Abstract
Predators of herbivorous animals can affect plant populations by altering herbivore density, behavior, or both. To test whether the indirect effect of predators on plants arises from density or behavioral responses in a herbivore population, we experimentally examined the dynamics of terrestrial food chains comprised of old field plants, leaf-chewing grasshoppers, and spider predators in Northeast Connecticut. To separate the effects of predators on herbivore density from the effects on herbivore behavior, we created two classes of spiders: (i) risk spiders that had their feeding mouth parts glued to render them incapable of killing prey and (ii) predator spiders that remained unmanipulated. We found that the effect of predators on plants resulted from predator-induced changes in herbivore behavior (shifts in activity time and diet selection) rather than from predator-induced changes in grasshopper density. Neither predator nor risk spiders had a significant effect on grasshopper density relative to a control. This demonstrates that the behavioral response of prey to predators can have a strong impact on the dynamics of terrestrial food chains. The results make a compelling case to examine behavioral as well as density effects in theoretical and empirical research on food chain dynamics.
Keywords: herbivore-mediated effects, predation, old field food chains, grasshoppers
The ecological literature contains numerous examples of the influence of predators on natural communities. A trophic cascade, the indirect interaction between predators and the resources consumed by the predators’ prey, has been the focus of much of this attention. In a three-trophic level system comprised of carnivores, herbivores, and plants, a trophic cascade describes an increase in plant abundance that results from carnivore effects on herbivore populations (1–3).
Carnivores can affect the impact that herbivores have on plants in two ways (4). They may cause changes in herbivore density through direct consumption of herbivores. This density-mediated effect between predators and plants results from a reduction in the number of herbivores feeding on plants (5–7). Alternatively, the presence of predators in itself represents a risk of predation to herbivores that can mediate predator–plant interactions by altering herbivore prey foraging behavior (4, 8–10). This behavioral mediation results from reduced feeding time and shifts in diet selection, both of which can cause a decrease in herbivore impact on plants.
Increasing evidence suggests that density- and behavior-mediated effects may be important causes of trophic cascades (4, 8–11). Although the existence of prey behavioral responses to predators is well established (4, 12), especially in aquatic systems, there is still little evidence for their effects and importance in terrestrial food web dynamics (4, 13). Few studies have even tested for trophic cascades in terrestrial systems (7, 13–20), in contrast to the multitude of studies in aquatic systems (21).
We report on a field study that provides considerable evidence that a trophic cascade in a terrestrial food chain can be driven solely by behavior-mediated prey population responses to predation. We discovered that predator-induced changes in herbivore feeding time and diet choice, rather than herbivore mortality due to predation, were the primary causes of a trophic cascade.
Study Site.
The research was conducted at the Yale–Myers Research Forest in northeastern Connecticut, a 3240-ha northeastern hardwood ecosystem interspersed with old fields (see ref. 17). Our research fields support a variety of grass and herb species, the most abundant being the grasses (monocots) Phleum pratense and Poa pratensis and the herbs (dicots) Solidago rugosa, Aster novaeangliae, Trifolium repens, and Daucus carota. The research focused on a natural, three-level food chain that included the abundant herbaceous plants, a generalist leaf-chewing grasshopper, Melanoplus femurrubrum, and the nursery web hunting spider, Pisaurina mira.
Background.
The primary challenge of evaluating behavioral mediation of trophic cascades is to create a predator that does not have the capacity to consume prey but is not compromised in its ability to display hunting behavior or to signal risk to the prey in other forms (17, 22, 23). We created two classes of spiders, predation and risk, for our experiments. The predation spiders were used to quantify density-mediated herbivore impacts on resources. Risk spiders were used to quantify the behavior-mediated herbivore impacts on resources. We created risk spiders by gluing their chelicera with nontoxic surgical cement so that they were unable to subdue the prey, removing all chance of density effects (17). The information presented below summarizes the results of tests and previous research (7, 17, 24) to determine whether this spider manipulation had any effects on spider behavior or grasshopper perception.
Spider Behavior.
In a series of replicated feeding trials to evaluate the ability of spiders to consume grasshopper prey (7, 14), we found that there was a linear relationship between the size of a spider predator and the maximum size of a grasshopper prey it could consume. Consistent with previous work (7, 25–27), our research (17) demonstrated that spiders were capable of consuming grasshoppers 10–30% larger than their own body size.
Successful tests of the behavioral-mediation hypothesis assumes that the risk treatment has no adverse effect on spider hunting behavior. We found that neither the amount nor pattern of movement, over a 24-h period, differed between risk and predation spiders in the presence of grasshopper prey (17). This result is consistent with another study examining the effects of mouth part manipulation on spider hunting behavior (25). Desiccation, a certain difficulty for spiders without prey, is not avoided. But starvation trials in which we placed P. mira spiders in terraria in a laboratory with ample moisture and leaf litter but no prey revealed that activity can be maintained without resources for up to 2 months.
Grasshopper Behavior.
We experimentally tested for the ability of grasshoppers to discern among predation and risk spiders (17). We found that the amount and pattern of grasshopper movement was the same when faced with risk or predation spiders (17).
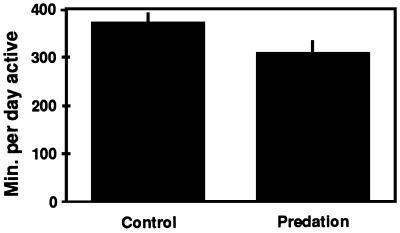
We tested for the effect of spiders on grasshopper activity time. Daily activity in the presence and in the absence of predators was measured on grasshoppers housed individually in 0.22-m3 enclosures placed in a field. Eight of 16 grasshoppers were assigned randomly to either a no predator (control) or predator (predation) treatment. Grasshopper location in the cage was recorded every 30 min between 7:00 a.m. and 6:00 p.m. for 2 days. Grasshoppers were considered active if their position had changed between consecutive 0.5-h periods. Activity time was calculated by summing active 30-min periods over the 11 h of observation each day. This method has been calibrated with more frequent and detailed measures of activity time and shown to give consistent and accurate results (28). We observed a significant 65-min (18%) reduction in grasshopper daily activity time in the presence of a spider predator (Fig. 1; Student’s t test, df = 14, P < 0.05).
Figure 1.
Grasshopper activity time reduction caused by the presence of a spider predator. Data show means and SE of eight replicates of each treatment.
Finally, Rothley et al. (24) found that grasshopper diets in the absence of spiders consisted primarily of grasses. The presence of spider predators, however, caused a significant decrease in the dietary proportion of grass and a significant increase in the dietary proportion of herbs, consistent with theory on adaptive consumer behavior in response to predation risk (24).
METHODS
We tested for density- and behavior-mediated trophic cascade patterns in our three trophic level system of spiders, grasshoppers, and plants based on the ability for spiders to subdue grasshoppers, alter grasshopper time budgets, and shift grasshopper diets. Trophic cascades in three trophic level systems such as ours can be detected experimentally by manipulating food chain length and measuring the damage on plants (1, 7, 29). By adding herbivores to a food chain containing only plants, theory predicts that we should observe high levels of plant damage (6, 7, 29). Adding predators to these two-level food chains containing plants and herbivores should decrease the damage inflicted on plants relative to food chains containing only plants and herbivores.
To test whether the indirect effect of predators on plants arises from density or behavioral responses in the herbivore population, we assembled food chains of varying length (experimental treatments) in 0.25 m2 × 0.8 m screen enclosures containing: (i) plants only, (ii) plants plus late developmental stage juvenile grasshoppers (iii) plants, grasshoppers, and risk spiders, (iv) plants, grasshoppers, and predation spiders. Spider sizes ranged from 18 to 22 mm. Grasshoppers body sizes during the experiment (male −20.0 mm ± 0.9 SE; female −24.2 mm ± 1.1 SE) were well within the size range that could be subdued by the spiders used in the experiments.
Experimental food chains were assembled in early August of each of 2 years. The experiment was conducted for 2 successive years to verify that our results were consistent over time. Enclosures were placed over natural vegetation and stocked with locally collected grasshoppers and spiders. Grasshopper stocking densities (6/0.25 m2) were intentionally higher than field densities (3–4/0.25 m2) to produce a pulse perturbation that would cause densities to decline toward a steady-state set by the limiting factors that vary by cage location in the field (see refs. 17 and 30). Such a pulse perturbation has no adverse effects on plant populations (16), and it tests the steady-state assumption associated with theoretical models of trophic dynamics (i.e., a pulse perturbation is the empirical analog of a linear perturbation in theoretical models that is used to assess the stability of a steady state or equilibrium). Spiders were stocked at 1/0.25 m2.
All treatments were assigned to the enclosures in a randomized block design with each treatment assigned once to each of 10 blocks per year. We monitored grasshopper and spider survival by censusing each enclosure every 2 days during the entire lifespan of the late stage juvenile and adult grasshoppers, ≈60 days. Risk and predation spiders consistently were observed in the cages during censuses; there was no spider mortality during the course of the experiment.
Experiments were terminated in early October, before insect-killing frosts. Upon termination, all above ground green biomass was clipped, sorted to species, dried at 60°C for 48 h and weighed. Percentage of plant damage in an experimental block was calculated as:
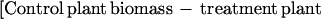 |
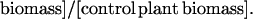 |
RESULTS
We did not detect any density effects of predation or risk spiders on the experimental grasshopper populations. ANOVA revealed that grasshopper densities with and without spider treatments were not significantly different (Table 1).
Table 1.
Densities of grasshoppers in experimental mesocosms under different predator treatments
| Treatment
|
ANOVA
|
||||
|---|---|---|---|---|---|
| Control | Risk spider | Predation spider | d/f | F | P |
| 2.9 ± 0.34 | 3.2 ± 0.50 | 3.1 ± 0.50 | 2, 57 | 0.29 | 0.25 |
Values are mean ± 1 SE.
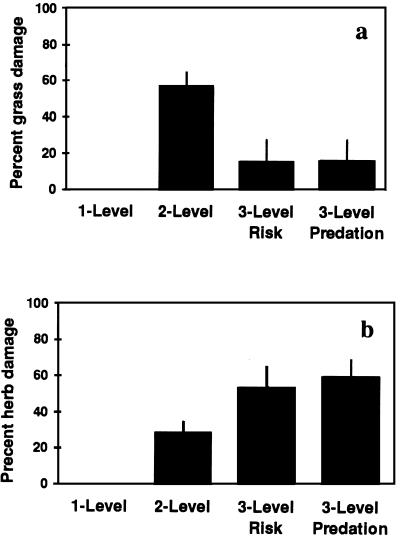
However, we detected significant changes in herbivore damage to grass with varying food chain length in ways that were consistent with the predicted pattern of a trophic cascade (Fig. 2a). Herbivore damage (percentage change in biomass relative to control biomass) on grasses in the absence of predators (two-level) was significantly higher than damage in the presence of risk or predation spiders (three-level) (ANOVA followed by Tukey test on arcsin-transformed data, P < 0.05, df = 3, 76). There was no difference in effect between risk and predation spider (three-level) treatments.
Figure 2.
Damage inflicted on plants by grasshoppers in food chains of varying length, providing evidence for a trophic cascade. (a) Herbivore damage on grasses without predators (two-level) is significantly higher than damage when grasshoppers were in the presence of risk or predation spiders (three-level). There is no difference between risk and predation spider (three-level) treatments. (b) Increased damage on herbs due to predator treatments. Herbivore damage on herbs without predators (two-level) is significantly less than herbivore damage when grasshoppers are in the presence of risk or predation spiders (three-level). but there is no difference between risk and predator spider (three-level) treatments.
We detected significant changes in herbivore damage to herb vegetation with varying food chain length in ways that were consistent with the predicted pattern of an inverse trophic cascade (Fig. 2b). In an inverse cascade, plant damage is lowest in two-level food chains and highest in three-level food chains. Both predation and risk spider treatments resulted in increased damage to herbs (Fig. 2b). Herbivore damage on herbs without predators (two-level) was significantly less than herbivore damage when grasshoppers were in the presence of risk or predation spiders (three-level) (ANOVA followed by Tukey test on arcsin-transformed data, P < 0.05, df = 3, 76). Again, there was no difference between risk and predation spider (three-level) treatments.
DISCUSSION
Behaviorally mediated effects were largely responsible for a trophic cascade in the grass plants and for an inverse cascade in herbs in our old field study system. Grasshoppers mortality was unlikely to be the mechanism causing these patterns; neither predation nor predation risk had a significant effect on grasshopper densities relative to a control (Table 1). Behavioral responses to predators are also important mechanisms of population dynamics in many aquatic ecosystems (9–11, 31, 32). However, although density effects are also important in aquatic systems (2, 33, 34), we found little indication that density-mediated effects were important in our experimental study system. This may be the result of our focus on the adult life stage when direct predation may be more prevalent in juvenile stages (17, 35, 36). Our study nevertheless represents one step toward an integrated evaluation of the food web implications of ontogenetic responses, via behavior- and density-mediated mechanisms, to predation in terrestrial food webs.
The patterns of plant damage in our experiment, a trophic cascade in grass plants and an inverse cascade in herbs, are consistent with expectations from a model of adaptive shifts in diet selection that predict an increased diet proportion of herbs as predation risk increases (24). However, because grasshoppers are small relative to the size of herbs and grasses, grasshoppers may perceive these two resources as separate habitats in space. Thus, the increased proportion of herbs in the grasshopper diet also may reflect habitat shifts toward the structurally more complex herb vegetation while seeking refuge from predators (cf. refs. 9, 10, 13). Regardless of whether it is a diet shift or a habitat shift, the fact remains that the indirect effects observed at the food web level are propagated entirely by grasshopper behavioral responses to predation rather than density responses.
Although there were no measurable differences in the effects of predators on grasshopper densities in the enclosures, mortality via predation or starvation may simply be “compensated” by mortality from nonpredator sources (37): The level of mortality caused by predators may be less than or equal to the level of natural mortality caused by density-dependent (e.g., intraspecific competition) and density-independent (e.g., weather) factors within the herbivore population. Thus, the habitat shift, and corresponding diet shift in response to predation risk, may represent a low cost behavioral adjustment of the grasshoppers (no excess mortality incurred) with surprisingly strong impact on the plants in the food chain.
It is well established that predators can exert a greater pressure on prey populations via behavioral rather than density-mediated mechanisms (4). Research is now beginning to reveal that community level consequences of behavioral responses to predators (e.g., indirect effects such as a trophic cascade) may add to or override density-mediated effects (9–11). It is through theory and experiments that address the magnitude and time scale on which these population responses to behavior- and density-based interactions occur that food web ecology will advance in its predictive capabilities (4, 38).
The mechanism behind the trophic cascade and other indirect effects has major implications for the type of mathematical models of community dynamics currently used for generating food web dynamic predictions. The predictability of food web dynamics, and the response to perturbations, has to date relied primarily on an assumption of density-mediated interactions (3, 4, 8, 39, 40). The inclusion of behavior-mediated interactions will likely require different types of models with a modified focus (4, 22). When addressing density- and behavior-mediated effects, modeling and field research must address interaction strengths measured as a density response and as a population response to trade-offs associated with behavior-based interactions (4, 8, 38, 39). Depending on the relative magnitude of density- and behavior-mediated effects and the time scale on which population responses occur, model complexity and the clarity of predictions may increase or decrease (8, 38–40).
Our research demonstrates that, in certain life stages, the behavioral response of prey to predators can have a strong indirect impact on terrestrial food chain interactions such as the trophic cascade. This evidence, and the increasingly large amount of data from aquatic systems, demonstrates the need to integrate examinations of these mechanisms more thoroughly into research and modeling efforts in food web ecology (e.g., 4, 8, 22).
Acknowledgments
We thank T. W. Schoener and D. K. Skelly and two anonymous reviewers for comments. This work was supported by National Science Foundation Grant DEB-9508604 to O.J.S.
References
- 1.Menge B A. Ecol Monogr. 1995;65:21–74. [Google Scholar]
- 2.Carpenter S R, Kitchell J F, Hodgson J R. Bioscience. 1985;35:634–639. [Google Scholar]
- 3.Paine R T. J Anim Ecol. 1980;49:667–685. [Google Scholar]
- 4.Abrams P A. In: Food Webs in Theory and Practice. Polis G, Winemiller K, editors. New York: Chapman & Hall; 1996. pp. 113–121. [Google Scholar]
- 5.Rosenzweig M L. Am Nat. 1973;107:275–294. [Google Scholar]
- 6.Oksanen L, Fretwell SD, Arruda J, Niemalä P. Am Nat. 1981;126:328–346. [Google Scholar]
- 7.Schmitz O J. Oecologia. 1993;93:327–335. doi: 10.1007/BF00317874. [DOI] [PubMed] [Google Scholar]
- 8.Abrams P A. Am Nat. 1992;140:573–600. [Google Scholar]
- 9.Turner A M, Mittelbach G. Ecology. 1990;71:2241–2254. [Google Scholar]
- 10.Huang C, Sih A. Oecologia. 1991;85:530–536. doi: 10.1007/BF00323765. [DOI] [PubMed] [Google Scholar]
- 11.McIntosh A R, Townsend C R. Oecologia. 1996;108:174–181. doi: 10.1007/BF00333229. [DOI] [PubMed] [Google Scholar]
- 12.Werner E E. Am Nat. 1992;140:S5–S32. [Google Scholar]
- 13.Moran M D, Rooney T P, Hurd L E. Ecology. 1996;77:2219–2227. [Google Scholar]
- 14.Oksanen L. Am Nat. 1983;122:45–52. [Google Scholar]
- 15.Spiller D A, Schoener T. Nature (London) 1990;347:469–472. [Google Scholar]
- 16.Schmitz O J. Proc Natl Acad Sci USA. 1994;91:5364–5367. doi: 10.1073/pnas.91.12.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schmitz O J, Beckerman A P, O’Brien K M. Ecology. 1997;78:1388–1399. [Google Scholar]
- 18.Hartvigsen G D, Wait A, Coleman J S. Oikos. 1995;74:463–468. [Google Scholar]
- 19.Chase J M. Oikos. 1996;77:495–506. [Google Scholar]
- 20.Carter P E, Rypstra A L. Oikos. 1995;72:433–439. [Google Scholar]
- 21.Brett M T, Goldman C R. Proc Natl Acad Sci USA. 1996;93:7723–7726. doi: 10.1073/pnas.93.15.7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Werner E E, Anholt B R. Ecology. 1996;77:157–169. [Google Scholar]
- 23.Wissinger S, McGrady J. Ecology. 1993;74:207–218. [Google Scholar]
- 24.Rothley, K. D., Schmitz, O. J. & Cohon, J. L. (1997) Behav. Ecol., in press.
- 25.Rovner J S. J Arach. 1980;8:201–215. [Google Scholar]
- 26.Ahad M A, Shahjahan M, Talukder F A. Pakistan J Sci Ind Res. 1995;38:230–232. [Google Scholar]
- 27.Fagan W F, Hurd L E. Am Midl Nat. 1991;126:380–384. [Google Scholar]
- 28.Belovsky G E, Slade J B. Oecologia. 1986;70:53–62. doi: 10.1007/BF00377110. [DOI] [PubMed] [Google Scholar]
- 29.Wootton J T. Annu Rev Ecol Syst. 1994;25:443–466. [Google Scholar]
- 30.Schmitz O J. Ecology. 1997;78:55–69. [Google Scholar]
- 31.Werner E E, Hall D J. Ecology. 1988;69:1352–1366. [Google Scholar]
- 32.Skelly D K, Werner E E. Ecology. 1990;71:2313–2322. [Google Scholar]
- 33.Power M E. Science. 1990;250:811–813. doi: 10.1126/science.250.4982.811. [DOI] [PubMed] [Google Scholar]
- 34.Wellborn G A, Skelly D K, Werner E E. Annu Rev Ecol Syst. 1996;27:337–363. [Google Scholar]
- 35.Belovsky G E, Slade J B. Oikos. 1993;68:193–201. [Google Scholar]
- 36.Joern A, Rudd N T. Oecologia. 1982;55:42–46. doi: 10.1007/BF00386716. [DOI] [PubMed] [Google Scholar]
- 37.Rosenzweig M L. Q Rev Biol. 1977;52:371–380. [Google Scholar]
- 38.Abrams P A, Menge B A, Mittlebach G G, Spiller D, Yodzis P. In: Food Webs in Theory and Practice. Polis G, Winemiller K, editors. New York: Chapman & Hall; 1996. pp. 371–395. [Google Scholar]
- 39.Yodzis P. In: Food Webs in Theory and Practice. Polis G, Winemiller K, editors. New York: Chapman & Hall; 1996. pp. 192–200. [Google Scholar]
- 40.Schoener T W. In: Mutualism and Community Organization: Behavioral, Theoretical and Food-Web Approaches. Kawanabe H, Cohen J E, Iwasaki K, editors. New York: Oxford Univ. Press; 1993. pp. 365–411. [Google Scholar]