Abstract
The salient feature of dendritic cells (DC) is the initiation of appropriate adaptive immune responses by discriminating between pathogens. Using a prototypic model of intracellular infection, we previously showed that Leishmania major parasites prime human DC for efficient interleukin-12 (IL-12) secretion. L. major infection is associated with self-limiting cutaneous disease and powerful immunity. In stark contrast, the causative agent of visceral leishmaniasis, Leishmania donovani, does not prime human DC for IL-12 production. Here, we report that DC priming by L. major infection results in the early activation of NF-κB transcription factors and the up-regulation and nuclear translocation of interferon regulatory factor 1 (IRF-1) and IRF-8. The inhibition of NF-κB activation by the pretreatment of DC with caffeic acid phenethyl ester blocks L. major-induced IRF-1 and IRF-8 activation and IL-12 expression. We further demonstrate that IRF-1 and IRF-8 obtained from L. major-infected human DC specifically bind to their consensus binding sites on the IL-12p35 promoter, indicating that L. major infection either directly stimulates a signaling cascade or induces an autocrine pathway that activates IRF-1 and IRF-8, ultimately resulting in IL-12 transcription.
Leishmania major is the causative agent of cutaneous leishmaniasis, which is characterized by the development of lesions at sand fly bite sites. These cutaneous lesions ulcerate, resolve, and ultimately stimulate powerful immunity against the disease. Robust induction of this immunity is the basis of leishmanization, an effective vaccination procedure in which the inoculation of live L. major has been used with great success (12); safety concerns, however, have led to the abandonment of such vaccination (17). In contrast to L. major, Leishmania donovani causes visceral leishmaniasis, a severe systemic illness that is often fatal if untreated.
Healing of cutaneous leishmaniasis has been attributed to the development of a strong Th1 immune response in the vertebrate host. Interleukin-12 (IL-12) is up-regulated in L. major-infected human dendritic cells (DC), whereas visceral leishmaniasis agents do not induce IL-12 production (28). Those previous studies revealed that L. donovani does not actively inhibit IL-12 production; rather, L. major primes human DC for IL-12 production. This strong induction of IL-12 by L. major-infected DC sets the stage for a strong and robust Th1 immune response that leads to lesion healing and immunity against the disease. Elucidation of the mechanisms that mediate the strong immunity elicited by L. major will undoubtedly have consequences for vaccine development against all Leishmania species as well as other infections, where strong cell-mediated immune responses are essential for resistance.
IL-12 belongs to a family of cytokines including IL-23, IL-27, and ciliary neutrotrophic factor receptor (CNTFR). The bioactive form of this proinflammatory cytokine is a unique heterodimeric protein composed of p35 and p40 subunits that are encoded by independent genes located on separate chromosomes (40, 45). The induction and secretion of bioactive IL-12 (IL-12p70) are regulated by the independent activation of p35 and p40 subunits. The IL-12p40 subunit can be secreted as homodimers or monomers and is tightly regulated by IL-12p40 gene expression in activated DC and other hematopoietic phagocytes (macrophages, monocytes, and neutrophils) (49).
The secretion of free IL-12p40 and IL-12p70 by activated macrophages and DC is dependent on the regulation of the IL-12p40 gene by an array of transcription factors including CAAT/enhancer binding protein (C/EBP) (39), Rel proteins (39), interferon regulatory factor 1 (IRF-1) (21), and gamma interferon (IFN-γ) consensus sequence binding protein, also known as IRF-8 (52). Although IL-12p70 secretion is specifically dependent on IL-12p40 secretion, recent studies indicate that IL-12p35 expression is also tightly regulated, and hence, it plays an important role in the regulation of IL-12p70 expression. The IL-12p35 subunit is regulated at both the transcriptional and translational levels (43, 48, 57). Putative common transcription factors between murine and human IL-12p35 promoters include activator protein 2 (AP-2), Sp-1, C/EBP, CREB (CRE binding protein), AP-1, Rel proteins, IRF-1, and IRF-8 (18, 21). IRF-1 has been shown to actively regulate IL-12p35 induction (22), while the activities of the other transcription factors remain to be elucidated.
Quantitative reverse transcription (RT)-PCR analysis of IL-12p35 and IL-12p40 expression revealed a 10-fold increase in the message level of IL-12p35 and IL-12p40 due to L. major as opposed to L. donovani infection (28). These data suggest that the L. major-specific up-regulation of IL-12 production is dependent on the regulation of both the IL-12p35 and IL-12p40 genes. The IL-12p35 and IL-12p40 promoters share DNA elements and likely attract a number of common transcription factors including IRF-1, IRF-8, c-Rel, and NF-κBp50, suggesting a specific role for these factors in the activation of both subunits in response to L. major infection. Here, we delineate the upstream molecular events of IL-12p70 induction in human DC infected with L. major and demonstrate that NF-κB and IRF transcription factors play a specific role in the induction of IL-12 in L. major-infected DC.
MATERIALS AND METHODS
DC generation.
CD14+ monocytes were isolated from peripheral blood mononuclear cells of healthy human donors (Central Indiana Regional Blood Center, Indianapolis, IN) by positive selection (AutoMACS separator; Miltenyi Biotec, Auburn, CA). The purity of separated monocytes was determined by flow cytometry analysis of CD14, CD19, CD45, and CD3 expression. Enriched monocytes were treated with granulocyte-macrophage colony-stimulating factor (1,000 U/ml) and IL-4 (400 U/ml) on days 0, 3, and 6 after plating in six-well plates at 2.5 × 106 cells/ml in complete RPMI medium (10% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine). Immature DC were harvested on day 7 and plated in 48-well plates at 2 × 105 cells/300 μl per well in the absence of exogenous cytokine. The phenotypes of DC were determined similarly by flow cytometry analysis of CD1a, CD14, HLA-DR, CD40, CD80, and CD86 expression. Antibody-stained cells were evaluated using an MCL500 flow cytometer (Beckman Coulter, Inc., Fullerton, CA).
Parasites and infection.
All infections were performed with L. major NIH Friedlin V1 (MHOM/IL/80/FN), isolated from a patient with localized cutaneous leishmaniasis in Israel, and L. donovani strain 9515 (MHOM/IN/95/9515), isolated from a splenic aspirate of a patient with visceral leishmaniasis in India. Infective-stage metacyclic promastigotes were isolated by use of a Ficoll gradient (47) and opsonized with 5% normal human serum prior to infection at a concentration of five parasites to one dendritic cell. Infection rates were determined at the end of each experiment by DiffQuick staining of cytospins and visualization by light microscopy. In all cases, there were no significant differences between infecting species. All parasite strains were cultured at 26°C without CO2 in medium 199 containing 20% heat-inactivated fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 2 mM l-glutamine,40 mM HEPES, 0.1 mM adenine (in 50 mM HEPES), 5 mg/ml hemin (in 50% triethanolamine), and 1 mg/ml biotin. Parasites tested negative for mycoplasma using a Mycoplasma PCR detection set (Takara Bio, Inc., Madison, WI) and tested below the detection limits for endotoxin using a Limulus Amoebocyte Lysate kit (Charles River Endosafe, NC).
Western blot analysis.
Nuclear extracts were obtained from L. major- and L. donovani-infected DC using NE-PER nuclear and cytoplasmic extraction reagents (Pierce Biotechnology, Rockford, IL). Twenty to thirty micrograms of nuclear extract was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis using the Novex system (Invitrogen Life Technologies, Carlsbad, CA). After transfer onto a polyvinylidene difluoride membrane (Millipore, Billerica, MA), blots were blocked in 1% bovine serum albumin in phosphate-buffered saline for 1 h at room temperature. Primary antibodies diluted in 1% bovine serum albumin in phosphate-buffered saline were used at concentrations suggested by the manufacturer. Blots were extensively washed before the addition of secondary antibody (BD Biosciences, Franklin Lakes, NJ). Proteins were detected using Supersignal West stable peroxide solution (Pierce Endogen, Rockford, IL), followed by exposure to X-ray film. Densitometry analysis was performed with a Bio-Rad GS-800 densitometer and QuantityOne software. The optical density of the transcription factors was normalized to lamin A/C from each condition.
EMSA.
Electrophoretic mobility shift assays (EMSAs) were performed using IRF-1 (24) and IRF-8 (10) consensus probes. The minimal inducible promoter region carrying the consensus regions 5′-TGAAATTCCC-3′ was labeled with [32P]ATP and mixed with nuclear extracts of Leishmania-infected human DC. The complexes were fractionated through 4% polyacrylamide gels, and the shifted complexes were detected by autoradiography.
ChIP analysis.
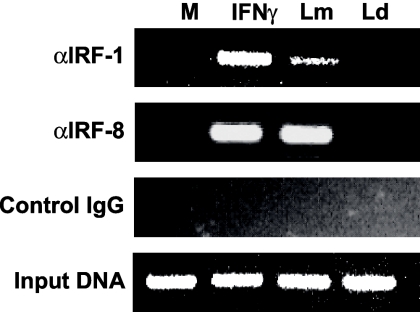
Chromatin immunoprecipitation (ChIP) was performed using an assay kit according to the manufacturer's instructions (Upstate Biotechnology, Charlottesville, VA). Briefly, 106 DC were infected with L. major or L. donovani and then cross-linked with 1% formaldehyde for 10 min at 37°C. Cross-linked chromatin was sheared by sonication to obtain DNA fragments ranging from 200 to 1,000 bp. Sheared chromatin was precleared by protein A-agarose beads, followed by overnight immunoprecipitation with 2 μg anti-IRF-1, anti-IRF-8, or isotype control antibody at 4°C. Cross-linking was reversed by heating at 65°C for 4 h, followed by proteinase K digestion and ethanol-chloroform purification of immunoprecipitated DNA. Input and purified immunoprecipitated DNAs were subjected to PCR using primers encompassing the IRF-1 site in the human IL-12p35 promoter (5′ primer GCGAACATTTCGCTTTCATT and 3′ primer ACTTTCCCGGGACTCTGGT). Products were amplified for 34 cycles using GoTaq (Promega Corp., Madison, WI) and analyzed on a 2% agarose gel.
Cytokines and antibodies.
The following antibodies were used for flow cytometry: anti-CD14-phycoerythrin (PE) (clone M5E2), immunoglobulin G2a(κ) [IgG2a(κ)]-PE (clone MOPC-173), anti-CD1a-PE (clone HI149), IgG1(κ)-PE (clone MOPC-2), anti-HLA-DR-PE (clone L243), IgG2b(κ)-PE (clone MPC-11), anti-CD40-PE (clone HB14), anti-CD80-PE (clone 2D10), and anti-CD86-PE (clone IT2.2), purchased from BioLegend, Inc. (San Diego, CA), and anti-CD19-PE (clone J4.119), IgG1-PE (clone 679.1Mc7), anti-CD3-fluorescein isothiocyanate (clone UCHT.1), and anti-CD45-PE-Cy5 (clone Immu19.2), purchased from Beckman Coulter, Inc. (Fullerton, CA). The following antibodies were used for Western blotting: anti-lamin A/C (clone N-18), anti-IRF-1 (clone C-20), anti-IRF-8 (clone H-70), anti-NF-κBp50 (clone E-10), anti-NF-κBp65 (clone F6), and anti-c-Rel (clone N), purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA), and anti-pSTAT1 (polyclonal) and anti-tSTAT1 (polyclonal), purchased from Cell Signaling Technology, Inc. (Danvers, MA). Horseradish peroxidase-conjugated secondary antibodies were purchased from BD Biosciences (Franklin Lakes, NJ). Recombinant human granulocyte-macrophage colony-stimulating factor (specific activity of ≥1 × 107 units/mg), IL-4 (specific activity of ≥5 × 106 units/mg), and IFN-γ (specific activity of ≥2 × 107 units/mg) were purchased from Peprotech, Inc. (Rocky Hill, NJ).
Real-time quantitative RT-PCR (Taqman).
Relative levels of IL-12p35, IL-12p40, and IL-23p19 mRNA were determined by real-time PCR. Total mRNA was prepared with the RNeasy Mini kit (Qiagen, Inc., Valencia, CA). One microgram of total mRNA was reverse transcribed using random primers with the Superscript III synthesis system for RT-PCR (Invitrogen, Carlsbad, CA) according to the manufacturer's instructions. For analysis of IL-12p35, IL-12p40, IL-23p19, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene mRNA expression, Taqman predeveloped assay kits were purchased from Applied Biosystems (Foster City, CA). Taqman reactions were performed according to the manufacturer's recommendations by using an ABI Prism 7500 sequence detection system (Perkin-Elmer, Foster City, CA). The relative number of mRNA copies for p35, p40, and p19 were determined using the comparative method by following formula: number of copies = 2−ΔΔCT, where ΔΔCT equals ΔCT(experimental) − ΔCT(calibrator), ΔCT equals CT(experimental) − CT(GAPDH), CT is the cycle number where a statistically significant increase in the emission intensity over the background is detected, and ΔCT(calibrator) equals the mean ΔCT for the uninfected and unstimulated control from each donor.
Statistical analyses.
A paired Student's t test on raw (quantitative RT-PCR) or log10-transformed (Western blot) data was utilized to determine statistical differences between experimental groups.
RESULTS
Transcriptional induction of IL-12p35 and IL-12p40 in L. major-infected DC.
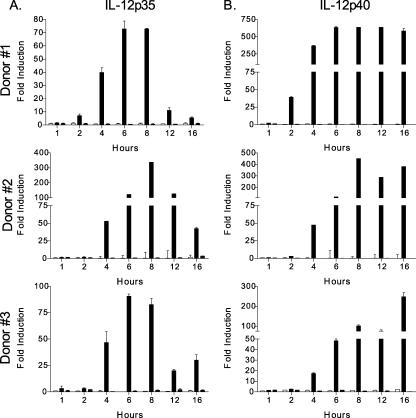
Although to a lesser extent than in the presence of CD40L, both IL-12p35 and IL-12p40 mRNAs are induced in DC after 8 h of L. major infection in the absence of CD40L (28). In order to determine the kinetics of this transcriptional induction, DC were infected with L. major and L. donovani for 1, 2, 4, 6, 8, 12, and 16 h, followed by the isolation of total RNA at these time points and quantification of IL-12 (IL-12p35 and IL-12p40) by real-time quantitative RT-PCR. The levels of IL-12p35 (Fig. 1A) and IL-12p40 (Fig. 1B) were significantly up-regulated in DC infected with L. major by 4 h, as opposed to L. donovani-infected DC. Generally, the highest level of message was detected by 8 h postinfection, followed by a drop in IL-12p35 levels 12 h postinfection and sustained expression of IL-12p40. IL-23p19 was also induced preferentially by L. major infection (data not shown). The similar kinetic inductions of IL-12p35 and IL-12p40 gene expression after L. major infection of DC suggest the activation of both genes by common transcription factors.
FIG. 1.
Kinetics of IL-12p35 and IL-12p40 induction in Leishmania-infected DC. RNA isolated from uninfected DC (open bars) or DC infected with L. major (black bars) or L. donovani (gray bars) for 1, 2, 4, 6, 8, 12, and 16 h was analyzed for IL-12p35 (A) and IL-12p40 (B) transcript levels by quantitative real-time RT-PCR. The induction of IL-12p35 and IL-12p40 transcripts in Leishmania-infected DC was calculated using the comparative ΔΔCT method and normalized to GAPDH. Values are means ± standard errors of the means (SEM) of triplicate culture wells. L. major-infected DC from all donors exhibited significant up-regulation of IL-12p35 and IL-12p40 mRNAs by 4 h postinfection (P ≤ 0.05).
Gene expression in Leishmania-infected DC.
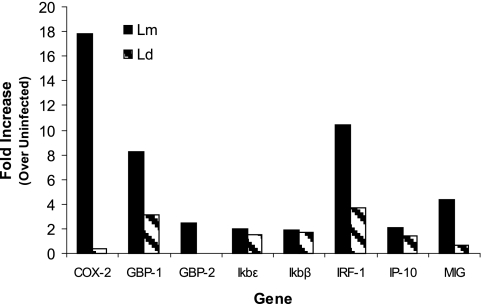
Assessment of the expression of genes known to be regulated by specific transcription factors can be a first clue to the signaling pathways that are activated in response to specific stimuli or infections. To identify transcription factors involved in signaling cascades initiated by L. major infection in human DC, we reanalyzed previously published microarray data. These microarray experiments were carried out using total RNA obtained from human DC infected with L. major and L. donovani for 16 h. Pooled cRNAs from seven donors were hybridized to the Affymetrix HU95A microarray (4). Here, we reanalyzed these data to assess changes in genes regulated by transcription factors known to bind IL-12 promoters. Genes regulated by IFN-γ-stimulated response elements (ISREs) (guanylate binding protein 1 [GBP-1], GBP-2, IP-10, and COX-2), GAS (IFN-γ-activated sequence) (IRF-1 and MIG), or NF-κB (IκBɛ, KκBβ, COX-2, and IRF-1) binding elements were assessed.
The reanalysis of the microarray information indicated the up-regulation of certain genes that are regulated by IRF-1 and IRF-8 in DC infected with L. major (Fig. 2). Among the genes that are preferentially up-regulated by L. major infection, guanylate binding proteins (GBP-1 and GBP-2) (8) and COX-2 (51) possess ISREs that bind IRF-1. In addition, IRF-1 mRNA levels are up-regulated specifically by L. major infection, further implicating IRF-1 as a mediator of IL-12 production in L. major-infected DC. IP-10, however, is not differentially regulated in L. major- and L. donovani-infected DC. Although the IP-10 promoter contains an ISRE, previous studies indicated that IRF-1 binding is not essential for ISRE-mediated transcription of the human IP-10 promoter (26). Interestingly, IRF-1 (38, 46) and MIG (56), genes which contain GAS promoter elements, are preferentially up-regulated in L. major-infected DC (Fig. 2), suggesting that there is an increase in IRF-8 binding.
FIG. 2.
Up-regulation of genes in L. major-infected DC. Data obtained from microarray analysis of total RNA obtained from DC infected with L. major (Lm) or L. donovani (Ld) for 16 h were reanalyzed (4) to examine genes regulated by NF-κB, ISRE, and GAS regulatory elements. Indicated changes in transcript levels in Leishmania-infected DC are over basal levels present in uninfected DC.
Both COX-2 (51) and IRF-1 (46) promoters have NF-κB elements, implicating a role for NF-κB activation. Of interest is that IκB gene promoters also contain NF-κB binding sites (13); these genes are not induced preferentially by L. major 16 h postinfection in DC (Fig. 2). Although NF-κB has been implicated in several nuances of IL-12 regulation (32, 35), it appears that it does not play a role in L. major-specific priming for IL-12 secretion by DC according to the microarray analysis (Fig. 2). However, it is possible that IκB proteins might be regulated by other members of the NF-κB family in this system or that the induction of the IκB genes occurs earlier than 16 h after infection of DC with L. major.
RT-PCR analysis of IRF-1 and IRF-8 in L. major-infected DC.
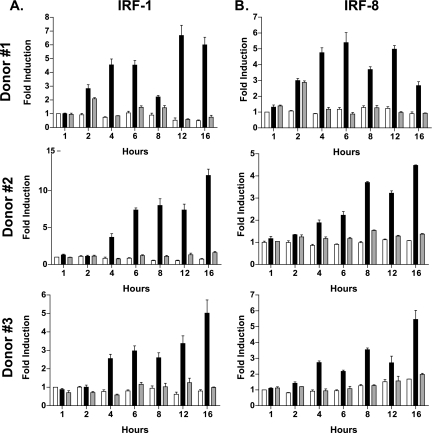
Other studies have shown that IRF-1 and IRF-8 are critical for the activation of both IL-12p35 and IL-12p40 promoters in murine macrophage systems (21, 22, 49), and our previous results strongly implicate these factors in response to L. major infection. In order to gauge the transcriptional induction of IRF-1 and IRF-8 in L. major-infected DC, we analyzed mRNA levels of IRF-1 and IRF-8 by real-time quantitative RT-PCR. Our results indicate that IRF-1 mRNA levels steadily increase in L. major-infected DC compared to L. donovani-infected DC starting at 4 h (Fig. 3A) postinfection. A similar kinetic pattern of induction was revealed for IRF-8 expression (Fig. 3B).
FIG. 3.
Kinetics of IRF-1 and IRF-8 induction in Leishmania-infected DC. RNA isolated from uninfected DC (open) or DC infected with L. major (black bars) or L. donovani (gray bars) for 1, 2, 4, 6, 8, 12, and 16 h was analyzed for IRF-1 (A) and IRF-8 (B) transcript levels by quantitative real-time RT-PCR. The induction of IRF-1 and IRF-8 transcripts in Leishmania-infected DC was calculated using the comparative ΔΔCT method and normalized to GAPDH. Values are means ± SEM of triplicate culture wells. L. major-infected DC from all donors exhibited significant up-regulation of IRF-1 and IRF-8 mRNAs by 4 h postinfection (P ≤ 0.05).
Nuclear translocation of IRF-1 and IRF-8.
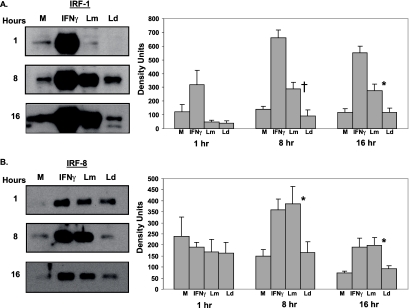
To directly assess the activation of the implicated transcription factors, nuclear extracts of L. major-infected DC were analyzed using Western blotting, probing for IRF-1 and IRF-8 (Fig. 4). As the kinetics of p35 and p40 induction revealed a time-dependent up-regulation of transcripts in response to L. major infection, nuclear extracts were obtained from L. major-infected DC at 1, 8, and 16 h postinfection to observe changes in nuclear translocation of the above-mentioned transcription factors. The significant increase in IRF-1 transcript levels in L. major-infected DC starting at 4 h (Fig. 3A) corresponds to the increased nuclear translocation of IRF-1 in L. major-infected DC, as opposed to L. donovani-infected DC by 8 h postinfection (Fig. 4A). This increase in IRF-1 nuclear translocation observed in L. major-infected DC is sustained at 16 h postinfection. Similar activation kinetics were observed for IRF-8 by L. major infection (Fig. 4B). Thus, increased nuclear translocation of IRF-1 and IRF-8 in L. major-infected DC at 8 h and 16 h of infection suggests that both these transcription factors are likely related to the induction of the IL-12p35 and IL-12p40 genes. IFN-γ stimulation of DC was used as a positive control for the nuclear translocation of both transcription factors; IFN-γ activation was further substantiated by increases in transcript levels of IRF-1 and IRF-8 in DC treated with IFN-γ (data not shown).
FIG. 4.
Nuclear translocation of IRF-1 and IRF-8 in L. major-infected DC. Nuclear extracts obtained from DC infected with L. major (Lm) or L. donovani (Ld) for 1, 8, or 16 h were analyzed by Western blotting for IRF-1 (A) and IRF-8 (B). IFN-γ (1,000 U/ml)-stimulated DC were used as a positive control. Densitometry readings for each blot were normalized to lamin A/C. Western blots for one representative donor are shown. Values are means ± SEM of 3 to 12 donors. †, P ≤ 0.1; *, P ≤ 0.05. M, medium control.
L. major-induced IRF-1 and IRF-8 interact with the IL-12p35 promoter.
The up-regulation of both IL-12p35 and IL-12p40 transcription and the increased nuclear translocation of IRF-1 and IRF-8 in L. major-infected DC compared to that of L. donovani-infected DC suggest a role for IRF-1 and IRF-8 in the activation of L. major-induced IL-12 expression. To determine if these factors are active in L. major-infected DC, we assessed the abilities of IRF-1 and IRF-8 present in nuclear extracts obtained from L. major-infected DC to bind their consensus binding sites by EMSA. This experiment revealed that crude nuclear extracts from L. major-infected DC do bind to IRF-1 and IRF-8 consensus sequences (data not shown). In order to determine if this binding occurs endogenously in L. major-infected DC, ChIP assays were performed on Leishmania-infected DC. Following 6 h of infection, the protein-DNA complexes were cross-linked with paraformaldehyde and immunoprecipitated with antibodies reacting to IRF-1 or IRF-8 (Fig. 5). Binding to the IL-12p35 promoter was assessed by amplifying the IRF-E promoter element by PCR. Binding of both IRF-1 and IRF-8 to the IL-12p35 promoter was observed specifically in L. major-infected DC compared to L. donovani-infected DC (Fig. 5).
FIG. 5.
Endogenous IRF-1 and IRF-8 interact with the IL-12p35 promoter in L. major-infected DC. DC were infected with L. major (Lm) and L. donovani (Ld) for 6 h, followed by cross-linking and immunoprecipitation of protein-DNA complexes with antibodies against IRF-1 and IRF-8 or an isotype control. The presence of the IL-12p35 IRF-E element was assessed by PCR. IFN-γ (1,000 units/ml)-treated DC served as a positive control at all time points. Input DNA indicates that all samples contain equal amounts of DNA. One representative of two independent experiments is shown. M, medium control.
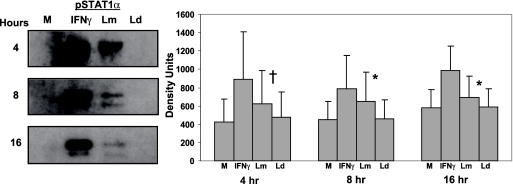
Up-regulation of phosphorylated STAT1 in L. major-infected DC.
The IRF-8 promoter contains a GAS element, raising the possibility that STAT1α mediates the activation of this promoter in L. major-infected DC. Therefore, we investigated the nuclear translocation of STAT1α in Leishmania-infected DC. As STAT1α only is active when phosphorylated, we utilized an anti-STAT1α-phosphospecific antibody. Our results indicate elevated levels of phosphorylated STAT1α in nuclear extracts of L. major-infected DC as early as 4 h postinfection compared to L. donovani-infected DC (Fig. 6). Phosphorylation of STAT1α in the cytosol of L. major-infected DC was not evident until 2 to 4 h postinfection (data not shown).
FIG. 6.
Activation and nuclear translocation of STAT1 in L. major-infected DC. Nuclear extracts obtained from DC infected with L. major (Lm) or L. donovani (Ld) for 4, 8, and 16 h were analyzed for phosphorylated STAT1α. IFN-γ (1,000 U/ml)-treated DC served as a positive control. Lamin A/C was used as a loading control. Western blots of one representative donor are shown. Values are means ± SEM for four to seven donors. †, P ≤ 0.1; *, P ≤ 0.05. M, medium control.
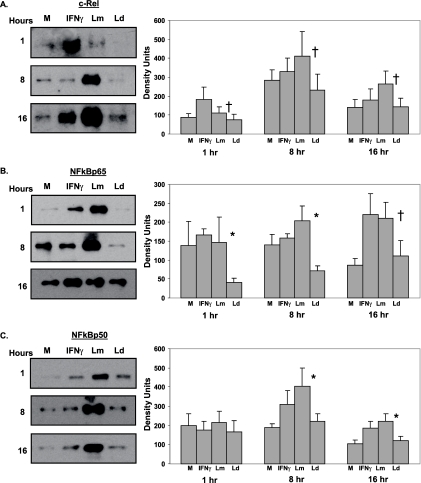
Nuclear translocation of c-Rel, NF-κBp65, and NF-κBp50.
The microarray analysis of L. major- and L. donovani-infected DC indicated that some NF-κB-regulated genes may not be differentially induced by Leishmania species. However, both IRF-1 and IRF-8 promoters contain NF-κB elements and are specifically induced only by L. major infection as early as 4 h postinfection, suggesting that the timing of the microarray experiment may have hindered our ability to detect differentially expressed NF-κB-regulated genes. Therefore, we assessed the nuclear translocation of NF-κB transcription factors in Leishmania-infected DC as early as 1 h postinfection (Fig. 7). Our results demonstrate increased nuclear translocation of NF-κBp65 in DC infected with L. major for 1, 8, and 16 h (Fig. 7B) and NF-κBp50 at 8 and 16 h postinfection (Fig. 7C), suggesting a role for NF-κBp65 and NF-κBp50 in the activation of IRF-1 and IRF-8 by L. major infection. c-Rel has been associated strongly with the maturation of DC (3) in addition to the activation of IL-12p35 and IL-12p40. Although somewhat more variable than NF-κBp65, c-Rel also translocates into the nucleus after L. major infection compared to L. donovani infection (Fig. 7A).
FIG. 7.
Nuclear translocation of NF-κB transcription factors in L. major-infected DC. Nuclear extracts obtained from DC infected with L. major (Lm) or L. donovani (Ld) for 1, 8, or 16 h were analyzed by Western blotting for c-Rel (A), NF-κBp65 (B), and NF-κBp50 (C). IFN-γ (1,000 U/ml)-stimulated DC were used as a positive control. Densitometry readings for each blot were normalized to lamin A/C. Western blots of one representative donor are shown. Values are means ± SEM for three to nine donors. †, P ≤ 0.1; *, P ≤ 0.05. M, medium control.
NF-κB elements are required for induction of IL-12, IRF-8, and IRF-1.
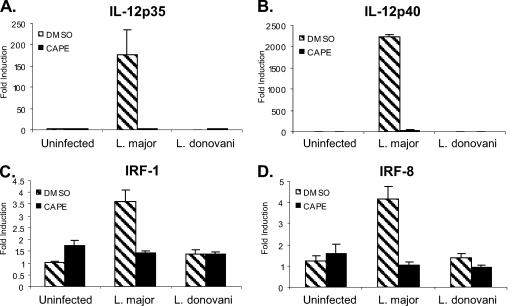
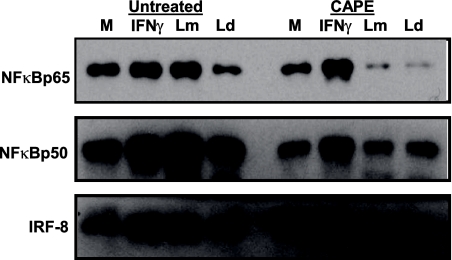
The increased nuclear translocation of NF-κBp50 and NF-κBp65 in L. major-infected DC at early time points after infection suggests a role for these transcription factors in the L. major-specific induction of IL-12p35 and IL-12p40. To determine the necessity of NF-κB for L. major-induced IL-12 expression, the NF-κB pathway in Leishmania-infected DC was blocked with caffeic acid phenyl ester (CAPE), a specific NF-κB inhibitor, and IL-12 expression was assessed by real-time quantitative RT-PCR. Although infection levels were not altered, IL-12p35 (Fig. 8A) and IL-12p40 (Fig. 8B) mRNA levels dropped drastically in L. major-infected DC pretreated with CAPE compared to untreated L. major-infected DC. CAPE treatment also affects IRF-8 and IRF-1 transcription, as evidenced by reduced IRF-1 (Fig. 8C) and IRF-8 (Fig. 8D) mRNA levels in treated L. major-infected DC compared to those in untreated L. major-infected DC. The reduction in IRF-8 transcription corresponds to reduced IRF-8 nuclear translocation in CAPE-treated L. major-infected DC, a reduction that was not observed in untreated L. major-infected DC (Fig. 9). Thus, it is evident that NF-κB family members coordinate gene activation during L. major infection, resulting in downstream events required for the activation of IL-12p35 and IL-12p40 promoters.
FIG. 8.
Inhibition of NF-κB blocks IL-12 and IRF up-regulation in L. major-infected human DC. DC were pretreated with 52 μM CAPE for 1 h prior to infection with either L. major or L. donovani or treated with IFN-γ (1,000 U/ml). RNA isolated at 8 h postinfection was analyzed for IL-12p35 (A), IL-12p40 (B), IRF-1 (C), and IRF-8 (D) by quantitative real time RT-PCR. The induction of IL-12 or IRF transcripts in Leishmania-infected DC was calculated using the comparative ΔΔCT method and normalized to GAPDH. Means ± SEM for three donors are shown. DMSO, dimethyl sulfoxide (vehicle control).
FIG. 9.
Inhibition of NF-κB blocks IRF-8 nuclear translocation in L. major-infected human DC. DC were pretreated with 52 μM CAPE for 1 h prior to infection with either L. major (Lm) or L. donovani (Ld) or treated with IFN-γ (1,000 U/ml). Nuclear extracts isolated 8 h posttreatment or postinfection were analyzed by Western blotting for NF-κBp65, NF-κBp50, and IRF-8. One representative of two independent experiments is shown. M, medium control.
To test if de novo protein synthesis is required for L. major-induced IL-12 transcription, we blocked protein translation with cycloheximide prior to infection. Cycloheximide pretreatment resulted in elevated amounts of IL-12p35 (169-fold ± 104-fold increase for L. major and 108-fold ± 168-fold increase for L. donovani over infection plus vehicle control) and IL-12p40 (28-fold ± 34-fold increase for L. major and 29-fold ± 43-fold increase for L. donovani over infection plus vehicle control), suggesting that protein synthesis is required for the production of a natural hemostatic mechanism that influences IL-12 production.
DISCUSSION
The goal of this study was to elucidate the mechanism of IL-12 regulation in L. major-infected human DC. L. major initiates a proinflammatory Th1 immune response mediated by IL-12 to establish and sustain immunity. Here, we investigated the specific induction of IL-12p35 and IL-12p40 in human DC in response to L. major infection and demonstrate a critical role for NF-κB, IRF-1, and IRF-8 in mediating this IL-12 production.
Elevated levels of IL-12p35 and IL-12p40 transcripts are induced in L. major-infected DC in the presence and absence of CD40L (28). The induction of both subunits of IL-12 by L. major in the absence of CD40L was observed at 8 h after infection of DC. However, information regarding the initial transcriptional events of IL-12 expression in L. major-infected DC was lacking. This information was provided by a detailed kinetic analysis of IL-12p35 and IL-12p40 transcription at various time points after infection of DC with either L. major or L. donovani (Fig. 1). Both IL-12 genes follow a similar pattern of up-regulation in DC in response to various durations of infection with L. major. These data add to a growing body of literature that reveals that along with IL-12p40 transcriptional control, IL-12p35 is also tightly regulated. In L. major-infected DC, both subunits seem to contribute equally to the induction of IL-12 in DC.
The parallel inductions of IL-12p35 and IL-12p40 in L. major-infected DC suggest that these genes are activated by a common set of transcription factors. This speculation is supported by data from a previously published microarray analysis of L. major- and L. donovani-infected DC (4). We utilized these data by segregating genes into three categories based on promoter regulator elements. The L. major-specific up-regulation of IRF-1 mRNA, in addition to genes regulated by IRF-1 (GBP genes and COX-2) (Fig. 2), strongly implicates IRF-1-mediated activation of these latter genes as well as IL-12p35 and IL-12p40 by L. major infection. The importance of IRF-1 is not surprising, as previous studies highlighted a key role for IRF-1 in IL-12 activation (21, 22), and murine studies demonstrated the necessity of IRF-1 in mediating resistance to L. major infection, a mechanism that requires IL-12 production (23, 27).
Our data also reveal that IRF-8 and MIG are preferentially induced by L. major infection in human DC (Fig. 2 and 3). Interestingly, IRF-1 (38, 46), MIG (56), and IRF-8 (15) genes are triggered through GAS elements. GAS sequences bind IRF-8 as well as STAT1; it has been suggested that IRF-8 is late-acting, potentiating or sustaining STAT1 activated immune activities (6). Here, we demonstrate that both IRF-8 (Fig. 4 and 5) and STAT1 (Fig. 6) are specifically activated by L. major infection, raising the possibility that either or both IRF-8 and STAT1 contribute to the L. major-induced IL-12 response in human DC. Interestingly, the lack of STAT1 in murine DC leads to lower IL-12 production in the draining lymph nodes and compromised Th1 cell responses during experimental L. major infection (14). One possible mechanism other than a direct activation of a STAT1-mediated signal transduction cascade by L. major to explain our results is the induction of autocrine-acting IFNs by L. major infection. We previously showed that neutralizing IFN-γ does not block L. major-induced CD40L-dependent IL-12p70 production (28); however, a role for type I IFNs or other cytokines remains to be elucidated.
Although IRF-8 was originally discovered as a gene repressor (34, 53, 54), it assumes the role of an activator with regard to IL-12p35 and IL-12p40 promoters (22, 52). Moreover, studies demonstrated the requirement of IRF-8-mediated IL-12 production for resistance to murine cutaneous leishmaniasis (11). In addition to a GAS element, binding either STAT1 (15) or IRF-8 (19), the IRF-8 promoter also binds IRF-1 (15). The activation of these codependent transcription factors by L. major likely induces a positive feedback loop, ensuring the activation of IL-12. The transactivation potential of IRF-8 is realized only when associated with other transcription factors including IRF-1, Rel proteins, and Pu.1 (19). Among the pool of transcription factors that might associate with IRF-8 to enable L. major-induced activation of IL-12, IRF-1 is the most likely candidate due to the following reasons: (i) the activation of the IL-12p35 promoter in human monocytes by a complex composed of IRF-1 and IRF-8 was reported previously (22), (ii) a large complex composed of IRF-1, IRF-8, Ets-2, and c-Rel has been implicated in the activation of the human IL-12p40 promoter (49), (iii) our ChIP analysis indicates the binding of both IRF-8 and IRF-1 to the IL-12p35 promoter in DC infected with L. major, and (iv) the early up-regulation of IRF-8 mRNA in DC infected with L. major correlates with an increased nuclear translocation of IRF-8 in L. major-infected DC, corresponding to a similar pattern for IRF-1.
Our ChIP analysis indicates that both IRF-1 and IRF-8 bind the IL-12p35 promoter in response to L. major infection, suggesting an interaction between IRF-8 and IRF-1. Of interest is that IRF-1 and IRF-8 do not bind the IL-12p35 promoter in DC infected with L. major at later times of infection (data not shown). This absence of IRF-1 and IRF-8 binding to the IL-12p35 promoter in DC infected with L. major for 12 h implies a subsequent halt of IL-12p35 promoter activation. Accordingly, our results indicate a clear drop in IL-12p35 levels in DC infected with L. major for 12 and 16 h (Fig. 1).
NF-κB actively regulates COX-2 (51), IRF-1 (46), and IRF-8 (15), in addition to IL-12p35 (18, 21) and IL-12p40 (39), all genes that are induced preferentially in human DC by L. major infection. By utilizing the NF-κB-specific inhibitor CAPE, we demonstrate here that the inhibition of NF-κB blocks L. major-induced IL-12p35 and IL-12p40 production (Fig. 8), supporting numerous previous studies implicating a specific role of NF-κB factors in IL-12 transcription (18, 21, 39). Both IL-12p35 and IL-12p40 promoters have DNA elements that have been shown to bind NF-κBp50 and c-Rel as part of larger complexes (49). Our densitometry analysis revealed significant c-Rel nuclear translocation in response to L. major infection at all time points (Fig. 7A). NF-κBp50 was also reproducibly activated in response to L. major at 8 and 16 h postinfection (Fig. 7C), indicating that these factors are likely involved in the L. major-specific induction of IL-12 production. Interestingly, NF-κBp65 (Fig. 7B) was preferentially activated by L. major infection compared to L. donovani infection as early as 1 h postinfection. However, it appears that NF-κBp65 activation may actually be inhibited by L. donovani infection, an observation that we are currently investigating. Pretreatment with CAPE blocked NF-κBp50, NF-κBp65, and IRF-8 nuclear translocation (Fig. 9). NF-κBp50 was previously shown to bind the IRF-8 promoter (15); our data indicating the early activation of NF-κBp65 in response to L. major infection imply that this factor may also be involved in IRF-8 and possibly IRF-1 transcription.
Our data clearly demonstrate that CAPE treatment inhibits NF-κB nuclear translocation, suggesting that NF-κB factors are required for the induction of IL-12p35, IL-12p40, IRF-1, and IRF-8 in response to L. major infection in human DC (Fig. 8). Interestingly, CAPE pretreatment did not inhibit NF-κB nuclear translocation in response to IFN-γ (Fig. 9). NF-κB activation is negatively regulated by IκB (25). In response to activation stimuli, IκB factors bound to NF-κB proteins in the cytoplasm undergo phosphorylation, ubiquitination, and eventual proteasome-mediated degradation, resulting in NF-κB nuclear translocation and promoter binding. While the JAK-STAT cascade is the major IFN-γ signaling pathway, IFN-γ induction of some genes requires NF-κB activation through a pathway that likely involves double-stranded RNA-dependent kinase (PKR) (16). The mechanism of CAPE inhibition of NF-κB activation is unclear; preventing IκB degradation in some studies (1), blocking nuclear translocation without significantly inhibiting IκB degradation in other studies (33), and also suppressing the interaction of NF-κB proteins with DNA (33). As the exact mechanism is unknown, it is tempting to surmise that the molecular basis for CAPE inhibition does not affect PKR activation of NF-κB.
The complex process of IL-12 transcription is coordinated by a group of transcription factors that respond to signaling cascades initiated by external stimuli. The induction of IL-12 has been best described in macrophage systems in response to IFN-γ and lipopolysaccharide stimulation (32, 39). Signaling cascades initiated by these stimuli activate a variety of transcription factors belonging to IRF, NF-κB, and the Pu.1 family (21, 22, 32) that ultimately activate IL-12p35 and IL-12p40 promoters. Given this scenario, the idea of L. major initiating similar signaling events in DC is attractive. Our results invoke a model whereby L. major initiates a signaling cascade leading to the activation of NF-κB elements followed by de novo synthesis and activation of IRF-1 and IRF-8 that further enhance IL-12p35 and IL-12p40 expression. This model predicts that blocking protein translation following L. major infection will inhibit the amplification of IL-12 in this system. Our attempts to test this model using cycloheximide revealed that the inhibition of protein synthesis resulted in large amounts of IL-12p35 and IL-12p40 mRNA under all conditions, suggesting that some fundamental protein necessary for regulating proinflammatory responses is absent in the presence of the inhibitor.
Signal transduction initiated by Leishmania in host cells results in the modulation of host immune responses. Specifically, L. major induces IL-12 production in DC, as opposed to L. donovani, resulting in strong Th1 immune responses followed by immunity. IL-12 production in response to L. major infection likely results from the initial interaction between the parasite and host receptors either by activating IL-12 transcription directly or by inducing an autocrine pathway. Many receptors have been implicated in binding Leishmania parasites, including galectins (36, 37), Toll-like receptors (9, 31, 41), complement receptors 1 and 3 (7, 29), Fc receptors (30), mannose receptor (2, 55), scavenger receptor (44), and DC-specific ICAM1-grabbing nonintegrin (5). The interaction of one receptor or multiple receptors with L. major parasites or products may result in intracellular signals affecting downstream events, leading to the activation of NF-κB, IRF-1, and IRF-8, ultimately activating IL-12 promoters.
Acknowledgments
This work was supported by National Institutes of Health grant 5R01AI056242 (to M.A.M.).
Editor: W. A. Petri, Jr.
Footnotes
Published ahead of print on 3 March 2008.
REFERENCES
- 1.Abdel-Latif, M. M., H. J. Windle, B. S. Homasany, K. Sabra, and D. Kelleher. 2005. Caffeic acid phenethyl ester modulates Helicobacter pylori-induced nuclear factor-kappa B and activator protein-1 expression in gastric epithelial cells. Br. J. Pharmacol. 1461139-1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blackwell, J. M., R. A. Ezekowitz, M. B. Roberts, J. Y. Channon, R. B. Sim, and S. Gordon. 1985. Macrophage complement and lectin-like receptors bind Leishmania in the absence of serum. J. Exp. Med. 162324-331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boffa, D. J., B. Feng, V. Sharma, R. Dematteo, G. Miller, M. Suthanthiran, R. Nunez, and H. C. Liou. 2003. Selective loss of c-Rel compromises dendritic cell activation of T lymphocytes. Cell. Immunol. 222105-115. [DOI] [PubMed] [Google Scholar]
- 4.Chaussabel, D., R. T. Semnani, M. A. McDowell, D. Sacks, A. Sher, and T. B. Nutman. 2003. Unique gene expression profiles of human macrophages and dendritic cells to phylogenetically distinct parasites. Blood 102672-681. [DOI] [PubMed] [Google Scholar]
- 5.Colmenares, M., A. L. Corbi, S. J. Turco, and L. Rivas. 2004. The dendritic cell receptor DC-SIGN discriminates among species and life cycle forms of Leishmania. J. Immunol. 1721186-1190. [DOI] [PubMed] [Google Scholar]
- 6.Contursi, C., I. M. Wang, L. Gabriele, M. Gadina, J. O'Shea, H. C. Morse III, and K. Ozato. 2000. IFN consensus sequence binding protein potentiates STAT1-dependent activation of IFNgamma-responsive promoters in macrophages. Proc. Natl. Acad. Sci. USA 9791-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Da Silva, R. P., B. F. Hall, K. A. Joiner, and D. L. Sacks. 1989. CR1, the C3b receptor, mediates binding of infective Leishmania major metacyclic promastigotes to human macrophages. J. Immunol. 143617-622. [PubMed] [Google Scholar]
- 8.Decker, T., D. J. Lew, and J. E. Darnell, Jr. 1991. Two distinct alpha-interferon-dependent signal transduction pathways may contribute to activation of transcription of the guanylate-binding protein gene. Mol. Cell. Biol. 115147-5153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Veer, M. J., J. M. Curtis, T. M. Baldwin, J. A. DiDonato, A. Sexton, M. J. McConville, E. Handman, and L. Schofield. 2003. MyD88 is essential for clearance of Leishmania major: possible role for lipophosphoglycan and Toll-like receptor 2 signaling. Eur. J. Immunol. 332822-2831. [DOI] [PubMed] [Google Scholar]
- 10.Driggers, P. H., D. L. Ennist, S. L. Gleason, W. H. Mak, M. S. Marks, B. Z. Levi, J. R. Flanagan, E. Appella, and K. Ozato. 1990. An interferon gamma-regulated protein that binds the interferon-inducible enhancer element of major histocompatibility complex class I genes. Proc. Natl. Acad. Sci. USA 873743-3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giese, N. A., L. Gabriele, T. M. Doherty, D. M. Klinman, L. Tadesse-Heath, C. Contursi, S. L. Epstein, and H. C. Morse III. 1997. Interferon (IFN) consensus sequence-binding protein, a transcription factor of the IFN regulatory factor family, regulates immune responses in vivo through control of interleukin 12 expression. J. Exp. Med. 1861535-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Greenblatt, C. L. 1988. Cutaneous leishmaniasis: the prospects for a killed vaccine. Parasitol. Today 453-54. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann, A., G. Natoli, and G. Ghosh. 2006. Transcriptional regulation via the NF-kappaB signaling module. Oncogene 256706-6716. [DOI] [PubMed] [Google Scholar]
- 14.Johnson, L. M., and P. Scott. 2007. STAT1 expression in dendritic cells, but not T cells, is required for immunity to Leishmania major. J. Immunol. 1787259-7266. [DOI] [PubMed] [Google Scholar]
- 15.Kanno, Y., C. A. Kozak, C. Schindler, P. H. Driggers, D. L. Ennist, S. L. Gleason, J. E. Darnell, Jr., and K. Ozato. 1993. The genomic structure of the murine ICSBP gene reveals the presence of the gamma interferon-responsive element, to which an ISGF3α subunit (or similar) molecule binds. Mol. Cell. Biol. 133951-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karehed, K., A. Dimberg, S. Dahl, K. Nilsson, and F. Oberg. 2007. IFN-gamma-induced upregulation of Fcgamma-receptor-I during activation of monocytic cells requires the PKR and NFkappaB pathways. Mol. Immunol. 44615-624. [DOI] [PubMed] [Google Scholar]
- 17.Khamesipour, A., Y. Dowlati, A. Asilian, R. Hashemi-Fesharki, A. Javadi, S. Noazin, and F. Modabber. 2005. Leishmanization: use of an old method for evaluation of candidate vaccines against leishmaniasis. Vaccine 233642-3648. [DOI] [PubMed] [Google Scholar]
- 18.Kollet, J., C. Witek, J. D. Gentry, X. Liu, S. D. Schwartzbach, and T. M. Petro. 2001. Deletional analysis of the murine IL-12 p35 promoter comparing IFN-gamma and lipopolysaccharide stimulation. J. Immunol. 1675653-5663. [DOI] [PubMed] [Google Scholar]
- 19.Levi, B. Z., S. Hashmueli, M. Gleit-Kielmanowicz, A. Azriel, and D. Meraro. 2002. ICSBP/IRF-8 transactivation: a tale of protein-protein interaction. J. Interf. Cytok. Res. 22153-160. [DOI] [PubMed] [Google Scholar]
- 20.Reference deleted.
- 21.Liu, J., S. Cao, L. M. Herman, and X. Ma. 2003. Differential regulation of interleukin (IL)-12 p35 and p40 gene expression and interferon (IFN)-gamma-primed IL-12 production by IFN regulatory factor 1. J. Exp. Med. 1981265-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu, J., X. Guan, T. Tamura, K. Ozato, and X. Ma. 2004. Synergistic activation of interleukin-12 p35 gene transcription by interferon regulatory factor-1 and interferon consensus sequence-binding protein. J. Biol. Chem. 27955609-55617. [DOI] [PubMed] [Google Scholar]
- 23.Lohoff, M., D. Ferrick, H. W. Mittrucker, G. S. Duncan, S. Bischof, M. Rollinghoff, and T. W. Mak. 1997. Interferon regulatory factor-1 is required for a T helper 1 immune response in vivo. Immunity 6681-689. [DOI] [PubMed] [Google Scholar]
- 24.Ma, X., M. Neurath, G. Gri, and G. Trinchieri. 1997. Identification and characterization of a novel Ets-2-related nuclear complex implicated in the activation of the human interleukin-12 p40 gene promoter. J. Biol. Chem. 27210389-10395. [DOI] [PubMed] [Google Scholar]
- 25.Magnani, M., R. Crinelli, M. Bianchi, and A. Antonelli. 2000. The ubiquitin-dependent proteolytic system and other potential targets for the modulation of nuclear factor-kB (NF-kB). Curr. Drug Targets 1387-399. [DOI] [PubMed] [Google Scholar]
- 26.Majumder, S., L. Z. Zhou, P. Chaturvedi, G. Babcock, S. Aras, and R. M. Ransohoff. 1998. p48/STAT-1alpha-containing complexes play a predominant role in induction of IFN-gamma-inducible protein, 10 kDa (IP-10) by IFN-gamma alone or in synergy with TNF-alpha. J. Immunol. 1614736-4744. [PubMed] [Google Scholar]
- 27.Mattner, F., K. Di Padova, and G. Alber. 1997. Interleukin-12 is indispensable for protective immunity against Leishmania major. Infect. Immun. 654378-4383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDowell, M. A., M. Marovich, R. Lira, M. Braun, and D. Sacks. 2002. Leishmania priming of human dendritic cells for CD40 ligand-induced interleukin-12p70 secretion is strain and species dependent. Infect. Immun. 703994-4001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mosser, D. M., and P. J. Edelson. 1985. The mouse macrophage receptor for C3bi (CR3) is a major mechanism in the phagocytosis of Leishmania promastigotes. J. Immunol. 1352785-2789. [PubMed] [Google Scholar]
- 30.Mosser, D. M., and L. A. Rosenthal. 1993. Leishmania-macrophage interactions: multiple receptors, multiple ligands and diverse cellular responses. Semin. Cell Biol. 4315-322. [DOI] [PubMed] [Google Scholar]
- 31.Muraille, E., C. De Trez, M. Brait, P. De Baetselier, O. Leo, and Y. Carlier. 2003. Genetically resistant mice lacking MyD88-adapter protein display a high susceptibility to Leishmania major infection associated with a polarized Th2 response. J. Immunol. 1704237-4241. [DOI] [PubMed] [Google Scholar]
- 32.Murphy, T. L., M. G. Cleveland, P. Kulesza, J. Magram, and K. M. Murphy. 1995. Regulation of interleukin 12 p40 expression through an NF-κB half-site. Mol. Cell. Biol. 155258-5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Natarajan, K., S. Singh, T. R. Burke, Jr., D. Grunberger, and B. B. Aggarwal. 1996. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc. Natl. Acad. Sci. USA 939090-9095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nelson, N., M. S. Marks, P. H. Driggers, and K. Ozato. 1993. Interferon consensus sequence-binding protein, a member of the interferon regulatory factor family, suppresses interferon-induced gene transcription. Mol. Cell. Biol. 13588-599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ouaaz, F., J. Arron, Y. Zheng, Y. Choi, and A. A. Beg. 2002. Dendritic cell development and survival require distinct NF-kappaB subunits. Immunity 16257-270. [DOI] [PubMed] [Google Scholar]
- 36.Pelletier, I., T. Hashidata, T. Urashima, N. Nishi, T. Nakamura, M. Futai, Y. Arata, K. I. Kasai, M. Hirashima, J. Hirabayashi, and S. Sato. 2003. Specific recognition of Leishmania major poly-beta-galactosyl epitopes by galectin-9. Possible implication of galectin-9 in interaction between L. major and host cells. J. Biol. Chem. 27822223-22230. [DOI] [PubMed] [Google Scholar]
- 37.Pelletier, I., and S. Sato. 2002. Specific recognition and cleavage of galectin-3 by Leishmania major through species-specific polygalactose epitope. J. Biol. Chem. 27717663-17670. [DOI] [PubMed] [Google Scholar]
- 38.Pine, R., A. Canova, and C. Schindler. 1994. Tyrosine phosphorylated p91 binds to a single element in the ISGF2/IRF-1 promoter to mediate induction by IFN alpha and IFN gamma, and is likely to autoregulate the p91 gene. EMBO J. 13158-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Plevy, S. E., J. H. Gemberling, S. Hsu, A. J. Dorner, and S. T. Smale. 1997. Multiple control elements mediate activation of the murine and human interleukin 12 p40 promoters: evidence of functional synergy between C/EBP and Rel proteins. Mol. Cell. Biol. 174572-4588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Podlaski, F. J., V. B. Nanduri, J. D. Hulmes, Y. C. Pan, W. Levin, W. Danho, R. Chizzonite, M. K. Gately, and A. S. Stern. 1992. Molecular characterization of interleukin 12. Arch. Biochem. Biophys. 294230-237. [DOI] [PubMed] [Google Scholar]
- 41.Ropert, C., L. R. Ferreira, M. A. Campos, D. O. Procopio, L. R. Travassos, M. A. Ferguson, L. F. Reis, M. M. Teixeira, I. C. Almeida, and R. T. Gazzinelli. 2002. Macrophage signaling by glycosylphosphatidylinositol-anchored mucin-like glycoproteins derived from Trypanosoma cruzi trypomastigotes. Microbes Infect. 41015-1025. [DOI] [PubMed] [Google Scholar]
- 42.Reference deleted.
- 43.Schoenhaut, D. S., A. O. Chua, A. G. Wolitzky, P. M. Quinn, C. M. Dwyer, W. McComas, P. C. Familletti, M. K. Gately, and U. Gubler. 1992. Cloning and expression of murine IL-12. J. Immunol. 1483433-3440. [PubMed] [Google Scholar]
- 44.Schonlau, F., K. Scharffetter-Kochanek, S. Grabbe, B. Pietz, C. Sorg, and C. Sunderkotter. 2000. In experimental leishmaniasis deficiency of CD18 results in parasite dissemination associated with altered macrophage functions and incomplete Th1 cell response. Eur. J. Immunol. 302729-2740. [DOI] [PubMed] [Google Scholar]
- 45.Sieburth, D., E. W. Jabs, J. A. Warrington, X. Li, J. Lasota, S. LaForgia, K. Kelleher, K. Huebner, J. J. Wasmuth, and S. F. Wolf. 1992. Assignment of genes encoding a unique cytokine (IL12) composed of two unrelated subunits to chromosomes 3 and 5. Genomics 1459-62. [DOI] [PubMed] [Google Scholar]
- 46.Sims, S. H., Y. Cha, M. F. Romine, P. Q. Gao, K. Gottlieb, and A. B. Deisseroth. 1993. A novel interferon-inducible domain: structural and functional analysis of the human interferon regulatory factor 1 gene promoter. Mol. Cell. Biol. 13690-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spath, G. F., and S. M. Beverley. 2001. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Exp. Parasitol. 9997-103. [DOI] [PubMed] [Google Scholar]
- 48.Tone, Y., S. A. Thompson, J. M. Babik, K. F. Nolan, M. Tone, C. Raven, and H. Waldmann. 1996. Structure and chromosomal location of the mouse interleukin-12 p35 and p40 subunit genes. Eur. J. Immunol. 261222-1227. [DOI] [PubMed] [Google Scholar]
- 49.Trinchieri, G. 2003. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 3133-146. [DOI] [PubMed] [Google Scholar]
- 50.Reference deleted.
- 51.Vila-del Sol, V., and M. Fresno. 2005. Involvement of TNF and NF-kappa B in the transcriptional control of cyclooxygenase-2 expression by IFN-gamma in macrophages. J. Immunol. 1742825-2833. [DOI] [PubMed] [Google Scholar]
- 52.Wang, I. M., C. Contursi, A. Masumi, X. Ma, G. Trinchieri, and K. Ozato. 2000. An IFN-gamma-inducible transcription factor, IFN consensus sequence binding protein (ICSBP), stimulates IL-12 p40 expression in macrophages. J. Immunol. 165271-279. [DOI] [PubMed] [Google Scholar]
- 53.Weisz, A., S. Kirchhoff, and B. Z. Levi. 1994. IFN consensus sequence binding protein (ICSBP) is a conditional repressor of IFN inducible promoters. Int. Immunol. 61125-1131. [DOI] [PubMed] [Google Scholar]
- 54.Weisz, A., P. Marx, R. Sharf, E. Appella, P. H. Driggers, K. Ozato, and B. Z. Levi. 1992. Human interferon consensus sequence binding protein is a negative regulator of enhancer elements common to interferon-inducible genes. J. Biol. Chem. 26725589-25596. [PubMed] [Google Scholar]
- 55.Wilson, M. E., and R. D. Pearson. 1988. Roles of CR3 and mannose receptors in the attachment and ingestion of Leishmania donovani by human mononuclear phagocytes. Infect. Immun. 56363-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wright, T. M., and J. M. Farber. 1991. 5′ regulatory region of a novel cytokine gene mediates selective activation by interferon gamma. J. Exp. Med. 173417-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yoshimoto, T., H. Nagase, T. Ishida, J. Inoue, and H. Nariuchi. 1997. Induction of interleukin-12 p40 transcript by CD40 ligation via activation of nuclear factor-kappaB. Eur. J. Immunol. 273461-3470. [DOI] [PubMed] [Google Scholar]