |
| TE151 | TCAATGAGACATCATTTCCTCGACCACTTCTTCGCTCATATGAATATCCTCCTTA |
| TE160 | ATCATATACAATGACTCTGGAGGAAACTGTTTTG |
| TE161 | TGCGGCCGCATGAGACATCATTTCCTCGACCAC |
| TE162 | ATCCGGCTGCATGAACACTAAAAT |
| TE163 | TGCGGCCGCTTTATATACAACCCTCATTGACCT |
The plasmids used in this work are also shown in Table 1. For each plasmid, the gene of interest was amplified using specific primers, HotStart Taq Mastermix (Qiagen), and Pfu polymerase (Invitrogen). Following PCR amplification, the genes were cloned into pBAD-TOPO using the TOPO TA method (Invitrogen). The sequence of each cloned gene was subsequently confirmed. The pBAD-TOPO clones expressed C-terminal V5, His-tagged protein fusions under arabinose control. To construct pAdfO (= pTHE073), the adfO-pBAD-TOPO plasmid (pTHE012) was digested with NdeI and religated with T4 DNA ligase. In pAdfO, the arabinose promoter was removed, and the endogenous adfO promoter was used to drive AdfO-V5-His expression.
Protein isolation and immunoblot analysis.
Strains harboring plasmids encoding V5, His-tagged proteins were grown in M9 medium containing glucose (0.2%), Casamino Acids (0.2%), and ampicillin (10 μg/ml) overnight at 37°C. Cultures were then diluted 1:50 into fresh M9 medium containing glucose (0.2%), Casamino Acids (0.2%), ampicillin (10 μg/ml), 44 mM NaHCO3, and l-arabinose (0.2%) and incubated for 16 h at 37°C without agitation. Following pelleting of the cells, culture supernatants (1.2 ml each) were transferred to fresh Eppendorf tubes, incubated in 10% tricarboxylic acid (Sigma) on ice for 2 h, and then centrifuged for 30 min at 13,000 × g at 4°C. Each pellet was washed with 500 μl of ice-cold acetone for 5 min at 4°C, air dried, and then resuspended in 20 μl of loading dye. Proteins were resolved on 10% NuPAGE gels and then transferred onto nitrocellulose and analyzed by immunoblotting with anti-V5 antibody (Invitrogen). Two-dimensional in-gel electrophoresis was used to compare the amounts of different proteins in culture supernatants from the adfO mutant and the wild-type strain (Applied Biomics, Hayward, CA).
Adherence assay.
Each EHEC strain was grown for 8 h in LB broth at 37°C with agitation. Bacterial cultures were then diluted 1:50 into Dulbecco modified Eagle medium (DMEM) (Gibco) supplemented with 100 mM HEPES (pH 7.0) and grown for 16 h at 37°C in the presence of 5% CO2 without agitation. For each assay, bacteria (10 μl) were added to 500 μl DMEM (low glucose; Gibco) containing 25 mM HEPES (pH 7.0), NaHCO3 (4 mM), and fetal calf serum (3%; Gibco) and transferred to a HeLa cell monolayer seeded on a glass coverslip in a 24-well dish. The multiplicity of infection was approximately 50 bacteria per HeLa cell. The 24-well dish was then centrifuged at 1,000 rpm for 10 min at room temperature and incubated at 37°C in the presence of 5% CO2. To induce protein expression in plasmid-bearing strains, l-arabinose (0.2%) was added 1 h after addition of the bacteria. Each well was washed three times with phosphate-buffered saline (PBS) after 3.5 h of incubation of bacteria with HeLa cells. Fresh DMEM (low glucose; Gibco) containing 25 mM HEPES (pH 7.0), NaHCO3 (4 mM), and fetal calf serum (3%; Gibco) was added to each well, and following an additional 2 h of incubation at 37°C in the presence of 5% CO2, the wells were washed five times with PBS. Bacteria and HeLa cells were then fixed using paraformaldehyde and were permeabilized using PBS-0.1% Triton X-100. Coverslips were stained with Alexa-Fluor 568-conjugated phalloidin (Invitrogen) and 4′,6′-diamidino-2-phenylindole (DAPI) (Invitrogen), washed, mounted, and sealed. This assay measured tight (T3SS-dependent) adherence of EHEC to HeLa cells; no binding was observed for strains unable to induce actin pedestals. In our hands, assays for diffuse (T3SS-independent) adherence did not give reproducible results.
We found that there was also day-to-day variation in the percentage of HeLa cells with adherent wild-type bacteria (EDL933). Therefore, for each set of adherence assays, the adherence of each bacterial strain was calculated relative to the adherence of the wild-type strain. Twenty microscopic fields were examined to determine the percentage of HeLa cells with adherent bacteria. To calculate the relative binding efficiency (RBE) of each strain, the percentage of HeLa cells with adherent bacteria of interest was divided by the percentage of HeLa cells with adherent wild-type bacteria. The data shown below for each mutant are the mean and standard deviation derived from three to nine independent experiments; in each experiment the assay was performed once. The one-sample t test was used to compare RBE values for the mutants and the wild type.
Competition assays with infant rabbits.
Three-day-old New Zealand White rabbits were orally inoculated with a 1:1 mixture of the wild-type strain (EDL933) and an isogenic derivative, either TEA009, TEA022, or TEA053, containing a total of ∼2.5 × 108 bacteria. Prior to inoculation, bacteria from overnight cultures were washed once and then resuspended in PBS. Seven days after inoculation, rabbits were sacrificed, and portions of the ileum, midcolon, and cecum were homogenized, diluted, and plated on sorbitol-MacConkey agar. After overnight growth, bacteria were replica plated on LB agar containing 10 μg/ml chloramphenicol (for TEA009 or TEA053) or 100 μg/ml spectinomycin (for TEA022) to determine the number of mutant bacteria. Competitive indices (CIs) were calculated using the following formula: CI = [(number of mutant bacteria in output)/(number of wild-type bacteria in output)]/[(number of mutant bacteria in input)/(number of wild-type bacteria in input)].
Growth curves.
Each strain was grown overnight in LB broth at 37°C and then diluted 1:1,000 in fresh LB broth. An aliquot (300 μl) was inoculated into a well of a 96-well plate and shaken at 37°C. The absorbance at 600 nm was determined for 16 h using a synergy HT plate reader (Biotek Instruments). Assays were performed in triplicate, and each strain was tested in three independent experiments.
RESULTS
AdfO is necessary for efficient adherence of EHEC to HeLa cells.
Because several EHEC virulence factors, including Shiga toxins 1 and 2, are encoded in prophages (37, 53), we hypothesized that additional genes that influence EHEC pathogenicity would be found in one or more of the 18 prophages in the genome of the prototype O157:H7 strain EDL933. We noticed that the cryptic prophage CP-933O (O-island 57) contains a gene, Z2053, whose predicted product has significant similarity to proteins of several other enteric pathogens that have been implicated in adherence to epithelial cells and/or intestinal colonization. For example, the predicted Z2053 amino acid sequence is 100% identical to the sequence of Paa, a porcine enteropathogenic E. coli protein that has been shown to promote this pathogen's colonization of epithelial cells in an ex vivo pig ileum explant model of infection (2). In addition, the Vibrio cholerae Z2053 protein orthologue, AcfC (50% identity to the Z2053 protein), is encoded on the V. cholerae TCP pathogenicity island and functions as an accessory intestinal colonization factor (39). Another Z2053 protein orthologue, PEB3 (53% identity to the Z2053 protein), is a major antigenic surface adhesin of Campylobacter jejuni (41).
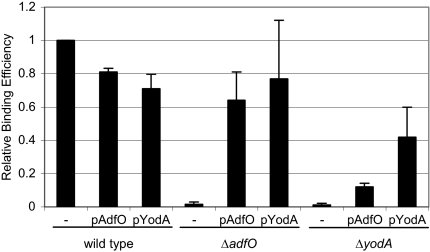
We deleted the Z2053 gene from EDL933 to investigate whether the Z2053 protein plays a role in EHEC pathogenesis. Initially, we explored the attachment of wild-type and EDL933 Z2053 mutant strains to cultured HeLa cell monolayers by assessing adherence by Giemsa staining and by quantitating attached CFU. However, these assays yielded highly unreproducible results with wild-type bacteria. Therefore, we instead assessed the frequency with which HeLa cells coincubated with bacteria generated actin pedestals (focal adhesions) visualized by fluorescent actin staining (FAS) underneath cell-associated bacteria. With wild-type strain EDL933 all cell-associated bacteria (stained with DAPI) were found to be adjacent to phalloidin-stained actin, and a majority of HeLa cells had adherent bacteria. Bacteria unable to induce FAS (e.g., an espFU mutant and a tir mutant) were rarely found to be cell associated, suggesting that under these experimental conditions actin rearrangement is required for bacterial adherence and that bacteria that did not tightly adhere were washed away. As there was some day-to-day variability in the results obtained with this assay, in each experiment the adherence of each strain relative to the adherence of the wild-type bacteria was calculated. Compared to the wild type, the Z2053 mutant exhibited a dramatic adherence defect; it had an RBE of 0.016 (P < 0.0001 in comparison with the wild type) (Fig. 1 and 2). Introduction of a plasmid-borne copy of the Z2053 gene driven from its native promoter increased the RBE of the Z2053 mutant to 0.64, confirming that deletion of the Z2053 gene accounted for the impaired adherence phenotype of this mutant (Fig. 2). Based on this observation that the Z2053 protein contributes to EHEC adherence to epithelial cells, we designated Z2053 AdfO (adherence factor encoded on CP-933O phage).
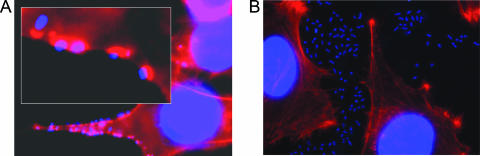
FIG. 1.
Adherence of (A) wild-type (EDL933) and (B) ΔadfO (TEA009) EHEC strains to HeLa cells. Bacteria and HeLa nuclei were visualized with DAPI (blue), and F-actin was visualized with Alexa-Fluor-586-phalloidin (red). The percentage of HeLa cells with adherent bacteria was calculated for 20 microscopic fields. The adherence of the wild-type strain was defined as 1; the RBE of the ΔadfO strain was found to be 0.016 ± 0.013.
FIG. 2.
RBE of the wild-type (EDL933), ΔadfO (TEA009), and ΔyodA (TEA053) strains carrying no plasmid (−), pAdfO, or pYodA with HeLa cells. The data are the means and standard deviations of results from at least three independent experiments.
AdfO influences protein secretion into the supernatant.
In the course of characterizing the adfO mutant, we compared the amounts of different proteins in culture supernatants from the adfO mutant and the wild type using two-dimensional in-gel electrophoresis. For the majority of the proteins in the supernatants, there was no AdfO-dependent change in the level. This was consistent with our observations that the adfO mutant did not exhibit a change in membrane integrity based on assays of sodium dodecyl sulfate and Triton X-100 tolerance (4; data not shown). However, we observed that the levels of four spots were reduced at least twofold in the mutant (data not shown). Two of these spots were picked from the gel and subject to matrix-assisted laser desorption ionization mass spectrometry analysis. Both of the proteins identified, the Z1931 and Z3065 proteins, had not been previously characterized in EHEC.
The gene encoding the Z1931 protein is located on the CP-933X prophage and has similarity to genes encoding members of the omptin family of outer membrane proteases. A number of omptin family members have been implicated in virulence, including Pla from Yersinia pestis and NspA from Neisseria meningitidis (21). The Z3065 protein was previously identified in the culture supernatant of EHEC grown in the tissue culture medium DMEM (29). The Z3065 protein is 99% identical to YodA from E. coli K-12, and the Z3065 gene and yodA are flanked by the same genes in their respective genomes. In E. coli K-12, YodA (recently renamed ZinT [24]) is a metal-binding protein that is secreted into the periplasm under stress conditions (6, 40).
YodA is required for efficient EHEC adherence to HeLa cells.
Since the amounts of the Z1931 and Z3065 proteins detected in culture supernatants were influenced by adfO, we reasoned that these proteins might contribute to EDL933 adherence to HeLa cells. We deleted the Z1931 and Z3065 genes from EDL933 to investigate this possibility. While the ΔZ1931 mutant did not have an adherence defect (data not shown), the ΔZ3065 mutant had a dramatic adherence defect whose magnitude (RBE, 0.011; P < 0.0001 in comparison with the wild type) was similar to the magnitude of the adherence defect of the adfO mutant (Fig. 2). Overexpression of the Z3065 gene in the ΔZ3065 mutant partially complemented the mutant's adherence defect (Fig. 2), increasing its binding efficiency to 0.42. These observations suggest that the Z3065 protein (referred to below as YodA) is also associated with efficient adherence of EHEC to cultured cells; however, it is possible that at least some of the adherence defect of the Z3065 mutant can be attributed to the growth defect of this strain (see below).
Since deletion of adfO and deletion of yodA resulted in similar profound adherence defects and because adfO influences the amount of YodA found in supernatants, we tested whether the adherence defect of the ΔadfO mutant could be bypassed by increasing the level of YodA. We found that overexpression of YodA in the adfO deletion mutant had a dramatic effect on the strain's adherence, increasing it from 0.016 to 0.77 (P < 0.0001) (Fig. 2). In contrast, overexpression of AdfO had only a minor effect on adherence of the yodA mutant (Fig. 2). Since overexpression of YodA could complement the adfO deletion, yodA appears to act downstream of adfO in EHEC adherence.
YodA is a T2SS substrate.
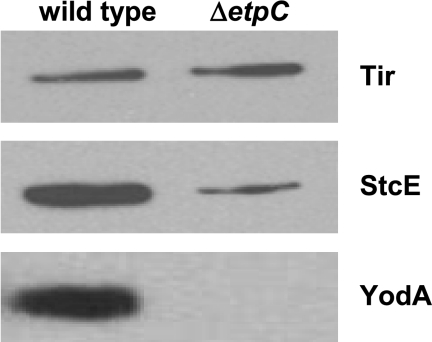
Since YodA was isolated in culture supernatants, we explored the possibility that secretion of this protein is facilitated by either the EHEC T2SS or the EHEC T3SS. YodA is predicted to have a signal sequence; such sequences are not associated with substrates for T3SS (45) but are usually present in T2SS substrates (46). We therefore tested whether YodA is a substrate for the EHEC T2SS. The O157 T2SS is encoded by the etpC to etpO genes located on pO157 (47). We constructed a T2SS mutant by replacing etpC, the first gene in the putative etp operon, with a spectinomycin resistance gene (aad-7) oriented in the direction opposite the etp genes. The ΔetpC::aad-7 mutant showed a marked reduction in the amount of StcE (the only known substrate of the O157 T2SS [28]) detected in the supernatant (Fig. 3). This observation suggests that the ΔetpC::aad-7 mutant was, as expected, deficient in type 2 secretion. In addition, there was almost no detectable YodA in the supernatant from the T2SS mutant, whereas this protein was readily identified in the supernatant from the wild-type strain (Fig. 3). In contrast, the ΔetpC::aad-7 mutation did not affect secretion of the T3SS effector Tir (Fig. 3), suggesting that this mutation specifically reduced type 2 secretion. Furthermore, there was no reduction in YodA secretion in a T3SS mutant (data not shown). These observations suggest that YodA can be a substrate of the EHEC T2SS. However, additional experiments revealed that not all YodA is secreted from the cell (data not shown).
FIG. 3.
Immunoblots of epitope-tagged Tir, StcE, and YodA in supernatants from wild-type EHEC strain EDL933 and a T2SS mutant (TEA022). Proteins in equal volumes of culture supernatants from EDL933 and TEA022 expressing plasmid-encoded V5-tagged Tir, StcE, or YodA were concentrated using tricarboxylic acid precipitation, resolved with 10% NuPAGE gels, and analyzed by immunoblotting with anti-V5 antibody.
Type 2 secretion is necessary for efficient adherence of EHEC to HeLa cells.
Since we found that YodA promotes EHEC adherence and that the T2SS is required for secretion of this protein, we tested whether the T2SS is important for EHEC adherence. The adherence of the T2SS mutant to HeLa cells also was found to be dramatically impaired (RBE, 0.003). Presumably, the EHEC T2SS is required for adherence to cultured monolayers because it enables the secretion of YodA and perhaps additional substrates as well.
T2SS is required for efficient EHEC colonization of the rabbit intestine.
Since EHEC adherence to epithelial cells is thought to be an important step in intestinal colonization, we investigated if adfO, yodA, and etpC contribute to intestinal colonization, as well as to adherence of EHEC to cultured monolayers. Intestinal colonization by the adfO, yodA, and etpC mutants was examined in competition assays with the wild-type strain using the infant rabbit model. In this assay, approximately 1:1 mixtures of mutant and wild-type strains were intragastrically inoculated into 3-day-old rabbits. Seven days later, the ratios of the numbers of mutant cells to the numbers of wild-type cells in different parts of the gastrointestinal tract were determined and expressed as CIs (see Materials and Methods).
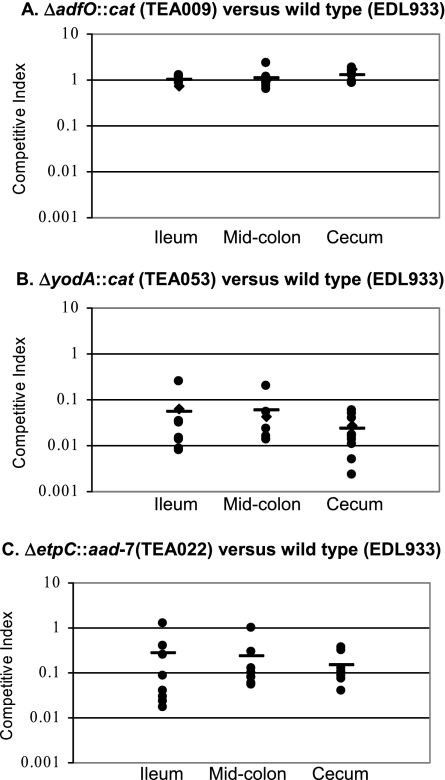
The adfO, yodA, and etpC mutants exhibited disparate phenotypes in this in vivo competition assay. When the adfO mutant and the wild-type strain were used, the CIs for the ileum, midcolon, and cecum were ∼1.0 (Fig. 4A). Thus, although the adherence of the adfO mutant to HeLa cells was markedly impaired, this strain was not attenuated in colonization of the infant rabbit intestine.
FIG. 4.
In vivo competition assays performed with the (A) ΔadfO (TEA009), (B) ΔyodA (TEA053), and (C) ΔetpC (TEA022) mutants and the isogenic wild-type strain (EDL933). TEA022, TEA009, or TEA053 and EDL933 were coinoculated into infant rabbits and harvested after 7 days of infection from the ileum, midcolon, and cecum. The CIs for TEA022 and TEA053 were significantly less than 1.0 (P < 0.01).
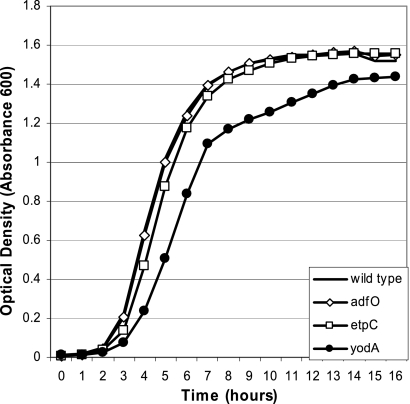
The yodA mutant was severely outcompeted by the wild type in the in vivo competition assay. In the infant rabbit model, the yodA mutant was outcompeted ∼10- to 100-fold in the ileum, midcolon, and cecum (Fig. 4B). However, monoculture growth analysis of the yodA mutant revealed that this strain had a delayed transition from the lag phase to the log phase, as well as a slight reduction in the growth rate (Fig. 5). This general growth deficiency of the yodA mutant may provide an explanation for the attenuated growth of this strain observed in the in vivo competition assay.
FIG. 5.
Growth curves for the wild type (EDL933) and the ΔadfO (TEA009), ΔetpC (TEA022), and ΔyodA (TEA053) mutants grown in LB medium.
Interestingly, the T2SS mutant did not exhibit a growth defect in vitro (Fig. 5) but displayed a colonization defect in vivo. Fewer mutant bacteria than wild-type bacteria were recovered from the ileum (CI, 0.26), midcolon (CI, 0.22), and cecum (CI, 0.15) (Fig. 4C). For all three of these regions of the gastrointestinal tract, the CIs were statistically different from the theoretical value, 1 (P < 0.01, Wilcoxon signed-rank test). Thus, the pO157-encoded T2SS contributes to efficient EHEC intestinal colonization.
DISCUSSION
Numerous EHEC genes have been shown to be important for adherence of this pathogen to tissue culture monolayers (60) and animal intestinal explants (34, 48) and to promote persistence in ruminant reservoir hosts (10, 11); however, with the exception of proteins encoded in the LEE pathogenicity island (9, 43, 44), the roles of candidate adhesion factors in animal models of disease are not known. Here, we identified three EHEC genes (adfO [Z2053], located on prophage CP-933O; yodA, a gene also found in E. coli K-12; and etpC, a gene located on pO157) that contribute to adherence of EHEC to HeLa cell monolayers. Using an infant rabbit model of infection, we found that the adfO mutant did not have a colonization defect but the etpC (T2SS) mutant had a ∼5-fold colonization defect. A strain deficient in YodA, which we found to be a substrate for the EHEC T2SS, had a larger colonization defect; however, this strain also exhibited a growth deficiency in vitro. Overall, our findings indicate that the pO157-encoded T2SS contributes to EHEC adherence and intestinal colonization and thus show that EHEC pathogenicity depends on type 2 secretion as well as type 3 secretion.
Tight adherence of EHEC to intestinal epithelia is thought to be mediated by the binding of the outer membrane protein intimin to Tir, a type 3 effector (23), and perhaps to host cell beta1 integrins (13) and/or nucleolin (50). Actin rearrangement and attaching and effacing lesion formation require that the T3SS translocate Tir and EspFU to host cells, and it is thought that LEE-encoded proteins and EspFU are necessary and sufficient for these processes. Since we assayed actin rearrangements rather than adherent bacteria, in principle, adfO, yodA, and etpC could directly influence the type 3 secretion process; alternatively, these genes may contribute to steps prior to or independent of type 3 secretion to promote adherence. Our assays to date have not allowed us to distinguish between these possibilities. Our attempts to monitor secretion of type 3 effectors have suggested that wild-type strain EDL933 releases very small amounts of these proteins into culture supernatants. Additional EHEC proteins are also likely to contribute to this organism's attachment to host cells, independent of the translocation of T3SS effectors. For example, fimbrial and nonfimbrial adhesins (25, 32, 52, 55, 56, 59, 60, 64) have been proposed to promote the binding of EHEC to host cells.
Recent studies revealed that pO157 contributes to EHEC adherence and long-term colonization of the bovine terminal rectum (49). Sheng et al. found that an EHEC strain cured of pO157 exhibited a >30-fold reduction in colonization of the bovine rectum compared to a complemented strain 7 days after infection (49). We observed only a ∼6-fold reduction in colonization of the infant rabbit intestine for an EHEC etpC mutant. The differences in the data may suggest that pO157-encoded factors in addition to the T2SS promote intestinal colonization. For example, the pO157-encoded serine protease EspP has been shown to promote EHEC colonization of the calf intestine (10, 11). Different proteins may augment adherence of EHEC to different regions of the ileum or large bowel; furthermore, distinct proteins may promote EHEC adherence in human and ruminant hosts.
Our data suggest that one or more of the substrates of the EHEC type 2 secretion machinery (such as YodA) contribute to EHEC adherence. However, it is not clear yet how a secreted protein could augment adhesion. The concept of a secreted adherence factor is not without precedent. The V. cholerae chitin binding protein GbpA is secreted by the V. cholerae T2SS and promotes this organism's adherence to intestinal epithelial cells, as well as to chitinaceous surfaces (26). A secreted protein could promote adherence by directly serving as an adhesin, by acting as a bridge between the pathogen and host cell, or indirectly by a variety of mechanisms. For example, StcE, the only known substrate of the EHEC T2SS besides YodA, is a zinc metalloprotease that cleaves the host protein C-1 esterase inhibitor, mucin, and glycoprotein 340 (28). StcE cleavage of proteins in the glycocalyx and mucin layers on top of enteric epithelial cells has been proposed to facilitate access of EHEC to the host cell membrane; this may explain the threefold decrease in EHEC intimate adherence (measured by the FAS test) of stcE mutants to HEp-2 cells (17). The defect in intimate adherence of our etpC mutant to HeLa cells cannot be explained by a defect in StcE secretion, since we observed that a stcE deletion mutant did not have a significant deficiency in adhesion to HeLa cells (data not shown); however, a defect in intraintestinal StcE secretion by the etpC mutant may contribute to this strain's colonization defect.
Information regarding YodA function garnered from studies of E. coli K-12 should provide some insight into the YodA function in EHEC since the predicted sequences of the two proteins are 99% identical. YodA was originally identified as a cadmium-induced protein that is translocated from the cytoplasm to the periplasm following exposure of E. coli K-12 to cadmium (40). The YodA crystal structure revealed that this protein binds to metal ions, including cadmium and zinc, and is structurally related to the lipocalin family of proteins (7). Members of this protein family are usually secreted and are often involved in transport (7). The growth of the E. coli K-12 yodA mutant was found to be impaired in copper- or zinc-limited conditions, leading the authors to propose that YodA may have a function in zinc homeostasis (24). Unlike the E. coli K-12 yodA mutant (24), the EHEC yodA mutant exhibited a growth defect in LB broth. This difference might be attributable to differences in the regulation of YodA production in EHEC and E. coli K-12 or to differences in the localization of the protein and/or its cellular role. We found that some portion of YodA is secreted by the EHEC T2SS. Secretion of YodA by E. coli K-12 has not been observed, perhaps because the genes encoding the E. coli K-12 T2SS are usually transcriptionally silenced (12). Only a portion of YodA is secreted from EHEC, and it is not yet clear whether cell-associated or secreted YodA influences adherence to cultured cells. Furthermore, additional studies are required to discern if YodA acts directly or indirectly to promote adherence. The growth defect of the yodA mutant makes it difficult to discern whether the colonization defect of this strain is simply the result of its reduced growth rate or reflects a specific role for YodA in promoting intestinal colonization.
Despite the marked deficiency in adherence of the adfO mutant to HeLa cells, this strain did not have an intestinal colonization defect in competition assays. We recently found that an EHEC espFU mutant has a similar phenotype; the espFU mutant is highly defective in generating actin rearrangements in HeLa cells, yet it had no defect in colonization of infant rabbits in competition assays (42). A four- to sixfold intestinal colonization defect was observed in the espFU mutant when single-infection experiments were performed, suggesting that production of EspFU by the wild-type strain in the competition experiments may have rescued the mutant in these assays (42). Single-strain infection studies with the adfO mutant suggested that transcomplementation of the mutant by AdfO produced by the wild-type strain in vivo is not the explanation for the absence of a colonization defect of the adfO mutant (data not shown). As mentioned above, there may be several EHEC proteins that act in a redundant fashion to promote adherence of EHEC to epithelial cells in the intestine, and other proteins may be able to substitute for AdfO in the rabbit intestine. Alternatively, it is possible that increased intraintestinal expression of type 3 effectors obviates the requirement for AdfO for attachment in vivo. Since AdfO orthologues are found in several pathogens and are linked to attachment to host cells and/or intestinal colonization in EHEC, porcine enteropathogenic E. coli (Paa), and V. cholerae (AcfC), additional investigation of the mechanism of action of AdfO is warranted.
Acknowledgments
We are grateful to Josee Harel for providing antisera. We thank John Leong for providing reagents and helpful comments on the manuscript. We thank the Tufts-NEMC GRASP Center for medium preparation and for providing HeLa cells.
This work was supported by NIH grant R21-AI67827 and by HHMI.
Editor: A. J. Bäumler
Footnotes
Published ahead of print on 3 March 2008.
REFERENCES
- 1.Baehler, A. A., and R. A. Moxley. 2002. Effect of culture conditions on Escherichia coli O157:H7-mediated attaching-effacing lesions in a bovine large intestinal mucosal explant model. FEMS Microbiol. Lett. 212107-110. [DOI] [PubMed] [Google Scholar]
- 2.Batisson, I., M. P. Guimond, F. Girard, H. An, C. Zhu, E. Oswald, J. M. Fairbrother, M. Jacques, and J. Harel. 2003. Characterization of the novel factor paa involved in the early steps of the adhesion mechanism of attaching and effacing Escherichia coli. Infect. Immun. 714516-4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campellone, K. G., D. Robbins, and J. M. Leong. 2004. EspFU is a translocated EHEC effector that interacts with Tir and N-WASP and promotes Nck-independent actin assembly. Dev. Cell 7217-228. [DOI] [PubMed] [Google Scholar]
- 4.Cascales, E., M. Gavioli, J. N. Sturgis, and R. Lloubes. 2000. Proton motive force drives the interaction of the inner membrane TolA and outer membrane pal proteins in Escherichia coli. Mol. Microbiol. 38904-915. [DOI] [PubMed] [Google Scholar]
- 5.Datsenko, K. A., and B. L. Wanner. 2000. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 976640-6645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.David, G., K. Blondeau, M. Renouard, and A. Lewit-Bentley. 2002. Crystallization and preliminary analysis of Escherichia coli YodA. Acta Crystallogr. Sect. D 581243-1245. [DOI] [PubMed] [Google Scholar]
- 7.David, G., K. Blondeau, M. Schiltz, S. Penel, and A. Lewit-Bentley. 2003. YodA from Escherichia coli is a metal-binding, lipocalin-like protein. J. Biol. Chem. 27843728-43735. [DOI] [PubMed] [Google Scholar]
- 8.de Grado, M., A. Abe, A. Gauthier, O. Steele-Mortimer, R. DeVinney, and B. B. Finlay. 1999. Identification of the intimin-binding domain of Tir of enteropathogenic Escherichia coli. Cell. Microbiol. 17-17. [DOI] [PubMed] [Google Scholar]
- 9.Donnenberg, M. S., S. Tzipori, M. L. McKee, A. D. O'Brien, J. Alroy, and J. B. Kaper. 1993. The role of the eae gene of enterohemorrhagic Escherichia coli in intimate attachment in vitro and in a porcine model. J. Clin. Investig. 921418-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dziva, F., A. Mahajan, P. Cameron, C. Currie, I. J. McKendrick, T. S. Wallis, D. G. Smith, and M. P. Stevens. 2007. EspP, a type V-secreted serine protease of enterohaemorrhagic Escherichia coli O157:H7, influences intestinal colonization of calves and adherence to bovine primary intestinal epithelial cells. FEMS Microbiol. Lett. 271258-264. [DOI] [PubMed] [Google Scholar]
- 11.Dziva, F., P. M. van Diemen, M. P. Stevens, A. J. Smith, and T. S. Wallis. 2004. Identification of Escherichia coli O157:H7 genes influencing colonization of the bovine gastrointestinal tract using signature-tagged mutagenesis. Microbiology 1503631-3645. [DOI] [PubMed] [Google Scholar]
- 12.Francetic, O., D. Belin, C. Badaut, and A. P. Pugsley. 2000. Expression of the endogenous type II secretion pathway in Escherichia coli leads to chitinase secretion. EMBO J. 196697-6703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frankel, G., O. Lider, R. Hershkoviz, A. P. Mould, S. G. Kachalsky, D. C. Candy, L. Cahalon, M. J. Humphries, and G. Dougan. 1996. The cell-binding domain of intimin from enteropathogenic Escherichia coli binds to beta1 integrins. J. Biol. Chem. 27120359-20364. [DOI] [PubMed] [Google Scholar]
- 14.Garmendia, J., G. Frankel, and V. F. Crepin. 2005. Enteropathogenic and enterohemorrhagic Escherichia coli infections: translocation, translocation, translocation. Infect. Immun. 732573-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garmendia, J., A. D. Phillips, M. F. Carlier, Y. Chong, S. Schuller, O. Marches, S. Dahan, E. Oswald, R. K. Shaw, S. Knutton, and G. Frankel. 2004. TccP is an enterohaemorrhagic Escherichia coli O157:H7 type III effector protein that couples Tir to the actin-cytoskeleton. Cell. Microbiol. 61167-1183. [DOI] [PubMed] [Google Scholar]
- 16.Girard, F., F. Dziva, P. van Diemen, A. D. Phillips, M. P. Stevens, and G. Frankel. 2007. Adherence of enterohemorrhagic Escherichia coli O157, O26, and O111 strains to bovine intestinal explants ex vivo. Appl. Environ Microbiol. 733084-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grys, T. E., M. B. Siegel, W. W. Lathem, and R. A. Welch. 2005. The StcE protease contributes to intimate adherence of enterohemorrhagic Escherichia coli O157:H7 to host cells. Infect. Immun. 731295-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartland, E. L., M. Batchelor, R. M. Delahay, C. Hale, S. Matthews, G. Dougan, S. Knutton, I. Connerton, and G. Frankel. 1999. Binding of intimin from enteropathogenic Escherichia coli to Tir and to host cells. Mol. Microbiol. 32151-158. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi, T., K. Makino, M. Ohnishi, K. Kurokawa, K. Ishii, K. Yokoyama, C. G. Han, E. Ohtsubo, K. Nakayama, T. Murata, M. Tanaka, T. Tobe, T. Iida, H. Takami, T. Honda, C. Sasakawa, N. Ogasawara, T. Yasunaga, S. Kuhara, T. Shiba, M. Hattori, and H. Shinagawa. 2001. Complete genome sequence of enterohemorrhagic Escherichia coli O157:H7 and genomic comparison with a laboratory strain K-12. DNA Res. 811-22. [DOI] [PubMed] [Google Scholar]
- 20.Ho, T. D., and M. K. Waldor. 2007. Enterohemorrhagic Escherichia coli O157:H7 gal mutants are sensitive to bacteriophage P1 and defective in intestinal colonization. Infect. Immun. 751661-1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hritonenko, V., and C. Stathopoulos. 2007. Omptin proteins: an expanding family of outer membrane proteases in Gram-negative Enterobacteriaceae. Mol. Membr. Biol 24395-406. [DOI] [PubMed] [Google Scholar]
- 22.Kaper, J. B., J. P. Nataro, and H. L. Mobley. 2004. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2123-140. [DOI] [PubMed] [Google Scholar]
- 23.Kenny, B., R. DeVinney, M. Stein, D. J. Reinscheid, E. A. Frey, and B. B. Finlay. 1997. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell 91511-520. [DOI] [PubMed] [Google Scholar]
- 24.Kershaw, C. J., N. L. Brown, and J. L. Hobman. 2007. Zinc dependence of zinT (yodA) mutants and binding of zinc, cadmium and mercury by ZinT. Biochem. Biophys. Res. Commun. 36466-71. [DOI] [PubMed] [Google Scholar]
- 25.Kim, S. H., and Y. H. Kim. 2004. Escherichia coli O157:H7 adherence to HEp-2 cells is implicated with curli expression and outer membrane integrity. J. Vet. Sci. 5119-124. [PubMed] [Google Scholar]
- 26.Kirn, T. J., B. A. Jude, and R. K. Taylor. 2005. A colonization factor links Vibrio cholerae environmental survival and human infection. Nature 438863-866. [DOI] [PubMed] [Google Scholar]
- 27.Knutton, S., T. Baldwin, P. H. Williams, and A. S. McNeish. 1989. Actin accumulation at sites of bacterial adhesion to tissue culture cells: basis of a new diagnostic test for enteropathogenic and enterohemorrhagic Escherichia coli. Infect. Immun. 571290-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lathem, W. W., T. E. Grys, S. E. Witowski, A. G. Torres, J. B. Kaper, P. I. Tarr, and R. A. Welch. 2002. StcE, a metalloprotease secreted by Escherichia coli O157:H7, specifically cleaves C1 esterase inhibitor. Mol. Microbiol. 45277-288. [DOI] [PubMed] [Google Scholar]
- 29.Li, M., I. Rosenshine, S. L. Tung, X. H. Wang, D. Friedberg, C. L. Hew, and K. Y. Leung. 2004. Comparative proteomic analysis of extracellular proteins of enterohemorrhagic and enteropathogenic Escherichia coli strains and their ihf and ler mutants. Appl. Environ Microbiol. 705274-5282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lim, J. Y., H. Sheng, K. S. Seo, Y. H. Park, and C. J. Hovde. 2007. Characterization of an Escherichia coli O157:H7 plasmid O157 deletion mutant and its survival and persistence in cattle. Appl. Environ Microbiol. 732037-2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu, H., L. Magoun, S. Luperchio, D. B. Schauer, and J. M. Leong. 1999. The Tir-binding region of enterohaemorrhagic Escherichia coli intimin is sufficient to trigger actin condensation after bacterial-induced host cell signalling. Mol. Microbiol. 3467-81. [DOI] [PubMed] [Google Scholar]
- 32.Low, A. S., F. Dziva, A. G. Torres, J. L. Martinez, T. Rosser, S. Naylor, K. Spears, N. Holden, A. Mahajan, J. Findlay, J. Sales, D. G. Smith, J. C. Low, M. P. Stevens, and D. L. Gally. 2006. Cloning, expression, and characterization of fimbrial operon F9 from enterohemorrhagic Escherichia coli O157:H7. Infect. Immun. 742233-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moon, H. W., S. C. Whipp, R. A. Argenzio, M. M. Levine, and R. A. Giannella. 1983. Attaching and effacing activities of rabbit and human enteropathogenic Escherichia coli in pig and rabbit intestines. Infect. Immun. 411340-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mundy, R., S. Schuller, F. Girard, J. M. Fairbrother, A. D. Phillips, and G. Frankel. 2007. Functional studies of intimin in vivo and ex vivo: implications for host specificity and tissue tropism. Microbiology 153959-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nataro, J. P., and J. B. Kaper. 1998. Diarrheagenic Escherichia coli. Clin. Microbiol. Rev. 11142-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naylor, S. W., A. J. Roe, P. Nart, K. Spears, D. G. Smith, J. C. Low, and D. L. Gally. 2005. Escherichia coli O157:H7 forms attaching and effacing lesions at the terminal rectum of cattle and colonization requires the LEE4 operon. Microbiology 1512773-2781. [DOI] [PubMed] [Google Scholar]
- 37.O'Brien, A. D., J. W. Newland, S. F. Miller, R. K. Holmes, H. W. Smith, and S. B. Formal. 1984. Shiga-like toxin-converting phages from Escherichia coli strains that cause hemorrhagic colitis or infantile diarrhea. Science 226694-696. [DOI] [PubMed] [Google Scholar]
- 38.Perna, N. T., G. Plunkett III, V. Burland, B. Mau, J. D. Glasner, D. J. Rose, G. F. Mayhew, P. S. Evans, J. Gregor, H. A. Kirkpatrick, G. Posfai, J. Hackett, S. Klink, A. Boutin, Y. Shao, L. Miller, E. J. Grotbeck, N. W. Davis, A. Lim, E. T. Dimalanta, K. D. Potamousis, J. Apodaca, T. S. Anantharaman, J. Lin, G. Yen, D. C. Schwartz, R. A. Welch, and F. R. Blattner. 2001. Genome sequence of enterohaemorrhagic Escherichia coli O157:H7. Nature 409529-533. [DOI] [PubMed] [Google Scholar]
- 39.Peterson, K. M., and J. J. Mekalanos. 1988. Characterization of the Vibrio cholerae ToxR regulon: identification of novel genes involved in intestinal colonization. Infect. Immun. 562822-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Puskarova, A., P. Ferianc, J. Kormanec, D. Homerova, A. Farewell, and T. Nystrom. 2002. Regulation of yodA encoding a novel cadmium-induced protein in Escherichia coli. Microbiology 1483801-3811. [DOI] [PubMed] [Google Scholar]
- 41.Rangarajan, E. S., S. Bhatia, D. C. Watson, C. Munger, M. Cygler, A. Matte, and N. M. Young. 2007. Structural context for protein N-glycosylation in bacteria: the structure of PEB3, an adhesin from Campylobacter jejuni. Protein Sci. 16990-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ritchie, J. M., M. J. Brady, K. N. Riley, T. D. Ho, K. G. Campellone, I. M. Herman, A. Donohue-Rolfe, S. Tzipori, M. K. Waldor, and J. M. Leong. 2008. EspF(U), a type III-translocated effector of actin assembly, fosters epithelial association and late-stage intestinal colonization by E. coli O157:H7. Cell. Microbiol. 10836-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ritchie, J. M., C. M. Thorpe, A. B. Rogers, and M. K. Waldor. 2003. Critical roles for stx2, eae, and tir in enterohemorrhagic Escherichia coli-induced diarrhea and intestinal inflammation in infant rabbits. Infect. Immun. 717129-7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ritchie, J. M., and M. K. Waldor. 2005. The locus of enterocyte effacement-encoded effector proteins all promote enterohemorrhagic Escherichia coli pathogenicity in infant rabbits. Infect. Immun. 731466-1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roe, A. J., D. E. Hoey, and D. L. Gally. 2003. Regulation, secretion and activity of type III-secreted proteins of enterohaemorrhagic Escherichia coli O157. Biochem. Soc Trans. 3198-103. [DOI] [PubMed] [Google Scholar]
- 46.Sandkvist, M. 2001. Type II secretion and pathogenesis. Infect. Immun. 693523-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmidt, H., B. Henkel, and H. Karch. 1997. A gene cluster closely related to type II secretion pathway operons of gram-negative bacteria is located on the large plasmid of enterohemorrhagic Escherichia coli O157 strains. FEMS Microbiol. Lett. 148265-272. [DOI] [PubMed] [Google Scholar]
- 48.Schuller, S., Y. Chong, J. Lewin, B. Kenny, G. Frankel, and A. D. Phillips. 2007. Tir phosphorylation and Nck/N-WASP recruitment by enteropathogenic and enterohaemorrhagic Escherichia coli during ex vivo colonization of human intestinal mucosa is different to cell culture models. Cell. Microbiol. 91352-1364. [DOI] [PubMed] [Google Scholar]
- 49.Sheng, H., J. Y. Lim, H. J. Knecht, J. Li, and C. J. Hovde. 2006. Role of Escherichia coli O157:H7 virulence factors in colonization at the bovine terminal rectal mucosa. Infect. Immun. 744685-4693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sinclair, J. F., and A. D. O'Brien. 2002. Cell surface-localized nucleolin is a eukaryotic receptor for the adhesin intimin-gamma of enterohemorrhagic Escherichia coli O157:H7. J. Biol. Chem. 2772876-2885. [DOI] [PubMed] [Google Scholar]
- 51.Stevens, M. P., A. J. Roe, I. Vlisidou, P. M. van Diemen, R. M. La Ragione, A. Best, M. J. Woodward, D. L. Gally, and T. S. Wallis. 2004. Mutation of toxB and a truncated version of the efa-1 gene in Escherichia coli O157:H7 influences the expression and secretion of locus of enterocyte effacement-encoded proteins but not intestinal colonization in calves or sheep. Infect. Immun. 725402-5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stevens, M. P., P. M. van Diemen, G. Frankel, A. D. Phillips, and T. S. Wallis. 2002. Efa1 influences colonization of the bovine intestine by Shiga toxin-producing Escherichia coli serotypes O5 and O111. Infect. Immun. 705158-5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Strockbine, N. A., L. R. Marques, J. W. Newland, H. W. Smith, R. K. Holmes, and A. D. O'Brien. 1986. Two toxin-converting phages from Escherichia coli O157:H7 strain 933 encode antigenically distinct toxins with similar biologic activities. Infect. Immun. 53135-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Su, C., and L. J. Brandt. 1995. Escherichia coli O157:H7 infection in humans. Ann. Intern. Med. 123698-714. [DOI] [PubMed] [Google Scholar]
- 55.Tarr, P. I., S. S. Bilge, J. C. Vary, Jr., S. Jelacic, R. L. Habeeb, T. R. Ward, M. R. Baylor, and T. E. Besser. 2000. Iha: a novel Escherichia coli O157:H7 adherence-conferring molecule encoded on a recently acquired chromosomal island of conserved structure. Infect. Immun. 681400-1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tatsuno, I., M. Horie, H. Abe, T. Miki, K. Makino, H. Shinagawa, H. Taguchi, S. Kamiya, T. Hayashi, and C. Sasakawa. 2001. toxB gene on pO157 of enterohemorrhagic Escherichia coli O157:H7 is required for full epithelial cell adherence phenotype. Infect. Immun. 696660-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tatsuno, I., H. Kimura, A. Okutani, K. Kanamaru, H. Abe, S. Nagai, K. Makino, H. Shinagawa, M. Yoshida, K. Sato, J. Nakamoto, T. Tobe, and C. Sasakawa. 2000. Isolation and characterization of mini-Tn5Km2 insertion mutants of enterohemorrhagic Escherichia coli O157:H7 deficient in adherence to Caco-2 cells. Infect. Immun. 685943-5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tobe, T., S. A. Beatson, H. Taniguchi, H. Abe, C. M. Bailey, A. Fivian, R. Younis, S. Matthews, O. Marches, G. Frankel, T. Hayashi, and M. J. Pallen. 2006. An extensive repertoire of type III secretion effectors in Escherichia coli O157 and the role of lambdoid phages in their dissemination. Proc. Natl. Acad. Sci. USA 10314941-14946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Torres, A. G., K. J. Kanack, C. B. Tutt, V. Popov, and J. B. Kaper. 2004. Characterization of the second long polar (LP) fimbriae of Escherichia coli O157:H7 and distribution of LP fimbriae in other pathogenic E. coli strains. FEMS Microbiol. Lett. 238333-344. [DOI] [PubMed] [Google Scholar]
- 60.Torres, A. G., and J. B. Kaper. 2003. Multiple elements controlling adherence of enterohemorrhagic Escherichia coli O157:H7 to HeLa cells. Infect. Immun. 714985-4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Torres, A. G., X. Zhou, and J. B. Kaper. 2005. Adherence of diarrheagenic Escherichia coli strains to epithelial cells. Infect. Immun. 7318-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tzipori, S., F. Gunzer, M. S. Donnenberg, L. de Montigny, J. B. Kaper, and A. Donohue-Rolfe. 1995. The role of the eaeA gene in diarrhea and neurological complications in a gnotobiotic piglet model of enterohemorrhagic Escherichia coli infection. Infect. Immun. 633621-3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wagner, P. L., and M. K. Waldor. 2002. Bacteriophage control of bacterial virulence. Infect. Immun. 703985-3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xicohtencatl-Cortes, J., V. Monteiro-Neto, M. A. Ledesma, D. M. Jordan, O. Francetic, J. B. Kaper, J. L. Puente, and J. A. Giron. 2007. Intestinal adherence associated with type IV pili of enterohemorrhagic Escherichia coli O157:H7. J. Clin. Investig. 1173519-3529. [DOI] [PMC free article] [PubMed] [Google Scholar]