The mammalian immune system comprises multiple physical, chemical, and cellular components that are traditionally classified as innate and adaptive immunity. The innate immune response is the first line of host defense against pathogens, depending on barrier structures, phagocytic cells (neutrophils, macrophages, and dendritic cells [DCs]), natural killer (NK) cells, and molecules such as complement proteins, cytokines, and antimicrobial peptides. Once the bacterium infects the host, the innate immunity provides immediate protection. After 4 to 5 days, the T-cell- and B-cell-mediated adaptive immune response begins to provide organism-specific protection and has a long-lasting immunological memory. In most cases, the bacterium will be eliminated from the host by the synergistic effect of both innate and adaptive immunity.
Yersinia pestis, a gram-negative bacterium and plague pathogen, is classified as a category A agent of bioterrorism (18, 19). It gains its notoriety from causing three massive pandemics in history that killed hundreds of millions of people (66). If not treated with proper antimicrobial drugs, the bacteria rapidly escape from containment in the lymph node, spread systemically through the blood, and cause fatal sepsis (66). One of its forms, pneumonic plague, is difficult to treat because of the speed of the disease's progress (a typical incubation period is 1 to 3 days), and by the time individuals are symptomatic, they are often close to death.
To survive inside of the host and maintain a persistent infection, Y. pestis uses a variety of mechanisms to evade or overcome the host immune system, especially the innate immune system. Since the interaction of Y. pestis and host immune system is such a large area of research that it is difficult to cover all aspects in full detail, this review will focus on the following subtopics.
HOW Y. PESTIS OVERCOMES THE INNATE IMMUNE RESPONSE
The innate immune system (nonspecific immunity) is able to discriminate between self and a variety of pathogens by recognizing the highly conserved sets of molecular structures specific to microbes (pathogen-associated molecular patterns [PAMPs]) via a limited number of germ line-encoded pattern recognition receptors (PRRs). Different PRRs react to specific pathogen-associated molecular patterns, exhibit distinct expression patterns, and activate immune cells directly to induce the expression of a variety of genes involved in the innate and adaptive immunity (for reviews, see references 3, 64, and 85). PRRs activate the complement pathway of innate immunity and induce production of cytokines such as interleukin-1β (IL-1β), IL-6, tumor necrosis factor (TNF), and chemokines to collectively induce inflammatory responses to pathogens, recruit neutrophils to the infection site, and activate macrophages to kill the microbes.
By the bite of an infected rodent flea, Y. pestis may invade directly into the host through the barrier structure of the host skin and encounter phagocytes such as polymorphonuclear leukocytes (PMNs) (predominantly neutrophils) and macrophages at the site of invasion. Most of them might be killed by neutrophils. However, the facultative intracellular Y. pestis preferentially infects host macrophages, possibly via recognition of specific surface-associated CCR5 molecules (33), and survives inside of macrophages at the early stage of infection. After proliferation and expression of various virulence determinants in macrophages, Y. pestis can be released into the extracellular compartment and spread systemically with acquisition of phagocytosis resistance. During this process, Y. pestis may circumvent destruction by the components of the host innate immune system (Fig. 1).
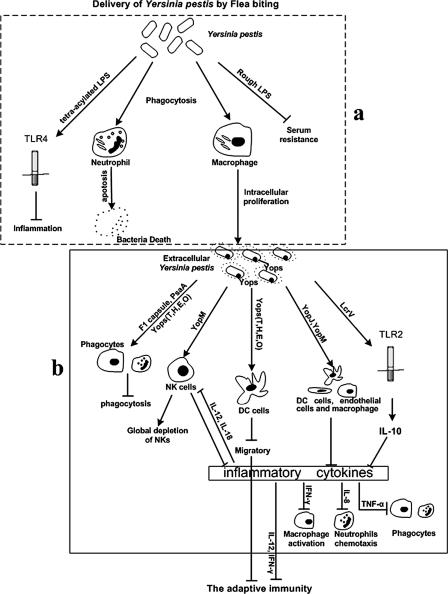
FIG. 1.
Y. pestis defense mechanisms against host innate immunity. (a) Defense mechanisms at the early stage of infection. The LPS structure diversities of Y. pestis during transition between flea and host temperatures make the bacteria resistant to the serum-mediated lysis and suppress the proinflammatory response. Meanwhile, the bacteria phagocytosed by macrophages can proliferate and express various virulence determinants to act on host immune responses. (b) Defense mechanisms after the release of Y. pestis from macrophages. The bacteria released from macrophages acquire the ability to resist phagocytosis and can inhibit the production of proinflammatory cytokines, which also attenuate the host's adaptive immunity.
DEFENSE MECHANISMS OF THE HOST INNATE IMMUNITY AT AN EARLY STAGE OF INFECTION
Inhibition of TLR4-mediated activation in the mammalian host.
Lipopolysaccharide (LPS) is a major component of the outer membrane in gram-negative bacteria and a ligand for Toll-like receptor 4 (TLR4), one kind of PRR (85). The expression of potent LPS by some pathogens might be beneficial for the host by providing early recognition of infection and effective initiation of immune signaling. The number and length of fatty acid side chains of LPS lipid A are diverse, and functional structure analysis indicates that the number and length of acyl side chains are critical for TLR4 signaling in humans. Hexa-acylated lipid A with side chains 12 to 14 carbons in length maximally stimulates immunological responses in humans, whereas altering the number or length of the attached fatty acids reduces the magnitude of the signal (55).
At different host-specific environments, the expression and formation of LPS in Y. pestis can change accordingly (74, 86). Lien and coworkers demonstrated that Y. pestis may well contain a mixture of stimulatory and nonstimulatory LPS species, especially during transition between flea and host temperatures (56). The bacteria grown in flea gut (21 to 26°C) produce a typical hexa-acylated LPS, which activates the TLR4-mediated immune signaling to induce proinflammatory cytokines such as TNF, IL-1, IL-6, and IL-8. However, after the temperature transition from the flea (26°C) to the mammalian host (37°C), Y. pestis immediately begins to produce tetra-acylated LPS, which is not only nonstimulatory for TLR4 but also acts as an antagonist for the stimulatory hexa-acylated form of LPS (30, 56). These changes may prevent activation of macrophages, secretion of proinflammatory cytokines, and activation and maturation of DCs that are required for the induction of adaptive immunity.
Serum resistance.
In order to survive and/or grow in blood for transmission between its flea vector and the mammalian host, Y. pestis has developed resistance to complement-mediated lysis (serum resistance) (65, 89). In contrast to enteropathogenic yersiniae, which are resistant to complement when grown at 37°C but not when grown at 26°C (66), Y. pestis is resistant to complement at both 26 and 37°C (9). In enteropathogenic yersiniae, this function is mediated by YadA, Ail, and LPS. Y. pestis does not express YadA (14, 15). LPS seems to play a major role in the resistance to serum-mediated lysis by elevating its content of N-acetylglucosamine (9). However, the Y. pestis strains deficient in the expression of Ail are extremely sensitive to complement. Alteration in the structure of LPS may also interfere the expression and the surface exposure of Ail. The study performed by Plano and coworkers suggested that Ail might be the sole complement resistance factor due to its requirement for full protection of Y. pestis from complement-mediated lysis in vitro (12).
Replication in macrophages and acquisition of phagocytosis resistance.
Phagocytes are essential effector cells of innate immunity by ingestion (engulfment) and destruction of microorganisms. At the early stage of infection, Y. pestis is phagocytosed by CD11b+ macrophages and Ly-6G+ neutrophils at the infection site (50). Histological evidence indicates that Y. pestis was killed within neutrophils, and bacterial cultivation and flow cytometric analyses also suggest that at up to 2 days postinfection host neutrophils were able to control Y. pestis growth (50). However, Y. pestis can survive and proliferate in the phagolysosome of macrophages to express various virulence determinants (72, 83). This intracellular growth is essential for the pathogenesis of Y. pestis. First, macrophages provide a niche, allowing bacteria to proliferate and acquire the ability to evade phagocytosis. Second, intracellular growth in macrophages provides a protected environment for the bacteria to avoid contact with other components of the host immune system. Furthermore, the bacteria within macrophages can be trafficked to the local draining lymph node (73, 96). Between 4 and 5 days of postinfection, the numbers of Y. pestis increase rapidly in vivo, and the bacteria can escape from macrophages into the extracellular compartment with phagocytosis resistance. The two-component system PhoP/PhoQ may regulate one or more genes important for the intracellular survival of Y. pestis in macrophages (61).
DEFENSE MECHANISM OF BACTERIA RELEASED FROM MACROPHAGES
After 1 to 4 h infection of the macrophage cell line, Y. pestis demonstrated the rapid expression of virulence markers such as Yops, F1 antigen, and V antigen (66). At 1 to 2 days postinfection, the virulence markers begin to disturb the host immune responses by different mechanisms, such as inducing apoptosis, suppressing the production of proinflammatory cytokines (e.g., TNF-α), inhibiting Fc receptor-mediated phagocytosis, and preventing neutrophil chemotaxis.
F1- and PsaA-mediated resistance to phagocytosis.
When Y. pestis replicates within macrophages, F1 protein (fraction 1 antigen) is expressed and forms a capsule around the bacterium. This capsule increases bacterial resistance to engulfment by both macrophages and neutrophils through a mechanism different from that of the type III secretion system (T3SS), presumably by preventing interactions of receptors that could potentially result in the uptake of pathogens (29).
The fimbrial structure of Y. pestis, PsaA (pH 6 antigen) has been shown to be induced at 37°C in acidic media, an environment close to the phagolysosome of macrophages (49, 70). A recent report demonstrated that purified PsaA selectively bound to apolipoprotein B (apoB)-containing lipoproteins (LDL) in human plasma (51). At concentrations close to the physiological concentration in human blood (250 μg of human LDL/ml), LDL nearly abolished the interaction of the purified PsaA with macrophages. This process could prevent recognition of pathogens by the host defense systems (38, 51).
Roles of Yops against host immune system.
The T3SS proteins encoded by pCD1 plasmid are important factors in the progression of acute systemic infection by Y. pestis. When Y. pestis replicates within macrophages at 37°C, the expression of T3SS proteins is increased and forms a needle-like complex on the surface of the bacterium (23). Once released from macrophages and interacted with target cells, the bacterium injects six different T3SS effector proteins (YopE, YopJ/YopP, YopM, YopH, YopT, and YpkA/YopO) into the cells and inhibits the responses of the host immune system. Macrophages, DCs, and granulocytes/neutrophils are early target cells for the injection (for details, see references 1, 22, 27, 36, 40, and 58).
(i) Direct effects on the activation of immune cells.
. The NK cell is one kind of T lymphocyte that can arrive at the inflammatory sites and directly kill the infected cells without the recognition of antigenic peptides. Y. pestis can cause a global depletion of NK cells and decrease the secretion of IFN-γ, resulting in a reduced production of reactive nitrogen intermediates by macrophages. These effects depend on the presence of the effector YopM and are manifested by the second day after infection (41).
Phagocytes (macrophages and neutrophils) are the main target cells by bacterial T3SS for the injection of Yops. At least four Yops (YopH, YopE, YopT, and YopO) are involved in inhibiting the phagocytosis of yersiniae, either by interfering with the host cell actin regulation of Rho GTPases (YopE, YopT, and YopO) or specifically and rapidly inactivating host proteins associated with signaling from the receptor to actin (YopH) (1, 2, 6, 39, 77). Moreover, YopH can suppress the production of reactive oxygen intermediates by macrophages and PMNs (35).
(ii) Suppression of the production of proinflammatory factors.
Besides paralyzing phagocytic cells, Yops also inhibit the proinflammatory responses elicited by infected cells. YopP has been shown to inhibit TNF-α release by macrophages and IL-8 release by epithelial and endothelial cells (17). TNF-α is a proinflammatory cytokine, which is primarily released by activated macrophages and plays a crucial role in limiting the severity of the bacterial infection. In addition to YopP, YopM interacts with protein kinase C-like 2 and ribosomal protein S6 kinase, which are also involved in proinflammatory signaling (54). The suppression of the production of proinflammatory factors not only reduces the activation of NK cells and phagocytes, but also destroys the inflammatory environment for the adaptive immunity.
Role of LcrV on host immune system.
LcrV is a multifunctional protein that is involved in the formation of a tip complex at the tip of the T3SS needle. It activates the secretion and translocation of effectors by binding to the negative regulator LcrG (42) and works together with Yops B and D for delivering Yops into eukaryotic cells (24). It is also exposed on the bacterial cell surface prior to contact with mammalian cells and may play a role in cell-cell adhesion (67). After being secreted into the environment, LcrV exploits TLR2 and CD14 to trigger the release of IL-10 by host immune cells and suppresses the production of proinflammatory cytokines such as TNF-α and IFN-γ, as well as some other innate defense components required to combat the pathogenesis of plague (81, 82). An LcrV mutant strain with a deletion close to the C terminus (amino acid residues 271 to 300) protected animals from lethal plague infection and did not block proinflammatory capacity (60). However, recent studies showed a weak interaction of LcrV and TLR2, and LcrV did not contribute to the virulence of Y. pestis (69, 75). These studies suggest that the LcrV of Y. pestis does not efficiently activate TLR2 signaling and that TLR2-mediated immunomodulation is unlikely play a significant role in plague (69).
Inhibition of chemotaxis.
PMNs are one kind of leukocytes which can migrate to the infection site by chemotaxis and destroy the invading bacteria. It has been shown that the effector YopP/YopJ of T3SS can be injected into endothelial cells and reduce the expression of adhesion molecules, such as ICAM-1 and E-selectin, on endothelial and bronchial epithelial cells, which might inhibit the recruitment of PMNs to the infection site (16, 28, 97). In addition to YopP, LcrV also inhibits the chemotaxis of neutrophils in both in vivo and in vitro (91).
HOW Y. PESTIS DEFEATS THE ADAPTIVE IMMUNE RESPONSES
The adaptive immune responses use selective and clonal expansion of the immune cells (T and B cells) to recognize antigens from a pathogen, providing the specificity and long-lasting immunological memory. Activation of the T and B cells not only depends on the TCR-MHC/peptide interaction and the costimulatory signals, but also the induction of costimulatory molecules and secretion of cytokines and chemokines by the cells of the innate immune system. Y. pestis reduces the host adaptive immunity by both influencing the cytokine induction and acting directly on the immune cells involved in the adaptive immune responses (Fig. 2).
FIG. 2.
Defense mechanisms of Y. pestis against the host's adaptive immunity and the link between innate immunity and adaptive immunity. The adaptive immunity against Y. pestis was influenced not only by suppression of cytokines induction provided by the innate immunity but also by the action of Yops directly on the immune cells involved in the adaptive immune responses. In contrast, the inactivation of T cells, which reduce the production of IFN-γ and TNF-α, influences the activity of the innate immunity.
Inhibiting the maturation of DCs and destroying the antigen presentation of DCs.
DCs play a key role at the interface of innate and adaptive immunity. They reside in peripheral epithelial tissues in an immature state, where they serve as sentinels for the invading microorganisms. Contact with the microorganisms triggers a series of programmed events that are initiated by internalization of pathogens and a concomitant stimulation of PRRs such as TLRs, followed by the conversion of immature DCs into mature ones. This occurs together with the degradation of pathogens within the phagosome and the presentation of microbial antigens to the major histocompatibility complex (MHC) molecules. This in turn causes DCs to activate T cells by an MHC-specific manner (11). In addition to the control of the costimulatory pathway, DCs seem to contribute to T-cell activation by overcoming suppression mediated by CD4 and CD8 regulatory T cells by secretion of IL-6 (46).
Many pathogens have developed a variety of mechanisms to disarm the host defenses, such as impairing DC maturation, promoting the apoptosis of DCs, and inhibiting cytokine secretion to modify its functions. During the infection of Y. pestis, DCs are one of the early targets of T3SS effectors (52). Unlike the interaction of Y. enterocolitica and DCs, which abolishes the surface presentation of MHC class II and costimulatory molecules (79), the most pronounced effect of Y. pestis on DCs appears to be the paralysis of DCs movement by impairing the cytoskeleton rearrangement function. This effect can be destructive to the function of DCs in the presentation of Y. pestis antigens (90).
Impairing the T-cell activation.
Yersinia has the ability to influence adaptive immunity by directly suppressing T-lymphocyte activation. YopH is the first documented effector protein to inhibit the adaptive immune responses in a cell culture model (4, 94). It paralyzes T cells rapidly by inducing cells to undergo mitochondrially regulated programmed cell death but also ensures that the cells will not be recovered to induce a protective immune responses (21). The functional analysis of Y. pseudotuberculosis YopP shows that it can suppress the development of a CD8 T-cell response in a mouse infection model (88).
Unfortunately, our current understanding of the interaction of Yersinia and the immune system is mostly based on studies of Y. pseudotuberculosis and Y. enterocolitica, two species closely related to Y. pestis. Both yersinia species are enteropathogenic and are usually limited to lymphoid tissue, where they cause a chronic infection characterized by prolonged local inflation mediated by shared pCD/pYV-encoded T3SS (20). Although these species are closely related, one should be cautious in applying data from Y. pseudotuberculosis and Y. enterocolitica studies to Y. pestis because only the latter species causes systematic infection (20). For example, T3SS effector YopJ can cause apoptosis of professional phagocytes according to studies on Y. enterocolitica, but this effector is not delivered via the T3SS in Y. pestis (95). Moreover, the studies of Y. pestis and host immune system were mostly focused on T3SS proteins. Further understanding the interaction between Y. pestis and immune systems should be explored to better understand its unique pathogenesis.
HOST IMMUNE RESPONSES TO Y. PESTIS
The process of an infectious disease is a complex interaction between pathogen and host. To survive inside the host, bacteria must have mechanisms (such as the secreted T3SS proteins) to counteract the defense mechanisms possessed by the host, especially the host immune system. On the contrary, the host also utilizes its immune system to eliminate the invading bacteria. During Y. pestis infection, the host redeploys its specific humoral and cellular immunity and establishes the protective immunity. Classical humoral immune mechanisms could directly combat extracellular Y. pestis organisms and simultaneously aid cell-mediated immunity by neutralizing Y. pestis virulence factors that dampen cellular responses and delivering antibody/antigen complexes to B cells, macrophages, and/or DCs, thereby promoting T-cell activation. At the same time, classical cell-mediated immune mechanisms may aid humoral defense by eradicating intracellular Y. pestis reservoirs.
Therefore, characterization of the antimicrobial immune responses in the host will provide a wealth of information for illustrating bacterial virulence and promoting the development of specific countermeasures.
Humoral immune responses to Y. pestis.
After infection, the complex antigen structure of Y. pestis induces the production of a number of antibodies in plague patients and infected animals (Table 1). Proteomic technologies, such as protein microarray and antigenomics, represent powerful methods for discovering novel immunogens and protective antigens via profiling antibody responses to the invading pathogen (10, 59, 76). We have used an antigen microarray containing more than 140 Y. pestis virulence-associated proteins to detect the antibody responses in plague patients. Apart from F1, YopD, YopE, and pH6 antigens, which have been described previously as immunogens, we provided experimental evidence for the immunogenicity of 10 other novel proteins (YPO2090, YPO2091, YPO2102, YPO2112, YPO2118, YPO2131, YPO2190, YPMT1.12c, YPMT1.24c, and YPMT1.75c) (48).
TABLE 1.
Proteins that have been proved to be immunogenic
| Protein | Function | Reference(s) |
|---|---|---|
| LcrV | V antigen | 5,45,92 |
| YscF | Type III secretion apparatus component | 53,84 |
| YscC | Type III secretion apparatus component | 34 |
| YscJ | Type III secretion apparatus component | 37 |
| VirG | Targeting protein of the YscC complex | 37 |
| YopN | Type III membrane-bound Yop targeting protein | 8 |
| YscO | Type III secretion apparatus component | 37 |
| YscP | Type III secretion apparatus component | 37 |
| TyeA | Type III secretion and targeting protein | 37 |
| YopD | Type III targeting component | 8,13 |
| YopH | T3SS effector | 8,13 |
| YopE | T3SS effector | 8,13,43 |
| YopK | Type III virulence determinant protein | 8,13,43 |
| YopM | T3SS effector | 8,13 |
| YpkA | T3SS effector | 8 |
| OppA | Oligopeptide periplasmic binding protein | 87 |
| Pla | Coagulase/fibrinolysin precursor | 13,31 |
| PsaA | pH6 antigen | 47 |
| LPS | Lipopolysaccharides | 71 |
| YadC | Outer member protein | 57 |
| F1 | F1 capsule antigen | 92 |
The serum samples collected from plague convalescent patients can transfer passive protection to naive mice, indicating that humoral immunity plays an important role against Y. pestis challenge. Two proteins, F1 and LcrV, provide a high degree of protection, and subunit vaccines based on these proteins have demonstrated efficacy in small animal models (5, 7, 26, 44, 80). The mechanism of protection conferred by the vaccines is principally antibody mediated, and the antibody titers to F1 and LcrV in the mouse are correlated with the protection (78, 93). Besides F1 and LcrV, YopD, YpkA, YscF, YadC, and OppA are the only proteins providing partial protection against Y. pestis challenge (8, 43, 53, 57, 87).
Although the vaccines containing F1 and/or LcrV can provide protection in small animals, studies by the U.S. Army Medical Research Institute of Infectious Diseases, Fort Detrick, MD, in October 2004 found that a significant number of nonhuman primates immunized with the F1-V fusion protein vaccine succumbed to aerosol Y. pestis infection despite their possession of high-titer antibody at the time of challenge (M. L. Pitt, Animal Models and Correlates of Protection for Plague Vaccines Workshop, Gaithersburg, MD [http://www.fda.gov/cber/minutes/workshop-min.htm]). Thus, antibodies may not suffice in protection against pneumonic plague.
Cellular immune responses to Y. pestis.
More and more evidence shows that the cell-mediated immune responses also play an important role in defense against Y. pestis (32, 62, 63). Cell-mediated protection against bacteria often relies upon the development of type 1 immune responses, characterized by the expansion of pathogen-specific T cells that secrete IFN-γ and TNF-α and by the CD8+ cytotoxic T-lymphocyte response. Studies from the Trudeau Institute showed that IFN-γ, TNF-α, and nitric oxide synthase 2, key elements of cellular immunity, provided critical protective functions during humoral defense against lethal pulmonary Y. pestis infection. Vaccination with live Y. pestis (KIM5 pCD1+, pMT+, pPCP+, pgm−) primes CD4 and CD8 T cells that synergistically protect against lethal pulmonary Y. pestis infection (63, 68). Moreover, the transfer of the Y. pestis-primed T cells to naive μ-MT mice protects against lethal intranasal Y. pestis challenge. These studies confirm that cellular immunity, in the absence of antibody, can protect the animal against pulmonary Y. pestis infection (62, 63).
Thus, the development of more efficient subunit vaccines should aim at priming both humoral immunity and cellular immunity. It is not reasonable to assume the same antigens could prime both antibody- and T-cell-mediated immunity effectively. The results from the Trudeau Institute indicate that protective T cells likely recognize antigens distinct from those previously defined targets for humoral immunity and F1 is not a dominant T-cell antigen (68).
Identification of Y. pestis antigens that stimulate protective T cells would be one of the major goals for vaccine development. Advances in whole-genome sequencing, bioinformatics, and proteomics provide a unique opportunity to define T-cell antigens. Belisle and coworkers used a proteomic approach by analyzing the subcellular protein fractions of Mycobacterium tuberculosis against splenocytes of C57BL/6 mice infected with M. tuberculosis to identify the fractions that stimulate a dominant T-cell response by measuring the production of IFN-γ. A total of 17 novel T-cell antigens were identified (25). In order to recognize the T-cell antigens in Y. pestis, we used in silico computer analysis and an in vitro IFN-γ assay to identify potential T-cell antigens. In all, 34 individual proteins that stimulated a strong IFN-γ response from splenocytes of mice immunized with Y. pestis live attenuated vaccine EV76 has been identified, and their protection efficiencies are currently under evaluation (unpublished data).
PERSPECTIVES
Pathogen-host interaction is an important topic for understanding pathogenesis and hence developing effective countermeasures. Y. pestis is a notorious pathogen causing plague pandemics throughout history and is a selected agent of bioterrorism threatening public health. Understanding the complex interaction between Y. pestis and the host immune system will enable us design more effective vaccines. Both humoral and cellular immunity contribute to host defense against Y. pestis infection. The proteins F1 and LcrV have been found to be major protective antigens in protecting against bubonic plague in both mouse and nonhuman primate models. However, cellular immunity seems necessary for protection against pneumonic plague. Currently, we have limited information about cellular immunity during plague development. New technologies, including genomics, proteomics, antigenomics, bioinformatics, pharmacogenomics, and reverse vaccine methodologies, have provided us with a wealth of opportunities for developing more effective countermeasures against plague.
Acknowledgments
We thank Xinming Song, from the Veterinary Infectious Disease Organization, Saskatoon, Saskatchewan, Canada, for careful reading and revision of the full text.
Our research on plague vaccine and pathogenesis was funded by the National 863 Project (contract 2006AA02Z438), the National Natural Science Foundation of China (grant 30430620), and the National Science Fund of China for distinguished Young Scholars (contract 30525025).
Editor: J. B. Kaper
Footnotes
Published ahead of print on 4 February 2008.
REFERENCES
- 1.Aepfelbacher, M., and J. Heesemann. 2001. Modulation of Rho GTPases and the actin cytoskeleton by Yersinia outer proteins (Yops). Int. J. Med. Microbiol. 291269-276. [DOI] [PubMed] [Google Scholar]
- 2.Aepfelbacher, M., R. Zumbihl, and J. Heesemann. 2005. Modulation of Rho GTPases and the actin cytoskeleton by YopT of Yersinia. Curr. Top. Microbiol. Immunol. 291167-175. [DOI] [PubMed] [Google Scholar]
- 3.Akira, S., S. Uematsu, and O. Takeuchi. 2006. Pathogen recognition and innate immunity. Cell 124783-801. [DOI] [PubMed] [Google Scholar]
- 4.Alonso, A., N. Bottini, S. Bruckner, S. Rahmouni, S. Williams, S. P. Schoenberger, and T. Mustelin. 2004. Lck dephosphorylation at Tyr-394 and inhibition of T-cell antigen receptor signaling by Yersinia phosphatase YopH. J. Biol. Chem. 2794922-4928. [DOI] [PubMed] [Google Scholar]
- 5.Anderson, G. W., Jr., S. E. Leary, E. D. Williamson, R. W. Titball, S. L. Welkos, P. L. Worsham, and A. M. Friedlander. 1996. Recombinant V antigen protects mice against pneumonic and bubonic plague caused by F1-capsule-positive and -negative strains of Yersinia pestis. Infect. Immun. 644580-4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andersson, K., N. Carballeira, K. E. Magnusson, C. Persson, O. Stendahl, H. Wolf-Watz, and M. Fallman. 1996. YopH of Yersinia pseudotuberculosis interrupts early phosphotyrosine signaling associated with phagocytosis. Mol. Microbiol. 201057-1069. [DOI] [PubMed] [Google Scholar]
- 7.Andrews, G. P., D. G. Heath, G. W. Anderson, Jr., S. L. Welkos, and A. M. Friedlander. 1996. Fraction 1 capsular antigen (F1) purification from Yersinia pestis CO92 and from an Escherichia coli recombinant strain and efficacy against lethal plague challenge. Infect. Immun. 642180-2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Andrews, G. P., S. T. Strachan, G. E. Benner, A. K. Sample, G. W. Anderson, Jr., J. J. Adamovicz, S. L. Welkos, J. K. Pullen, and A. M. Friedlander. 1999. Protective efficacy of recombinant Yersinia outer proteins against bubonic plague caused by encapsulated and nonencapsulated Yersinia pestis. Infect. Immun. 671533-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anisimov, A. P., S. V. Dentovskaya, G. M. Titareva, I. V. Bakhteeva, R. Z. Shaikhutdinova, S. V. Balakhonov, B. Lindner, N. A. Kocharova, S. N. Senchenkova, O. Holst, G. B. Pier, and Y. A. Knirel. 2005. Intraspecies and temperature-dependent variations in susceptibility of Yersinia pestis to the bactericidal action of serum and to polymyxin B. Infect. Immun. 737324-7331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bacarese-Hamilton, T., A. Ardizzoni, J. Gray, and A. Crisanti. 2004. Protein arrays for serodiagnosis of disease. Methods Mol. Biol. 264271-283. [DOI] [PubMed] [Google Scholar]
- 11.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392245-252. [DOI] [PubMed] [Google Scholar]
- 12.Bartra, S. S., K. L. Styer, D. M. O'Bryant, M. L. Nilles, B. J. Hinnebusch, A. Aballay, and G. V. Plano. 2008. Resistance of Yersinia pestis to complement-dependent killing is mediated by the Ail outer membrane protein. Infect. Immun. 76612-622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benner, G. E., G. P. Andrews, W. R. Byrne, S. D. Strachan, A. K. Sample, D. G. Heath, and A. M. Friedlander. 1999. Immune response to Yersinia outer proteins and other Yersinia pestis antigens after experimental plague infection in mice. Infect. Immun. 671922-1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Biedzka-Sarek, M., R. Venho, and M. Skurnik. 2005. Role of YadA, Ail, and lipopolysaccharide in serum resistance of Yersinia enterocolitica serotype O:3. Infect. Immun. 732232-2244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bliska, J. B., and S. Falkow. 1992. Bacterial resistance to complement killing mediated by the Ail protein of Yersinia enterocolitica. Proc. Natl. Acad. Sci. USA 893561-3565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bohn, E., S. Muller, J. Lauber, R. Geffers, N. Speer, C. Spieth, J. Krejci, B. Manncke, J. Buer, A. Zell, and I. B. Autenrieth. 2004. Gene expression patterns of epithelial cells modulated by pathogenicity factors of Yersinia enterocolitica. Cell Microbiol. 6129-141. [DOI] [PubMed] [Google Scholar]
- 17.Boland, A., and G. R. Cornelis. 1998. Role of YopP in suppression of tumor necrosis factor alpha release by macrophages during Yersinia infection. Infect. Immun. 661878-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bossi, P., and F. Bricaire. 2003. The plague, possible bioterrorist act. Presse Med. 32804-807. (In French.) [PubMed] [Google Scholar]
- 19.Broussard, L. A. 2001. Biological agents: weapons of warfare and bioterrorism. Mol. Diagn. 6323-333. [DOI] [PubMed] [Google Scholar]
- 20.Brubaker, R. R. 2007. How the structural gene products of Yersinia pestis relate to virulence. Future Microbiol. 2377-385. [DOI] [PubMed] [Google Scholar]
- 21.Bruckner, S., S. Rhamouni, L. Tautz, J. B. Denault, A. Alonso, B. Becattini, G. S. Salvesen, and T. Mustelin. 2005. Yersinia phosphatase induces mitochondrially dependent apoptosis of T cells. J. Biol. Chem. 28010388-10394. [DOI] [PubMed] [Google Scholar]
- 22.Cornelis, G. R. 2002. Yersinia type III secretion: send in the effectors. J. Cell Biol. 158401-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cornelis, G. R. 2002. The Yersinia Ysc-Yop virulence apparatus. Int. J. Med. Microbiol. 291455-462. [DOI] [PubMed] [Google Scholar]
- 24.Cornelis, G. R., and H. Wolf-Watz. 1997. The Yersinia Yop virulon: a bacterial system for subverting eukaryotic cells. Mol. Microbiol. 23861-867. [DOI] [PubMed] [Google Scholar]
- 25.Covert, B. A., J. S. Spencer, I. M. Orme, and J. T. Belisle. 2001. The application of proteomics in defining the T-cell antigens of Mycobacterium tuberculosis. Proteomics 1574-586. [DOI] [PubMed] [Google Scholar]
- 26.DeBord, K. L., D. M. Anderson, M. M. Marketon, K. A. Overheim, R. W. DePaolo, N. A. Ciletti, B. Jabri, and O. Schneewind. 2006. Immunogenicity and protective immunity against bubonic plague and pneumonic plague by immunization of mice with the recombinant V10 antigen, a variant of LcrV. Infect. Immun. 744910-4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.DeLeo, F. R., and B. J. Hinnebusch. 2005. A plague upon the phagocytes. Nat. Med. 11927-928. [DOI] [PubMed] [Google Scholar]
- 28.Denecker, G., S. Totemeyer, L. J. Mota, P. Troisfontaines, I. Lambermont, C. Youta, I. Stainier, M. Ackermann, and G. R. Cornelis. 2002. Effect of low- and high-virulence Yersinia enterocolitica strains on the inflammatory response of human umbilical vein endothelial cells. Infect. Immun. 703510-3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du, Y., R. Rosqvist, and A. Forsberg. 2002. Role of fraction 1 antigen of Yersinia pestis in inhibition of phagocytosis. Infect. Immun. 701453-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dziarski, R. 2006. Deadly plague versus mild-mannered TLR4. Nat. Immunol. 71017-1019. [DOI] [PubMed] [Google Scholar]
- 31.Easterbrook, T. J., K. Reddin, A. Robinson, and N. Modi. 1995. Studies on the immunogenicity of the Pla protein from Yersinia pestis. Contrib. Microbiol. Immunol. 13214-215. [PubMed] [Google Scholar]
- 32.Elvin, S. J., and E. D. Williamson. 2004. Stat 4 but not Stat 6 mediated immune mechanisms are essential in protection against plague. Microb. Pathog. 37177-184. [DOI] [PubMed] [Google Scholar]
- 33.Elvin, S. J., E. D. Williamson, J. C. Scott, J. N. Smith, G. Perez De Lema, S. Chilla, P. Clapham, K. Pfeffer, D. Schlondorff, and B. Luckow. 2004. Evolutionary genetics: ambiguous role of CCR5 in Yersinia pestis infection. Nature 430417. [DOI] [PubMed] [Google Scholar]
- 34.Goodin, J. L., R. W. Raab, R. L. McKown, G. L. Coffman, B. S. Powell, J. T. Enama, J. A. Ligon, and G. P. Andrews. 2005. Yersinia pestis outer membrane type III secretion protein YscC: expression, purification, characterization, and induction of specific antiserum. Protein Expr. Purif. 40152-163. [DOI] [PubMed] [Google Scholar]
- 35.Green, S. P., E. L. Hartland, R. M. Robins-Browne, and W. A. Phillips. 1995. Role of YopH in the suppression of tyrosine phosphorylation and respiratory burst activity in murine macrophages infected with Yersinia enterocolitica. J. Leukoc. Biol. 57972-977. [DOI] [PubMed] [Google Scholar]
- 36.Grosdent, N., I. Maridonneau-Parini, M. P. Sory, and G. R. Cornelis. 2002. Role of Yops and adhesins in resistance of Yersinia enterocolitica to phagocytosis. Infect. Immun. 704165-4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hill, J., C. D. Underwood, L. Sundberg, H. Astrom, S. E. Leary, A. Forsberg, and R. W. Titball. 2003. Immunological characterisation of sub-units of the Yersinia type III secretion apparatus. Adv. Exp. Med. Biol. 529415-417. [DOI] [PubMed] [Google Scholar]
- 38.Huang, X., and L. E. Lindler. 2004. The pH 6 antigen is an antiphagocytic factor produced by Yersinia pestis independent of Yersinia outer proteins and capsule antigen. Infect. Immun. 727212-7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iriarte, M., and G. R. Cornelis. 1998. YopT, a new Yersinia Yop effector protein, affects the cytoskeleton of host cells. Mol. Microbiol. 29915-929. [DOI] [PubMed] [Google Scholar]
- 40.Juris, S. J., F. Shao, and J. E. Dixon. 2002. Yersinia effectors target mammalian signalling pathways. Cell Microbiol. 4201-211. [DOI] [PubMed] [Google Scholar]
- 41.Kerschen, E. J., D. A. Cohen, A. M. Kaplan, and S. C. Straley. 2004. The plague virulence protein YopM targets the innate immune response by causing a global depletion of NK cells. Infect. Immun. 724589-4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lawton, D. G., C. Longstaff, B. A. Wallace, J. Hill, S. E. Leary, R. W. Titball, and K. A. Brown. 2002. Interactions of the type III secretion pathway proteins LcrV and LcrG from Yersinia pestis are mediated by coiled-coil domains. J. Biol. Chem. 27738714-38722. [DOI] [PubMed] [Google Scholar]
- 43.Leary, S. E., K. F. Griffin, E. E. Galyov, J. Hewer, E. D. Williamson, A. Holmstrom, Forsberg, and R. W. Titball. 1999. Yersinia outer proteins (YOPS) E, K and N are antigenic but non-protective compared to V antigen, in a murine model of bubonic plague. Microb. Pathog. 26159-169. [DOI] [PubMed] [Google Scholar]
- 44.Leary, S. E., K. F. Griffin, H. S. Garmory, E. D. Williamson, and R. W. Titball. 1997. Expression of an F1/V fusion protein in attenuated Salmonella typhimurium and protection of mice against plague. Microb. Pathog. 23167-179. [DOI] [PubMed] [Google Scholar]
- 45.Leary, S. E., E. D. Williamson, K. F. Griffin, P. Russell, S. M. Eley, and R. W. Titball. 1995. Active immunization with recombinant V antigen from Yersinia pestis protects mice against plague. Infect. Immun. 632854-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee, H. K., and A. Iwasaki. 2007. Innate control of adaptive immunity: dendritic cells and beyond. Semin. Immunol. 1948-55. [DOI] [PubMed] [Google Scholar]
- 47.Li, B., L. Jiang, Q. Song, J. Yang, Z. Chen, Z. Guo, D. Zhou, Z. Du, Y. Song, J. Wang, H. Wang, S. Yu, and R. Yang. 2005. Protein microarray for profiling antibody responses to Yersinia pestis live vaccine. Infect. Immun. 733734-3739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li, B., D. Zhou, Z. Wang, Z. Song, H. Wang, M. Li, and R. Yang. 2008. Antibody profiling in plague patients by protein microarray. Microbes Infect. 1045-51. [DOI] [PubMed] [Google Scholar]
- 49.Lindler, L. E., and B. D. Tall. 1993. Yersinia pestis pH 6 antigen forms fimbriae and is induced by intracellular association with macrophages. Mol. Microbiol. 8311-324. [DOI] [PubMed] [Google Scholar]
- 50.Lukaszewski, R. A., D. J. Kenny, R. Taylor, D. G. Rees, M. G. Hartley, and P. C. Oyston. 2005. Pathogenesis of Yersinia pestis infection in BALB/c mice: effects on host macrophages and neutrophils. Infect. Immun. 737142-7150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Makoveichuk, E., P. Cherepanov, S. Lundberg, A. Forsberg, and G. Olivecrona. 2003. pH6 antigen of Yersinia pestis interacts with plasma lipoproteins and cell membranes. J. Lipid Res. 44320-330. [DOI] [PubMed] [Google Scholar]
- 52.Marketon, M. M., R. W. DePaolo, K. L. DeBord, B. Jabri, and O. Schneewind. 2005. Plague bacteria target immune cells during infection. Science 3091739-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Matson, J. S., K. A. Durick, D. S. Bradley, and M. L. Nilles. 2005. Immunization of mice with YscF provides protection from Yersinia pestis infections. BMC Microbiol. 538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McDonald, C., P. O. Vacratsis, J. B. Bliska, and J. E. Dixon. 2003. The yersinia virulence factor YopM forms a novel protein complex with two cellular kinases. J. Biol. Chem. 27818514-18523. [DOI] [PubMed] [Google Scholar]
- 55.Miller, S. I., R. K. Ernst, and M. W. Bader. 2005. LPS, TLR4 and infectious disease diversity. Nat. Rev. Microbiol. 336-46. [DOI] [PubMed] [Google Scholar]
- 56.Montminy, S. W., N. Khan, S. McGrath, M. J. Walkowicz, F. Sharp, J. E. Conlon, K. Fukase, S. Kusumoto, C. Sweet, K. Miyake, S. Akira, R. J. Cotter, J. D. Goguen, and E. Lien. 2006. Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat. Immunol. 71066-1073. [DOI] [PubMed] [Google Scholar]
- 57.Murphy, B. S., C. R. Wulff, B. A. Garvy, and S. C. Straley. 2007. Yersinia pestis YadC: a novel vaccine candidate against plague. Adv. Exp. Med. Biol. 603400-414. [DOI] [PubMed] [Google Scholar]
- 58.Navarro, L., N. M. Alto, and J. E. Dixon. 2005. Functions of the Yersinia effector proteins in inhibiting host immune responses. Curr. Opin. Microbiol. 821-27. [DOI] [PubMed] [Google Scholar]
- 59.Neuman de Vegvar, H. E., and W. H. Robinson. 2004. Microarray profiling of antiviral antibodies for the development of diagnostics, vaccines, and therapeutics. Clin. Immunol. 111196-201. [DOI] [PubMed] [Google Scholar]
- 60.Overheim, K. A., R. W. Depaolo, K. L. Debord, E. M. Morrin, D. M. Anderson, N. M. Green, R. R. Brubaker, B. Jabri, and O. Schneewind. 2005. LcrV plague vaccine with altered immunomodulatory properties. Infect. Immun. 735152-5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oyston, P. C., N. Dorrell, K. Williams, S. R. Li, M. Green, R. W. Titball, and B. W. Wren. 2000. The response regulator PhoP is important for survival under conditions of macrophage-induced stress and virulence in Yersinia pestis. Infect. Immun. 683419-3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parent, M. A., K. N. Berggren, L. W. Kummer, L. B. Wilhelm, F. M. Szaba, I. K. Mullarky, and S. T. Smiley. 2005. Cell-mediated protection against pulmonary Yersinia pestis infection. Infect. Immun. 737304-7310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Parent, M. A., L. B. Wilhelm, L. W. Kummer, F. M. Szaba, I. K. Mullarky, and S. T. Smiley. 2006. Gamma interferon, tumor necrosis factor alpha, and nitric oxide synthase 2, key elements of cellular immunity, perform critical protective functions during humoral defense against lethal pulmonary Yersinia pestis infection. Infect. Immun. 743381-3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pasare, C., and R. Medzhitov. 2005. Toll-like receptors: linking innate and adaptive immunity. Adv. Exp. Med. Biol. 56011-18. [DOI] [PubMed] [Google Scholar]
- 65.Perry, R. D., and R. R. Brubaker. 1983. Vwa+ phenotype of Yersinia enterocolitica. Infect. Immun. 40166-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perry, R. D., and J. D. Fetherston. 1997. Yersinia pestis: etiologic agent of plague. Clin. Microbiol. Rev. 1035-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pettersson, J., A. Holmstrom, J. Hill, S. Leary, E. Frithz-Lindsten, A. von Euler-Matell, E. Carlsson, R. Titball, A. Forsberg, and H. Wolf-Watz. 1999. The V-antigen of Yersinia is surface exposed before target cell contact and involved in virulence protein translocation. Mol. Microbiol. 32961-976. [DOI] [PubMed] [Google Scholar]
- 68.Philipovskiy, A. V., and S. T. Smiley. 2007. Vaccination with live Yersinia pestis primes CD4 and CD8 T cells that synergistically protect against lethal pulmonary Y. pestis infection. Infect. Immun. 75878-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pouliot, K., N. Pan, S. Wang, S. Lu, E. Lien, and J. D. Goguen. 2007. Evaluation of the role of LcrV-Toll-like receptor 2-mediated immunomodulation in the virulence of Yersinia pestis. Infect. Immun. 753571-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Price, S. B., M. D. Freeman, and K. S. Yeh. 1995. Transcriptional analysis of the Yersinia pestis pH 6 antigen gene. J. Bacteriol. 1775997-6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Prior, J. L., P. G. Hitchen, D. E. Williamson, A. J. Reason, H. R. Morris, A. Dell, B. W. Wren, and R. W. Titball. 2001. Characterization of the lipopolysaccharide of Yersinia pestis. Microb. Pathog. 3049-57. [DOI] [PubMed] [Google Scholar]
- 72.Pujol, C., and J. B. Bliska. 2003. The ability to replicate in macrophages is conserved between Yersinia pestis and Yersinia pseudotuberculosis. Infect. Immun. 715892-5899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pujol, C., and J. B. Bliska. 2005. Turning Yersinia pathogenesis outside in: subversion of macrophage function by intracellular yersiniae. Clin. Immunol. 114216-226. [DOI] [PubMed] [Google Scholar]
- 74.Rebeil, R., R. K. Ernst, C. O. Jarrett, K. N. Adams, S. I. Miller, and B. J. Hinnebusch. 2006. Characterization of late acyltransferase genes of Yersinia pestis and their role in temperature-dependent lipid A variation. J. Bacteriol. 1881381-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reithmeier-Rost, D., J. Hill, S. J. Elvin, D. Williamson, S. Dittmann, A. Schmid, G. Wilharm, and A. Sing. 2007. The weak interaction of LcrV and TLR2 does not contribute to the virulence of Yersinia pestis. Microbes Infect. 9997-1002. [DOI] [PubMed] [Google Scholar]
- 76.Robinson, W. H. 2006. Antigen arrays for antibody profiling. Curr. Opin. Chem. Biol. 1067-72. [DOI] [PubMed] [Google Scholar]
- 77.Rosqvist, R., A. Forsberg, M. Rimpilainen, T. Bergman, and H. Wolf-Watz. 1990. The cytotoxic protein YopE of Yersinia obstructs the primary host defense. Mol. Microbiol. 4657-667. [DOI] [PubMed] [Google Scholar]
- 78.Sabhnani, L., M. Manocha, D. Tomar, D. Shashikiran, and D. N. Rao. 2003. Yersinia pestis F1 antigen: a correlation between antibody titres and subclass distribution with differential avidity in different inbred mouse strains. Int. Immunopharmacol. 31413-1418. [DOI] [PubMed] [Google Scholar]
- 79.Schoppet, M., A. Bubert, and H. I. Huppertz. 2000. Dendritic cell function is perturbed by Yersinia enterocolitica infection in vitro. Clin. Exp. Immunol. 122316-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Simpson, W. J., R. E. Thomas, and T. G. Schwan. 1990. Recombinant capsular antigen (fraction 1) from Yersinia pestis induces a protective antibody response in BALB/c mice. Am. J. Trop. Med. Hyg. 43389-396. [DOI] [PubMed] [Google Scholar]
- 81.Sing, A., D. Rost, N. Tvardovskaia, A. Roggenkamp, A. Wiedemann, C. J. Kirschning, M. Aepfelbacher, and J. Heesemann. 2002. Yersinia V-antigen exploits Toll-like receptor 2 and CD14 for interleukin 10-mediated immunosuppression. J. Exp. Med. 1961017-1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sodhi, A., R. K. Sharma, and H. V. Batra. 2005. Yersinia rLcrV and rYopB inhibits the activation of murine peritoneal macrophages in vitro. Immunol. Lett. 99146-152. [DOI] [PubMed] [Google Scholar]
- 83.Straley, S. C., and P. A. Harmon. 1984. Yersinia pestis grows within phagolysosomes in mouse peritoneal macrophages. Infect. Immun. 45655-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Swietnicki, W., B. S. Powell, and J. Goodin. 2005. Yersinia pestis Yop secretion protein F: purification, characterization, and protective efficacy against bubonic plague. Protein Expr. Purif. 42166-172. [DOI] [PubMed] [Google Scholar]
- 85.Takahashi, K., and T. Kawai. 2007. Pathogen recognition by Toll-like receptor. Nippon Rinsho. 65(Suppl. 2, Pt. 1)53-57. (In Japanese.) [PubMed] [Google Scholar]
- 86.Tan, L., and C. Darby. 2005. Yersinia pestis is viable with endotoxin composed of only lipid A. J. Bacteriol. 1876599-6600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tanabe, M., H. S. Atkins, D. N. Harland, S. J. Elvin, A. J. Stagg, O. Mirza, R. W. Titball, B. Byrne, and K. A. Brown. 2006. The ABC transporter protein OppA provides protection against experimental Yersinia pestis infection. Infect. Immun. 743687-3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Trulzsch, K., G. Geginat, T. Sporleder, K. Ruckdeschel, R. Hoffmann, J. Heesemann, and H. Russmann. 2005. Yersinia outer protein P inhibits CD8 T-cell priming in the mouse infection model. J. Immunol. 1744244-4251. [DOI] [PubMed] [Google Scholar]
- 89.Une, T., and R. R. Brubaker. 1984. In vivo comparison of avirulent Vwa- and Pgm- or Pstr phenotypes of yersiniae. Infect. Immun. 43895-900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Velan, B., E. Bar-Haim, A. Zauberman, E. Mamroud, A. Shafferman, and S. Cohen. 2006. Discordance in the effects of Yersinia pestis on the dendritic cell functions manifested by induction of maturation and paralysis of migration. Infect. Immun. 746365-6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Welkos, S., A. Friedlander, D. McDowell, J. Weeks, and S. Tobery. 1998. V antigen of Yersinia pestis inhibits neutrophil chemotaxis. Microb. Pathog. 24185-196. [DOI] [PubMed] [Google Scholar]
- 92.Williamson, E. D., S. M. Eley, K. F. Griffin, M. Green, P. Russell, S. E. Leary, P. C. Oyston, T. Easterbrook, K. M. Reddin, and A. Robinson. 1995. A new improved sub-unit vaccine for plague: the basis of protection. FEMS Immunol. Med. Microbiol. 12223-230. [DOI] [PubMed] [Google Scholar]
- 93.Williamson, E. D., P. M. Vesey, K. J. Gillhespy, S. M. Eley, M. Green, and R. W. Titball. 1999. An IgG1 titre to the F1 and V antigens correlates with protection against plague in the mouse model. Clin. Exp. Immunol. 116107-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yao, T., J. Mecsas, J. I. Healy, S. Falkow, and Y. Chien. 1999. Suppression of T and B lymphocyte activation by a Yersinia pseudotuberculosis virulence factor, yopH. J. Exp. Med. 1901343-1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zauberman, A., S. Cohen, E. Mamroud, Y. Flashner, A. Tidhar, R. Ber, E. Elhanany, A. Shafferman, and B. Velan. 2006. Interaction of Yersinia pestis with macrophages: limitations in YopJ-dependent apoptosis. Infect. Immun. 743239-3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhou, D., Y. Han, and R. Yang. 2006. Molecular and physiological insights into plague transmission, virulence, and etiology. Microbes Infect. 8273-284. [DOI] [PubMed] [Google Scholar]
- 97.Zhou, D., Z. Tong, Y. Song, Y. Han, D. Pei, X. Pang, J. Zhai, M. Li, B. Cui, Z. Qi, L. Jin, R. Dai, Z. Du, J. Wang, Z. Guo, P. Huang, and R. Yang. 2004. Genetics of metabolic variations between Yersinia pestis biovars and the proposal of a new biovar, microtus. J. Bacteriol. 1865147-5152. [DOI] [PMC free article] [PubMed] [Google Scholar]