Abstract
Borrelia burgdorferi synthesizes a variety of differentially regulated outer surface lipoproteins in the tick vector and in vertebrate hosts. Among these is OspD, a protein that is highly induced in vitro by conditions that mimic the tick environment. Using genetically engineered strains in which ospD is deleted, we demonstrate that this protein is not required for B. burgdorferi survival and infectivity in either the mouse or the tick. However, examination of both transcript levels and protein expression indicates that OspD expression is limited to a discrete window of time during B. burgdorferi replication within the tick. This time frame corresponds to tick detachment from the host following feeding, and expression of OspD continues during tick digestion of the blood meal but is low or undetectable after the tick has molted. The high level of OspD production correlates to the highest cell densities that B. burgdorferi is known to reach in vivo. Although OspD is nonessential to the infectious cycle of B. burgdorferi, the tight regulation of expression suggests a beneficial contribution of OspD to the spirochete during bacterial replication within the tick midgut.
Borrelia burgdorferi is the causative agent of Lyme disease, the leading vector-borne disease in the United States. An obligate parasite, B. burgdorferi alternates between Ixodes ticks, which act as vectors to disseminate the bacterium, and vertebrate hosts that serve as zoonotic reservoirs. The physiologies of the vector and hosts differ significantly from each other in many features, such as pH, temperature, nutrients, and immune systems. Even the vertebrate hosts can be physiologically diverse, including mammals, birds, and lizards (1, 8). One mechanism that B. burgdorferi uses to survive in these potentially lethal and contrasting conditions is the differential expression of outer surface lipoproteins (Osp) (15, 33, 36, 51, 52, 56, 60, 61).
Among the regulated surface proteins is OspA, which can serve as an adhesin to tick midgut tissue (39, 51, 61). The blood meal of the feeding tick triggers the downregulation of OspA, allowing migration of the parasite to the salivary glands and transmission to the host. OspB, cotranscribed in an operon with OspA, was recently reported to further aid in the adherence of B. burgdorferi to tick midgut tissues (33). In contrast, OspC expression is upregulated during tick feeding and is required for B. burgdorferi to successfully infect the mammalian host (15, 36, 52, 58). Members of the OspEF-related proteins and the complement regulator-acquiring surface proteins have been shown to bind the complement inhibitory proteins factor H and factor H-like protein 1, presumably to avoid complement-mediated killing in the mammalian host (16, 25, 55). Consistent with this hypothesis, OspEF-related protein and complement regulator-acquiring surface protein expression is increased during mammalian infection and tick feeding but downregulated in the unfed tick (31, 60). VlsE, a membrane protein that undergoes antigenic variation, is expressed in both the tick and the mammal but antigenically varies only in the mammalian host (17, 19, 35, 37, 62).
The temporal expression and function of the B. burgdorferi lipoprotein OspD, first characterized by Norris and colleagues in 1992, were unknown (34). The ospD locus was identified in the three genospecies of Borrelia that cause Lyme disease but not in all isolates examined, indicating that the gene is widespread but not universal (30, 34). Sequence analysis suggested that ospD is undergoing lateral transfer and dissemination throughout the Lyme disease spirochetes (30). However, ospD was not found in the closely related Borrelia species that cause relapsing fever, indicating that the function of the OspD protein relates specifically to the infectious cycle of the Lyme disease spirochetes. Several microarray experiments reported dramatic differential regulation of ospD under various culture conditions (5, 38, 59). The differential regulation of ospD may relate to the unusual genetic structure of the promoter region. In strain B31, seven direct repeats of 17 bp each comprise a portion of the promoter containing putative −35 and −10 sequences for sigma-70 binding (34). Although the numbers of repeats may vary among strains and genospecies, the repeat sequence itself is a set feature of the ospD promoter (30). The repeat motif purportedly could serve as a binding site for an unidentified regulatory protein controlling ospD expression (5, 30, 34).
Although OspD was first identified in 1992 (34), a systematic examination of OspD expression and function during the B. burgdorferi life cycle has only recently been investigated, both here and by Li et al. (29). Through genetic disruption of the ospD locus and analysis of RNA levels and protein expression patterns, we evaluated the requirement for this protein throughout the mouse-tick transmission cycle.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
B. burgdorferi strain B31 A3 is an infectious, clonal derivative (11) of the type strain B31 (ATCC 35210) (6). The genome sequence of strain B31 has been determined (7, 12). Borrelia cultures were grown in liquid Barbour-Stoenner-Kelly (BSK)-II medium supplemented with 6% rabbit serum (Pel Freez Biologicals, Rogers, AZ) at 35°C or in solid BSK medium incubated at 35°C under 2.5% CO2 (49). Escherichia coli TOP10 cells (Invitrogen, Carlsbad, CA) were used for all recombinant DNA cloning purposes.
OspD mutant construction and transformation of B. burgdorferi.
The entire coding region of ospD was deleted by allelic replacement with the kanamycin-resistance cassette described by Bono et al. (4). Primers A and B (Table 1) were used to amplify the area encompassing the ospD locus, including 662 bp of upstream and 590 bp of downstream flanking regions. The B. burgdorferi genome sequence was obtained from The Institute for Genomic Research (http://cmr.jcvi.org/tigrscripts/CMR/GenomePage.cgi?database=gbb) (7, 12). The PCR fragment was cloned into pGEM-T EZ (Promega, Inc., Madison, WI), and the coding region of ospD was deleted by inverse PCR using primers C and D (Table 1), producing a unique BglII restriction enzyme site in place of the gene. The BglII site was used to insert the kanamycin-resistance cassette, creating the allelic exchange vector pGEM::ΔOspD. All constructs were confirmed by sequencing.
TABLE 1.
Oligonucleotides used in this study
| Oligonucleotide designation | Sequence (5′-3′) | Function | Reference |
|---|---|---|---|
| A | GGCCATGGGAAGAAGGAG | Amplification of ospD and flanking regions | This study |
| B | CAATCCTATTACTGCGGG | Amplification of ospD and flanking regions | This study |
| C | AGATCTGATGGAAGTAAGGAAGGAAGa | Inverse PCR for construction of suicide vector | This study |
| D | AGATCTTATTTATACTCCTTAAATATAATGTCa | Inverse PCR for construction of suicide vector | This study |
| E | CACCGTTCATGATAAACAAGAATTATCb | Amplification of ospD for expression in E. coli | This study |
| F | AGTATTTAACAAGGCCACAAC | Amplification of ospD for expression in E. coli | This study |
| G | AGAAGCGGTTATAAATGCAGTT | ospD forward QPCR primer | This study |
| H | TCTGCCATTTGAGCTAAATCAT | ospD reverse QPCR primer | This study |
| I | AATTTCATCTGCTGCAGATCAAGTAAAAAGTGc | ospD QPCR probe | This study |
| flaB FWD | TCTTTTCTCTGGTGAGGGAGCT | flaB forward QPCR primer | 23 |
| flaB REV | TCCTTCCTGTTGAACACCCTCT | flaB reverse QPCR primer | 23 |
| flaB PROBE | AAACTGCTCAGGCTGCACCGGTTCc | flaB QPCR probe | 23 |
| J | ACGGATTCTAATGCGGTTTTACTT | ospC forward QPCR primer | This study |
| K | CAATAGCTTTAGCAGCAATTTCATCT | ospC reverse QPCR primer | This study |
| L | CTGTGAAAGAGGTTGAAGCGTTGCTGTCATc | ospC QPCR probe | This study |
| M | GATCATGTTCGAGACCTTCA | Tick actin forward QPCR primer | 28 |
| N | CGATACCCGTGGTACGA | Tick actin reverse QPCR primer | 28 |
| O | CCATCCAGGCCGTGCTCTCc | Tick actin QPCR probe | 28 |
The BglII restriction enzyme sequence is underlined.
Underlined nucleotides were added for directional cloning in the expression vector.
TaqMan probes were labeled at the 5′ end with FAM (6-carboxyfluorescein) and at the 3′ end with TAMRA (6-carboxytetramethylrhodamine).
Transformation of B. burgdorferi has previously been described (10, 11). The B. burgdorferi strain B31 A3 genome contains a large plasmid complement that contributes to infectivity in mice and ticks (7, 12, 14, 22, 44, 45, 47). Therefore, plasmid content was monitored by PCR (11) to ensure that strains were isogenic and that plasmid loss could not contribute to any observed phenotypic differences between strains.
OspD antibody production.
The ospD coding region, lacking the nucleotide sequence encoding the signal peptidase II leader sequence, was amplified with Vent polymerase (New England BioLabs, Ipswich, MA) using primers E and F and cloned into the pBAD202/D-TOPO expression vector (Invitrogen). When expressed from the vector, OspD is fused to a polyhistidine tag for purification and the thioredoxin protein to facilitate solubilization. OspD was overexpressed by growing 1 liter of bacteria at 37°C to an optical density at 600 nm of 0.6, adding 0.00002% arabinose (final concentration), incubating cells an additional 4 h, and then harvesting them by centrifugation. The cell pellet was resuspended in 1× buffer containing 5 mM imidazole, 0.5 M NaCl, and 20 mM Tris-HCl, pH 7.9. Cells were lysed by three passages through a cold French press cell at 14,000 lb/in2 (96 MPa), and the protein was purified using His-Bind quick columns, according to the manufacturer's recommendations (Novagen, Madison, WI). Purified OspD was used to raise polyclonal antiserum in a New Zealand White rabbit.
B. burgdorferi animal studies.
Rocky Mountain Laboratories (RML) is accredited by the International Association for Assessment and Accreditation of Laboratory Animal Care. Protocols for animal experiments were prepared according to the guidelines of the National Institutes of Health and approved by the RML Animal Care and Use Committee. RML mice and white-footed mice (Peromyscus leucopus) were used to assess infectivity of B. burgdorferi strains. RML mice are an outbred strain of Swiss-Webster mice. Both mice colonies are maintained onsite at RML. Mice were needle inoculated with 4 × 103 spirochetes intraperitoneally and 1 × 103 subcutaneously, according to our standard route and dose (11). Murine infectivity was assessed both by the immunoreactivity of mouse sera to B. burgdorferi antigens and by the reisolation of spirochetes from ear tissue, the bladder, and the rear ankle joint (13, 50).
An Ixodes scapularis colony maintained at RML was the source for all ticks used in this study. Ticks were allowed to feed on mice to repletion or were forcibly removed with forceps at specified time points. Approximately 100 to 200 larvae or 5 to 20 nymphs were allowed to feed on each mouse. Larval ticks were artificially infected as described by Policastro and Schwan (42), except that larval ticks were equilibrated to a lower relative humidity before immersion to enhance spirochete uptake (P. Policastro, personal communication).
IFAs of tick midgut tissue.
The ability of B. burgdorferi to infect ticks was assessed by immunofluorescence assays (IFAs) of tick midgut tissues (51). Spirochetes were detected using a 1:100 dilution of goat anti-B. burgdorferi antisera labeled with fluorescein isothiocyanate (Kirkegaard & Perry Laboratories, Inc., Gaithersburg, MD). Two antibodies were used to detect OspD: rabbit anti-OspD antiserum (1:500 dilution) and then a 1:50 dilution of goat anti-rabbit immunoglobulin G antibody labeled with tetramethyl rhodamine isothiocyanate (Kirkegaard & Perry Laboratories). IFAs of in vitro-cultivated B. burgdorferi were performed similarly, except that spirochetes were washed in phosphate-buffered saline plus MgCl2 buffer to remove medium components.
Isolation of nucleic acids from infected ticks.
Genomic DNA was isolated from batches of five nymphal ticks infected with B. burgdorferi. Ticks were snap-frozen in liquid nitrogen and ground with a plastic pestle in an Eppendorf tube, and genomic DNA was isolated using the DNeasy blood and tissue kit (Qiagen, Inc., Valencia, CA), according to the manufacturer's protocol for animal tissues. Similarly, total RNA was purified (five infected nymphal ticks per group) by snap-freezing in liquid nitrogen and grinding, and RNA was isolated using the Nucleospin RNA II kit (Clontech, Inc., Mountain View, CA), according to the manufacturer's recommendations.
QPCR analysis.
Quantitative PCR (QPCR) of genomic DNA was performed with 100 ng of total genomic DNA using the TaqMan universal PCR master mix kit (Applied Biosystems, Branchburg, NJ), following the manufacturer's recommendations. Primers and probes are described in Table 1. Reactions were carried out on an Applied Biosystems 7900HT sequence detection system. Cycling parameters for all QPCR reactions started with an initial cycle consisting of 50°C for 2 min and 95°C for 10 min, which was followed by 40 cycles consisting of 95°C for 15 s and 60°C for 1 min. A fragment of the B. burgdorferi flaB gene (23) was amplified to determine relative spirochete numbers and was compared to genomic DNA isolated from a known number of B. burgdorferi cells. Tick gene equivalents were determined by QPCR of a portion of the I. scapularis actin gene (28) and were normalized to a standard curve of a known number of DNA copies of the cloned actin gene fragment. The standard curve was calculated based on the molecular mass of the plasmid containing the actin gene fragment.
RNA was converted to cDNA using the high-capacity cDNA reverse transcription kit (Applied Biosystems); reaction mixtures consisted of 1 μg RNA and were converted to cDNA per the manufacturer's recommendations. The cDNA samples were diluted 1:10, and 1 μl was used per reaction. Negative control reactions were used to demonstrate that all genomic DNA had been removed; they consisted of RNA treated as described above but lacking reverse transcriptase. All QPCR samples were analyzed in triplicate.
RESULTS
Construction of OspD mutant strains.
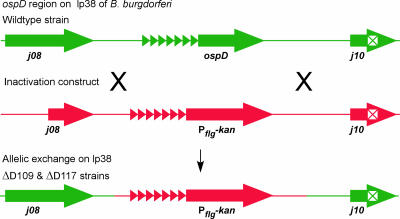
Two independently derived OspD mutant strains, ΔD109 and ΔD117, were obtained by allelic exchange with an inactivation construct in separate transformations (Fig. 1). Plasmid profiles were determined by PCR to ensure that strains were isogenic with the wild type (data not shown). Southern blottings of total genomic DNA from both mutant strains confirmed the absence of the ospD coding region and the presence of the gene encoding kanamycin resistance (data not shown). Additional confirmation was demonstrated by immunoblotting with cell lysates from in vitro-grown cultures, where OspD is abundantly expressed. Immunoblots hybridized with anti-OspD antisera bound to an appropriately sized protein in the wild-type cell lysate but not in the mutant lysates (Fig. 2A). IFAs using the OspD antisera bound to the surfaces of wild-type bacteria but not to those of the mutant strains (data not shown). Together, these data demonstrate that the mutant strains do not produce OspD. Attempts to complement the mutants with a wild-type copy of ospD, either in trans on a shuttle vector or in cis by integration, resulted in noninfectious transformants lacking the virulence plasmid lp25. A putative restriction/modification enzyme encoded on this linear plasmid acts as a barrier to successful transformation with some plasmid constructs (24, 26). Therefore, we continued the experiments using two independently isolated mutants obtained from separate transformations. We assumed that any spontaneous secondary mutations occurring in one strain would be unlikely to have arisen in a separately derived transformant. If the OspD mutant strains were unable to complete the infectious cycle, then complementation by other means would be necessary to confirm the contribution of OspD to the phenotype.
FIG. 1.
Strategy for deletion of ospD from the B. burgdorferi genome. The flanking regions of ospD, including portions of the gene encoding BBJ09 and the pseudogene BBJ10, were cloned with the kanamycin-resistance gene fused to the flgB promoter (PflgB) to construct an inactivation plasmid. The pseudogene is denoted by ⊠, and direct repeats upstream of ospD are indicated by ▸. Objects are not drawn to scale.
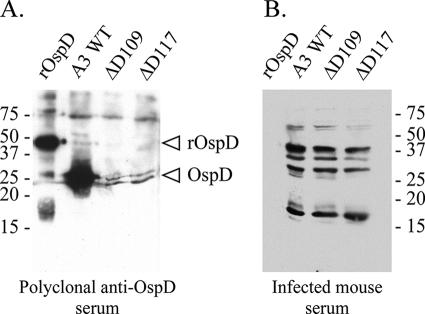
FIG. 2.
Immunoblots assessing OspD expression in various B. burgdorferi strains. (A) OspD antiserum reacts with a protein of the appropriate size (28 kDa) from a whole-cell lysate of the A3 wild-type (WT) strain, but not with whole-cell lysates from the OspD mutant strains (ΔD109 and ΔD117). The rOspD-thioredoxin fusion protein (∼42 kDa) was run in parallel as a positive control. (B) Individual serum samples from three mice infected by tick bite were incubated with immunoblots of purified rOspD protein and whole-cell lysates of B. burgdorferi wild-type and OspD mutant strains. A single, representative immunoblot is shown. Molecular mass standards (in kilodaltons) are indicated beside each panel.
OspD is not required for infection of mice.
B. burgdorferi expresses OspC during tick feeding, in preparation for establishing an infection in the mammalian host (15, 36, 46, 52). OspC mutant strains do not establish infections in mice, either by needle inoculation or by tick transmission (15, 56, 58). To assess whether OspD is similarly required for mammalian infection, mice were needle inoculated with OspD mutant strains or the wild-type strain. Infection was assessed by a serological response to B. burgdorferi 3 weeks after infection and by reisolating B. burgdorferi from mouse tissues 6 months postinfection (Table 2). All mice tested positively, both by serological detection and by culture, indicating that OspD is not required to infect or to persist in the murine host when B. burgdorferi is introduced by needle injection.
TABLE 2.
Infectivity of B. burgdorferi strains in mice
| B. burgdorferi strain | Inoculation method | Serologya | Reisolationb |
|---|---|---|---|
| B31 A3 (wild type) | Needle inoculation | 5/5 | 5/5 |
| Tick bitec | 4/4 | 4/4 | |
| ΔD109 | Needle inoculation | 5/5 | 5/5 |
| Tick bite | 3/4 | 3/4 | |
| ΔD117 | Needle inoculation | 5/5 | 5/5 |
| Tick bite | 3/4 | 3/4 |
Number of mice infected/number of mice inoculated with B. burgdorferi. Mouse infectivity was assessed >3 weeks postinfection by serological response against the early antigen P39 (BmpA) (53) and total B. burgdorferi cell lysate.
Number of mice culture positive/number of mice inoculated. The bladder, ear tissue, and rear ankle joint were dissected and cultured in liquid BSK-II medium and assessed for the presence of motile B. burgdorferi by dark-field microscopy. All three tissues per mouse were either positive or negative for B. burgdorferi growth.
Both larval and nymphal ticks were used, and all were artificially infected as larvae.
However, tick transmission of B. burgdorferi differs substantially from needle inoculation, both in the number of spirochetes delivered and the proteins present on the bacterial surface. In parallel to the needle inoculation experiment described above, we artificially infected larval ticks with different B. burgdorferi strains (42) and assessed the ability of the OspD mutant strains to establish murine infection by tick bite (Table 2). All strains, including the OspD mutants, successfully established mouse infections following tick challenge, indicating that OspD expression is not required for mammalian infection.
Mice do not produce antibodies to OspD during early infection.
Although the mouse infection studies described above demonstrate that OspD is not required for mammalian infection, it is still possible that OspD is expressed during infection of the host. Norris et al. used proteinase K digestion to demonstrate that OspD is surface exposed (34) and therefore should be antigenic in the mammalian host, if OspD is expressed during infection. To determine if mice infected with B. burgdorferi produced antibodies to OspD, serum samples from three different mice infected by tick bite were used to probe immunoblots of purified recombinant OspD (rOspD) and B. burgdorferi cell lysates (a representative panel is shown in Fig. 2B). The rOspD was not recognized by any of the serum samples tested, and no differences were detected in seroreactivity between wild-type and ospD mutant strains. Specifically, the serum samples from mice infected with wild-type or OspD mutant strains did not display any differences in reactivity with B. burgdorferi lysates at the OspD molecular mass size (28 kDa). Therefore, during murine infection, B. burgdorferi does not express sufficient levels of OspD to elicit a humoral immune response in mice.
OspD mutant persists throughout tick life cycle.
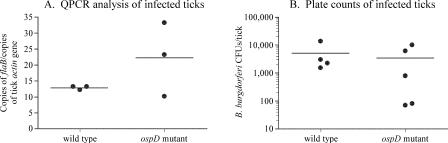
Midgut tissues of ticks infected with wild-type, ΔD109, and ΔD117 strains were examined by IFA for the presence of B. burgdorferi strains at various stages of the Ixodes life cycle. Both mutant strains and the wild type established infections in larval ticks and persisted through the blood meals to the adult stage (Table 3). The spirochete loads within fed I. scapularis nymphs were determined for both the wild type and the mutant strains by QPCR (Fig. 3A). Total genomic DNA from infected ticks was isolated, and copies of the chromosomal flaB gene were determined by QPCR and standardized to the copies of the tick actin gene, for comparison between strains. Although the number of spirochetes in ticks infected with the OspD mutant strain had a wider value spread, the average obtained was not significantly different from that of the wild type (P > 0.05, using a two-tailed, unpaired t test). Together, these data confirm that ospD is not required for B. burgdorferi persistence in the tick vector.
TABLE 3.
Infection of ticks with B. burgdorferi strains
| B. burgdorferi strain | No. of ticks infected with B. burgdorferi/total no. of ticks examineda
|
||
|---|---|---|---|
| Artificially infected larvae | Naturally infected larvae | Nymphal infectionb | |
| B31 A3 (wild type) | 6/6 | 9/9 | 8/10 |
| ΔD109 | 5/6 | 10/10 | 5/10 |
| ΔD117 | 1/2 | 15/15 | 3/6 |
Infection was assessed by IFA of tick midgut contents using polyclonal antiserum raised against B. burgdorferi. Ticks were artificially infected by immersion (42), allowed to feed on mice, and then examined. Naturally infected larvae acquired B. burgdorferi by feeding on infected mice and were examined within 3 days after detachment from the host.
Ticks were artificially infected as larvae, fed, and followed through the molt. Nymphs were assessed for infection after feeding.
FIG. 3.
Wild-type and OspD mutant strains colonize ticks equivalently, as assessed by QPCR and the plating of viable spirochetes. (A) Spirochete burdens in fed nymphal ticks, infected with either the wild type or the ospD mutant, were determined by QPCR of the B. burgdorferi flaB gene and normalized to the tick actin gene. Averages were not significantly different between strains (P > 0.05). Each data point is composed of five ticks. (B) Whole adult ticks (molted from nymphs that fed on P. leucopus mice) were macerated and plated to determine the spirochetal burden. No significant difference between the averages was observed (P > 0.05). Averages are denoted by horizontal bars, and P values were calculated using a two-tailed, unpaired t test.
Survival of the OspD mutant strain in a natural host: Peromyscus leucopus.
The studies described above were conducted using a laboratory-maintained, outbred mouse colony derived from Swiss-Webster mice. A natural reservoir for B. burgdorferi is the white-footed mouse, Peromyscus leucopus (2, 27). We hypothesized that OspD, although not required for survival within ticks that feed upon laboratory mice, might confer a selective advantage for survival or replication in ticks that feed on a natural host, such as P. leucopus. I. scapularis nymphs, infected with the wild-type or OspD mutant strains, were allowed to feed to repletion on P. leucopus mice and molt to adults. The adult ticks were macerated, resuspended in liquid BSK-II, and plated on solid medium. Colony counts did not differ significantly between the OspD mutant strains and the wild type (P > 0.05, using a two-tailed, unpaired t test) (Fig. 3B), indicating that OspD does not confer an obvious advantage to B. burgdorferi during tick digestion of P. leucopus blood. This result also confirms that the loss of ospD does not affect the fitness of B. burgdorferi, as assessed by QPCR as described above.
OspD is expressed after tick detachment from host.
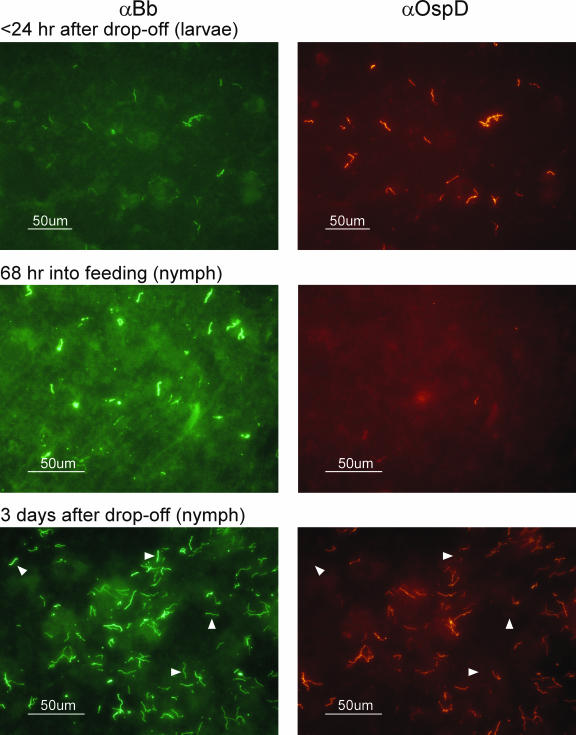
An in vitro microarray study of B. burgdorferi indicated that ospD was induced under tick-like conditions (38). Therefore, we assessed B. burgdorferi OspD expression throughout the life cycle of I. scapularis using an IFA (Table 4). The proportion of spirochetes producing OspD was determined by double fluorescence. The relative level of surface OspD expression on spirochetes in tick midgut tissues was determined by the intensity of fluorescence compared to that of in vitro-cultivated B. burgdorferi, which produces abundant levels of OspD protein. OspD was present at the highest levels detected within 24 h of tick detachment from the host, and virtually all spirochetes observed in the tick midgut tissues expressed OspD (Fig. 4 and Table 4). OspD expression continued to be strong for at least 1 week after detachment, although small numbers of spirochetes could be detected that lacked OspD at these later time points. OspD expression levels were low or undetectable at all other time points sampled, i.e., while the tick was attached and feeding on the mammalian host and after the molt. The window in which OspD is present on the surface of B. burgdorferi coincides with a decrease in the temperature of the tick vector after detachment, from the surface temperature of the mammal to the generally lower ambient temperature. These results agree with the in vitro microarray results of Ojaimi et al., in which the ospD transcript displayed the highest induction when B. burgdorferi transcripts from spirochetes grown at 23°C (tick) were compared to those from spirochetes grown at 35°C (mammal) (38). The continued expression of OspD on the spirochete surface during the days immediately following the tick blood meal also correlates to the period of the highest-known cell densities attained by B. burgdorferi in vivo (9, 41).
TABLE 4.
OspD expression during I. scapularis infection
| Time point | No. of ticks | % of spirochetes expressing OspDa | Relative expression levelb |
|---|---|---|---|
| 68 h into feedingc | 6 nymphs | 36 ± 31 | Weak |
| 1 day after drop-off | 4 larvae | 100 ± 0 | Strong |
| 3 days after drop-off | 6 nymphs | 84 ± 8.8 | Strong |
| 1 wk after drop-off | 5 larvae | 86 ± 16 | Moderate to strong |
| Unfed | 4 adults | 60 ± 28 | Weak |
Values expressed as percentages ± standard deviations.
The relative expression level is based on the intensity of the spirochete fluorescence observed from IFAs compared to control spirochetes cultured in vitro that express high levels of OspD (see Fig. 4).
Nymphs were forcibly removed 68 h after they had attached to the murine host.
FIG. 4.
IFAs of B. burgdorferi from tick midgut tissues at various stages of the I. scapularis life cycle. Spirochetes were double-labeled with fluorescein isothiocyanate-labeled anti-B. burgdorferi antisera (αBb; on the left) to identify entire spirochetes and anti-OspD antisera indirectly labeled with tetramethylrhodamine isothiocyanate (αOspD; on the right) to identify OspD-expressing spirochetes. Arrowheads in the bottom images indicate spirochetes within the population that no longer express OspD. Representative images are shown.
Transcript levels of ospD during and after tick feeding.
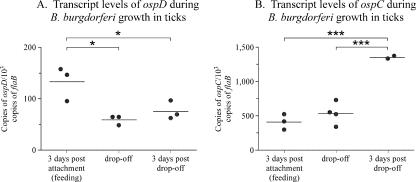
Since OspD protein appeared on the spirochete surface after ticks detached from their host, we assessed ospD transcript levels during and after tick feeding to identify time points when potential transcriptional regulation may occur. Total RNA was isolated from nymphal ticks during feeding (at 3 days postattachment of the tick), at detachment of the tick from the host (point of the highest level of protein expression), and 3 days postdetachment. QPCR was used to assess ospD transcript levels and was standardized to the levels of the constitutively synthesized flaB transcript, for comparison across strains (Fig. 5A). Transcript levels of ospD were highest at 3 days postattachment, the earliest time point examined, and were significantly different from subsequent time points (P < 0.05, as calculated using one-way analysis of variance using the Tukey-Kramer posttest). For comparison, the transcript levels of ospC were determined for the same time points and showed a significant increase in ospC expression at 3 days post drop-off (Fig. 5B) (P < 0.001, as determined for ospD as described above). The decrease in the ospD transcript over the time frame examined indicates that regulation of the ospD locus may occur at the transcriptional level. However, we cannot exclude the possibility that posttranscriptional regulation also controls ospD expression.
FIG. 5.
B. burgdorferi transcript levels during and after tick feeding. (A) B. burgdorferi transcript levels of ospD, normalized to 103 copies of the chromosomal flaB gene, are shown for selected time points. (B) For comparison, ospC transcript levels are shown for the same time points. Each data point is composed of five nymphal ticks, and averages are denoted by horizontal bars. Asterisks and brackets indicate significant differences between values (*, P < 0.05; ***, P < 0.001).
The direct repeat region of the ospD promoter is stable during bacterial survival in the tick vector.
The seven direct repeats are unique to the ospD promoter; although similar sequences are present upstream of three other genes, the sequences are not identical or fully reiterated (5). The repeats in the ospD promoter (Fig. 1) were speculated to undergo expansion or contraction by recombination (34) and subsequently shown to vary between B. burgdorferi strains (30). We tested whether a clonal population of B. burgdorferi, persisting in the tick midgut, might show variation in the number of repeats. DNA isolated from 3 groups of nymphal ticks (that had acquired the infection as larvae and were harvested after the nymphal blood meal) was used to PCR amplify a 351-bp region encompassing the direct repeats and 132 bp of the coding region of ospD. The PCR products migrated as a single, discreet band at the expected size on an agarose gel, indicating that the promoter region did not vary in size significantly within each pool (data not shown). Cloning and sequencing of the PCR fragment showed that all seven direct repeats were maintained intact in the 42 clones examined (data not shown). We conclude that there is no evidence for variation in the repeat region of a clonal population of B. burgdorferi during tick infection.
DISCUSSION
The results presented here demonstrate that OspD is not required during mammalian infection (Table 2). The ability of OspD mutant strains to persistently infect mice for over 6 months demonstrates the dispensability of OspD for infection of the mammalian host. Reinforcing these results are previous studies of both mammalian isolates of B. burgdorferi and other species of Lyme disease-inducing spirochetes that were shown to lack ospD (20, 21, 30, 40, 57). Further, we show that it is unlikely that OspD is expressed during murine infection. Mice infected with B. burgdorferi by tick bite do not produce antibodies that recognize OspD (Fig. 2), indicating that OspD is not expressed at immunologically significant levels. This is substantiated by the Probert and LeFebvre study in which OspD-vaccinated mice were not protected from needle-inoculated challenge with B. burgdorferi (43).
The OspD mutant strains are also capable of infecting I. scapularis, both by artificial inoculation and natural acquisition from infected mice, and persisting through the entire tick life cycle (Table 3). Therefore, we conclude that OspD is not essential to B. burgdorferi survival in vivo. Recently, Li and colleagues reported similar findings for OspD (29).
Surprisingly, this nonessential protein appears to be tightly regulated and synthesized only after fed ticks have detached from the host (Table 4 and Fig. 4). The protein remains on the outer surface during the tick's digestion of the blood meal, but after the subsequent molt, OspD expression is low or undetectable. This limited window of expression supports the finding that OspD is not required for mouse infectivity, as expression does not occur until after the tick detaches from the host. OspD expression therefore represents a unique regulation pattern for a B. burgdorferi protein.
A finely tuned regulatory mechanism for OspD expression had been suggested from genetic characterization of the ospD locus and in vitro microarray studies. The seven direct repeats upstream of the ospD gene were proposed binding sites for a putative regulator (34). The repeat elements vary in number between strains (30), and Norris and coworkers speculated that recombination between repeats may expand or contract this region (34). However, we saw no evidence for variation in the number of repeats during B. burgdorferi infection of the tick (data not shown). Furthermore, in vitro microarray data of B. burgdorferi cultivated under temperatures mimicking that of the tick (23°C) demonstrated dramatic increases in ospD expression (38). However, the results presented here, and those of Li et al. (29), found only small changes in ospD transcript levels over the time frame in which the protein appears on the spirochete surface (Fig. 4 and 5).
The regulatory mechanism(s) controlling OspD expression remains unclear. Possibly, the highest levels of ospD transcript may occur outside the time points that we examined. Alternatively, ospD regulation may occur posttranscriptionally, which would explain why the observed RNA levels do not vary despite the relatively rapid increase in the protein levels over the same time period.
Regardless, these results raise the question of why evolutionary pressures have not eliminated a gene such as ospD, which is likely to be energetically costly to the bacterium to regulate and synthesize at a high level, but nonessential to its life cycle. B. burgdorferi has evolved a tight regulatory system for ospD that differs from that for other known outer surface proteins, which suggests a positive pressure for retention and dissemination. The timing of OspD expression correlates to the period of the highest cell densities observed for B. burgdorferi in vivo (9, 41). A possible function for OspD may relate to cell signaling. Little is known about sensing cell densities in B. burgdorferi, but one component of a quorum sensing system, LuxS, has been identified in the genome (54). Like OspD, LuxS has been shown to be dispensable for B. burgdorferi survival in vivo (3, 18), and the luxS transcript is expressed in feeding ticks, but not unfed ticks (32). Intercellular signaling, while not essential to B. burgdorferi, may be important in a natural infection where other bacterial populations coexist. Alternatively, OspD expression also coincides with the tick's digestion of the blood meal, as the midgut hemolytic activity of Ixodes ticks is not detectable until 3 days after tick attachment to the host (48). OspD may be involved in the scavenging of nutrients during this period, which may provide an advantage in nature when B. burgdorferi may be in competition with other microorganisms. B. burgdorferi infects a variety of different hosts, and OspD may potentially provide a selective advantage for spirochete survival within ticks that have fed upon a specific host, other than the laboratory and white-footed mice we tested. Presumably, the evolutionary benefit provided by ospD outweighs the advantages of eliminating this nonessential locus and explains why it is widespread throughout Borrelia strains that cause Lyme disease.
Acknowledgments
We gratefully acknowledge the graphical talents of Gary Hettrick and Anita Mora in figure preparation. We thank Tom Schwan and Paul Policastro for technical advice on tick and IFA manipulations, Viveka Vadyvaloo for help and advice on RNA isolation, and Kit Tilly for critical reading of the manuscript and help with QPCR.
This research was supported by the Intramural Research Program of the NIH, NIAID.
Editor: D. L. Burns
Footnotes
Published ahead of print on 10 March 2008.
REFERENCES
- 1.Anderson, J. F., R. C. Johnson, L. A. Magnarelli, and F. W. Hyde. 1986. Involvement of birds in the epidemiology of the Lyme disease agent Borrelia burgdorferi. Infect. Immun. 51394-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson, J. F., and L. A. Magnarelli. 1984. Avian and mammalian hosts for spirochete-infected ticks and insects in a Lyme disease focus in Connecticut. Yale J. Biol. Med. 57627-641. [PMC free article] [PubMed] [Google Scholar]
- 3.Blevins, J. S., A. T. Revel, M. J. Caimano, X. F. Yang, J. A. Richardson, K. E. Hagman, and M. V. Norgard. 2004. The luxS gene is not required for Borrelia burgdorferi tick colonization, transmission to a mammalian host, or induction of disease. Infect. Immun. 724864-4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bono, J. L., A. F. Elias, J. J. Kupko III, B. Stevenson, K. Tilly, and P. Rosa. 2000. Efficient targeted mutagenesis in Borrelia burgdorferi. J. Bacteriol. 1822445-2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brooks, C. S., P. S. Hefty, S. E. Jolliff, and D. R. Akins. 2003. Global analysis of Borrelia burgdorferi genes regulated by mammalian host-specific signals. Infect. Immun. 713371-3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Burgdorfer, W., A. G. Barbour, S. F. Hayes, J. L. Benach, E. Grunwaldt, and J. P. Davis. 1982. Lyme disease: a tick-borne spirochetosis? Science 2161317-1319. [DOI] [PubMed] [Google Scholar]
- 7.Casjens, S., N. Palmer, R. van Vugt, W. M. Huang, B. Stevenson, P. Rosa, R. Lathigra, G. Sutton, J. Peterson, R. J. Dodson, D. Haft, E. Hickey, M. Gwinn, O. White, and C. Fraser. 2000. A bacterial genome in flux: the twelve linear and nine circular extrachromosomal DNAs in an infectious isolate of the Lyme disease spirochete Borrelia burgdorferi. Mol. Microbiol. 35490-516. [DOI] [PubMed] [Google Scholar]
- 8.Clark, K., A. Hendricks, and D. Burge. 2005. Molecular identification and analysis of Borrelia burgdorferi sensu lato in lizards in the southeastern United States. Appl. Environ. Microbiol. 712616-2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Silva, A. M., and E. Fikrig. 1995. Growth and migration of Borrelia burgdorferi in Ixodes ticks during blood feeding. Am. J. Trop. Med. Hyg. 53397-404. [DOI] [PubMed] [Google Scholar]
- 10.Elias, A. F., J. L. Bono, J. J. Kupko III, P. E. Stewart, J. G. Krum, and P. A. Rosa. 2003. New antibiotic resistance cassettes suitable for genetic studies in Borrelia burgdorferi. J. Mol. Microbiol. Biotechnol. 629-40. [DOI] [PubMed] [Google Scholar]
- 11.Elias, A. F., P. E. Stewart, D. Grimm, M. J. Caimano, C. H. Eggers, K. Tilly, J. L. Bono, D. R. Akins, J. D. Radolf, T. G. Schwan, and P. Rosa. 2002. Clonal polymorphism of Borrelia burgdorferi strain B31 MI: implications for mutagenesis in an infectious strain background. Infect. Immun. 702139-2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fraser, C. M., S. Casjens, W. M. Huang, G. G. Sutton, R. Clayton, R. Lathigra, O. White, K. A. Ketchum, R. Dodson, E. K. Hickey, M. Gwinn, B. Dougherty, J.-F. Tomb, R. D. Fleischmann, D. Richardson, J. Peterson, A. R. Kerlavage, J. Quackenbush, S. Salzberg, M. Hanson, R. van Vugt, N. Palmer, M. D. Adams, J. Gocayne, J. Weidmann, T. Utterback, L. Watthey, L. McDonald, P. Artiach, C. Bowman, S. Garland, C. Fujii, M. D. Cotton, K. Horst, K. Roberts, B. Hatch, H. O. Smith, and J. C. Venter. 1997. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature 390580-586. [DOI] [PubMed] [Google Scholar]
- 13.Grimm, D., A. F. Elias, K. Tilly, and P. A. Rosa. 2003. Plasmid stability during in vitro propagation of Borrelia burgdorferi assessed at a clonal level. Infect. Immun. 713138-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grimm, D., K. Tilly, D. M. Bueschel, M. A. Fisher, P. F. Policastro, F. C. Gherardini, T. G. Schwan, and P. A. Rosa. 2005. Defining plasmids required by Borrelia burgdorferi for colonization of tick vector Ixodes scapularis (Acari: Ixodidae). J. Med. Entomol. 42676-684. [DOI] [PubMed] [Google Scholar]
- 15.Grimm, D., K. Tilly, R. Byram, P. E. Stewart, J. G. Krum, D. M. Bueschel, T. G. Schwan, P. F. Policastro, A. F. Elias, and P. A. Rosa. 2004. Outer-surface protein C of the Lyme disease spirochete: a protein induced in ticks for infection of mammals. Proc. Natl. Acad. Sci. USA 1013142-3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hellwage, J., T. Meri, T. Heikkilä, A. Alitalo, J. Panelius, P. Lahdenne, J. T. Seppälä, and S. Meri. 2001. The complement regulator factor H binds to the surface protein OspE of Borrelia burgdorferi. J. Biol. Chem. 2768427-8435. [DOI] [PubMed] [Google Scholar]
- 17.Hodzic, E., S. Feng, K. J. Freet, and S. W. Barthold. 2003. Borrelia burgdorferi population dynamics and prototype gene expression during infection of immunocompetent and immunodeficient mice. Infect. Immun. 715042-5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hübner, A., A. T. Revel, D. M. Nolen, K. E. Hagman, and M. V. Norgard. 2003. Expression of a luxS gene is not required for Borrelia burgdorferi infection of mice via needle inoculation. Infect. Immun. 712892-2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Indest, K. J., J. K. Howell, M. B. Jacobs, D. Scholl-Meeker, S. J. Norris, and M. T. Philipp. 2001. Analysis of Borrelia burgdorferi vlsE gene expression and recombination in the tick vector. Infect. Immun. 697083-7090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iyer, R., O. Kalu, J. Purser, S. Norris, B. Stevenson, and I. Schwartz. 2003. Linear and circular plasmid content in Borrelia burgdorferi clinical isolates. Infect. Immun. 713699-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iyer, R., D. Liveris, A. Adams, J. Nowakowski, D. McKenna, S. Bittker, D. Cooper, G. P. Wormser, and I. Schwartz. 2001. Characterization of Borrelia burgdorferi isolated from erythema migrans lesions: interrelationship of three molecular typing methods. J. Clin. Microbiol. 392954-2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jacobs, M. B., S. J. Norris, K. M. Phillippi-Falkenstein, and M. T. Philipp. 2006. Infectivity of the highly transformable BBE02− lp56− mutant of Borrelia burgdorferi, the Lyme disease spirochete, via ticks. Infect. Immun. 743678-3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jewett, M. W., K. Lawrence, A. C. Bestor, K. Tilly, D. Grimm, P. Shaw, M. Vanraden, F. Gherardini, and P. A. Rosa. 2007. The critical role of the linear plasmid lp36 in the infectious cycle of Borrelia burgdorferi. Mol. Microbiol. 641358-1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawabata, H., S. J. Norris, and H. Watanabe. 2004. BBE02 disruption mutants of Borrelia burgdorferi B31 have a highly transformable, infectious phenotype. Infect. Immun. 727147-7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kraiczy, P., C. Skerka, M. Kirschfink, V. Brade, and P. F. Zipfel. 2001. Immune evasion of Borrelia burgdorferi by acquisition of human complement regulators FHL-1/reconectin and factor H. Eur. J. Immunol. 311674-1684. [DOI] [PubMed] [Google Scholar]
- 26.Lawrenz, M. B., H. Kawabata, J. E. Purser, and S. J. Norris. 2002. Decreased electroporation efficiency in Borrelia burgdorferi containing linear plasmids lp25 and lp56: impact on transformation of infectious B. burgdorferi. Infect. Immun. 7044798-44804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levine, J. F., M. L. Wilson, and A. Spielman. 1985. Mice as reservoirs of the Lyme disease spirochete. Am. J. Trop. Med. Hyg. 34355-360. [DOI] [PubMed] [Google Scholar]
- 28.Li, X., X. Liu, D. S. Beck, F. S. Kantor, and E. Fikrig. 2006. Borrelia burgdorferi lacking BBK32, a fibronectin-binding protein, retains full pathogenicity. Infect. Immun. 743305-3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li, X., G. Neelakanta, X. Liu, D. S. Beck, F. S. Kantor, D. Fish, J. F. Anderson, and E. Fikrig. 2007. Role of outer surface protein D in the Borrelia burgdorferi life cycle. Infect. Immun. 754237-4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marconi, R. T., D. S. Samuels, R. K. Landry, and C. F. Garon. 1994. Analysis of the distribution and molecular heterogeneity of the ospD gene among the Lyme disease spirochetes: evidence for lateral gene exchange. J. Bacteriol. 1764572-4582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller, J. C., K. von Lackum, K. Babb, J. D. McAlister, and B. Stevenson. 2003. Temporal analysis of Borrelia burgdorferi Erp protein expression throughout the mammal-tick infectious cycle. Infect. Immun. 716943-6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Narasimhan, S., M. J. Caimano, F. T. Liang, F. Santiago, M. Laskowski, M. T. Philipp, A. R. Pachner, J. D. Radolf, and E. Fikrig. 2003. Borrelia burgdorferi transcriptome in the central nervous system of non-human primates. Proc. Natl. Acad. Sci. USA 10015953-15958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Neelakanta, G., X. Li, U. Pal, X. Liu, D. S. Beck, K. Deponte, D. Fish, F. S. Kantor, and E. Fikrig. 2007. Outer surface protein B is critical for Borrelia burgdorferi adherence and survival within Ixodes ticks. PLoS Pathog. 3e33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Norris, S. J., C. J. Carter, J. K. Howell, and A. G. Barbour. 1992. Low-passage-associated proteins of Borrelia burgdorferi B31: characterization and molecular cloning of OspD, a surface-exposed, plasmid-encoded lipoprotein. Infect. Immun. 604662-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nosbisch, L. K., and A. M. de Silva. 2007. Lack of detectable variation at Borrelia burgdorferi vlsE locus in ticks. J. Med. Entomol. 44168-170. [DOI] [PubMed] [Google Scholar]
- 36.Ohnishi, J., J. Piesman, and A. M. de Silva. 2001. Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks. Proc. Natl. Acad. Sci. USA 98670-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohnishi, J., B. Schneider, W. B. Messer, J. Piesman, and A. M. de Silva. 2003. Genetic variation at the vlsE locus of Borrelia burgdorferi within ticks and mice over the course of a single transmission cycle. J. Bacteriol. 1854432-4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ojaimi, C., C. Brooks, S. Casjens, P. Rosa, A. Elias, A. G. Barbour, A. Jasinskas, J. Benach, L. Katona, J. Radolf, M. Caimano, J. Skare, K. Swingle, D. Akins, and I. Schwartz. 2003. Profiling temperature-induced changes in Borrelia burgdorferi gene expression using whole genome arrays. Infect. Immun. 711689-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pal, U., A. M. de Silva, R. R. Montgomery, D. Fish, J. Anguita, J. F. Anderson, Y. Lobet, and E. Fikrig. 2000. Attachment of Borrelia burgdorferi within Ixodes scapularis mediated by outer surface protein A. J. Clin. Investig. 106561-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Palmer, N., C. Fraser, and S. Casjens. 2000. Distribution of twelve linear extrachromosomal DNAs in natural isolates of Lyme disease spirochetes. J. Bacteriol. 1822476-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Piesman, J., J. R. Oliver, and R. J. Sinsky. 1990. Growth kinetics of the Lyme disease spirochete (Borrelia burgdorferi) in vector ticks (Ixodes dammini). Am. J. Trop. Med. Hyg. 42352-357. [DOI] [PubMed] [Google Scholar]
- 42.Policastro, P. F., and T. G. Schwan. 2003. Experimental infection of Ixodes scapularis larvae (Acari: Ixodidae) by immersion in low passage cultures of Borrelia burgdorferi. J. Med. Entomol. 40364-370. [DOI] [PubMed] [Google Scholar]
- 43.Probert, W. S., and R. B. LeFebvre. 1994. Protection of C3H/HeN mice from challenge with Borrelia burgdorferi through active immunization with OspA, OspB, or OspC, but not with OspD or the 83-kilodalton antigen. Infect. Immun. 621920-1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Purser, J. E., M. B. Lawrenz, M. J. Caimano, J. D. Radolf, and S. J. Norris. 2003. A plasmid-encoded nicotinamidase (PncA) is essential for infectivity of Borrelia burgdorferi in a mammalian host. Mol. Microbiol. 48753-764. [DOI] [PubMed] [Google Scholar]
- 45.Purser, J. E., and S. J. Norris. 2000. Correlation between plasmid content and infectivity in Borrelia burgdorferi. Proc. Natl. Acad. Sci. USA 9713865-13870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramamoorthi, N., S. Narasimhan, U. Pal, F. Bao, X. F. Yang, D. Fish, J. Anguita, M. V. Norgard, F. S. Kantor, J. F. Anderson, R. A. Koski, and E. Fikrig. 2005. The Lyme disease agent exploits a tick protein to infect the mammalian host. Nature 436573-577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Revel, A. T., J. S. Blevins, C. Almazan, L. Neil, K. M. Kocan, J. de la Fuente, K. E. Hagman, and M. V. Norgard. 2005. bptA (bbe16) is essential for the persistence of the Lyme disease spirochete, Borrelia burgdorferi, in its natural tick vector. Proc. Natl. Acad. Sci. USA 1026972-6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ribeiro, J. M. C. 1988. The midgut hemolysin of Ixodes dammini (Acari: Ixodidae). J. Parasitol. 74532-537. [PubMed] [Google Scholar]
- 49.Rosa, P., D. S. Samuels, D. Hogan, B. Stevenson, S. Casjens, and K. Tilly. 1996. Directed insertion of a selectable marker into a circular plasmid of Borrelia burgdorferi. J. Bacteriol. 1785946-5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schwan, T. G., W. Burgdorfer, M. E. Schrumpf, and R. H. Karstens. 1988. The urinary bladder, a consistent source of Borrelia burgdorferi in experimentally infected white-footed mice (Peromyscus leucopus). J. Clin. Microbiol. 26893-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schwan, T. G., and J. Piesman. 2000. Temporal changes in outer surface proteins A and C of the Lyme disease-associated spirochete, Borrelia burgdorferi, during the chain of infection in ticks and mice. J. Clin. Microbiol. 38382-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schwan, T. G., J. Piesman, W. T. Golde, M. C. Dolan, and P. A. Rosa. 1995. Induction of an outer surface protein on Borrelia burgdorferi during tick feeding. Proc. Natl. Acad. Sci. USA 922909-2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Simpson, W. J., W. Burgdorfer, M. E. Schrumpf, R. H. Karstens, and T. G. Schwan. 1991. Antibody to a 39-kilodalton Borrelia burgdorferi antigen (P39) as a marker for infection in experimentally and naturally inoculated animals. J. Clin. Microbiol. 29236-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stevenson, B., and K. Babb. 2002. LuxS-mediated quorum sensing in Borrelia burgdorferi, the Lyme disease spirochete. Infect. Immun. 704099-4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stevenson, B., N. El-Hage, M. A. Hines, J. C. Miller, and K. Babb. 2002. Differential binding of host complement inhibitor factor H by Borrelia burgdorferi Erp surface proteins: a possible mechanism underlying the expansive host range of Lyme disease spirochetes. Infect. Immun. 70491-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stewart, P. E., X. Wang, D. M. Bueschel, D. R. Clifton, D. Grimm, K. Tilly, J. A. Carroll, J. J. Weis, and P. A. Rosa. 2006. Delineating the requirement for the Borrelia burgdorferi virulence factor OspC in the mammalian host. Infect. Immun. 743547-3553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Terekhova, D., R. Iyer, G. P. Wormser, and I. Schwartz. 2006. Comparative genome hybridization reveals substantial variation among clinical isolates of Borrelia burgdorferi sensu stricto with different pathogenic properties. J. Bacteriol. 1886124-6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tilly, K., J. G. Krum, A. Bestor, M. W. Jewett, D. Grimm, D. Bueschel, R. Byram, D. Dorward, P. Stewart, and P. Rosa. 2006. Borrelia burgdorferi OspC protein required exclusively in a crucial early stage of mammalian infection. Infect. Immun. 743554-3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tokarz, R., J. M. Anderton, L. I. Katona, and J. L. Benach. 2004. Combined effects of blood and temperature shift on Borrelia burgdorferi gene expression as determined by whole genome DNA array. Infect. Immun. 725419-5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.von Lackum, K., J. C. Miller, T. Bykowski, S. P. Riley, M. E. Woodman, V. Brade, P. Kraiczy, B. Stevenson, and R. Wallich. 2005. Borrelia burgdorferi regulates expression of complement regulator-acquiring surface protein 1 during the mammal-tick infection cycle. Infect. Immun. 737398-7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang, X. F., U. Pal, S. M. Alani, E. Fikrig, and M. V. Norgard. 2004. Essential role for OspA/B in the life cycle of the Lyme disease spirochete. J. Exp. Med. 199641-648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang, J.-R., and S. J. Norris. 1998. Kinetics and in vivo induction of genetic variation of vlsE in Borrelia burgdorferi. Infect. Immun. 663689-3697. [DOI] [PMC free article] [PubMed] [Google Scholar]