Abstract
Currently, there is no animal model for Plasmodium falciparum challenge to evaluate malaria transmission-blocking vaccines based on the well-established Pfs25 target antigen. The biological activity of transmission-blocking antibodies is typically assessed using an assay known as the membrane feeding assay (MFA). It is an in vitro method that involves mixing antibodies with cultured P. falciparum gametocytes and feeding them to mosquitoes through an artificial membrane followed by assessment of infection in the mosquitoes. We genetically modified Plasmodium berghei to express Pfs25 and demonstrated that the transgenic parasites (TrPfs25Pb) are susceptible to anti-Pfs25 antibodies during mosquito-stage development. The asexual growth kinetics and mosquito infectivity of TrPfs25Pb were comparable to those of wild-type parasites, and TrPfs25Pb displayed Pfs25 on the surface of ookinetes. Immune sera from nonhuman primates immunized with a Pfs25-based vaccine when passively transferred to mice blocked transmission of TrPfs25Pb to Anopheles stephensi. Furthermore, mice immunized with Pfs25 DNA vaccine and challenged with TrPfs25Pb displayed reduced malaria transmission compared to mice immunized with wild-type plasmid. These studies describe development of an animal malaria model alternative to the in vitro MFA and show that the model can facilitate P. falciparum transmission-blocking vaccine evaluation based on the target antigen Pfs25. We believe that an animal model to test transmission-blocking vaccines would be superior to the MFA, since there may be additional immune factors that synergize the transmission-blocking activity of antibodies in vivo.
Annually, 300 to 500 million people worldwide suffer from clinical malaria, and approximately 1 million people, mainly children under 5 years old, die as a result of the disease (8, 18). Successful transmission of Plasmodium parasites relies on the uptake of sexual stages (gametocytes) of the pathogen by mosquitoes during a blood meal. Subsequent gametogenesis and fertilization of female and male gametes establishes a sexual reproduction phase in the mosquito midgut. Resulting zygotes transform into motile ookinetes, which traverse the peritrophic matrix and midgut epithelium, lodge on the basal lamina of the midgut, and develop into oocysts. Sporozoites produced in the oocysts are released into the hemocoel, then invade salivary glands, and are subsequently introduced to new hosts when the mosquito takes another blood meal. A number of stage-specific proteins have been shown to play important roles during fertilization and ookinete formation, and antibodies recognizing these antigens are potent blockers of parasite development in mosquitoes (12). Among the proteins that are expressed on the surface of zygotes and ookinetes, P25 and P28 have been shown to be crucial for successful transmission of Plasmodium parasites (7, 21). Both P25 and P28 are strong candidates for transmission-blocking vaccines (TBV), and phase I clinical trials for Pfs25 (Plasmodium falciparum) and Pv25 (Plasmodium vivax) have further confirmed their importance as vaccine candidates (14, 19). Previous studies have shown that P25 and P28 are expressed in a coordinated fashion during the zygote-to-ookinete transformation (13). Reverse genetics approaches (i.e., gene disruptions) have further established that the proteins have overlapping redundant functions, and in Plasmodium berghei, genes encoding P25 and P28 separated by a 1.4-kb intergenic region are located on chromosome 10 (21). A single gene disruption (either p25 or p28) caused only partial inhibition of parasite development; however, disruption of both p25 and p28 resulted in greater than 90% reduction of parasite development (21).
Currently, assessment of transmission-blocking antibodies entails membrane feeding assays (MFAs) (3, 9, 12), which are often unreliable and are only an in vitro method of assessment and may not truly represent the in vivo transmission-blocking potential of immune sera (22). This assay involves combining test antibodies in infectious gametocyte cultures and feeding the mixture to mosquitoes through an artificial membrane while maintaining a constant temperature of 37°C. MFAs are cumbersome, tedious, and rely on the availability of infectious gametocytes, produced either in culture (18 to 20 days) for P. falciparum or obtained from an infected chimpanzee or infected people for P. vivax.
By taking advantage of the functionally redundant activities of the P25 and P28 proteins, we sought to develop a Pfs25 transgenic murine malaria (P. berghei) model to evaluate P. falciparum transmission-blocking antibodies induced by a Pfs25 vaccine in vivo. A recently published paper described the generation of transgenic P. berghei parasites expressing Pvs25 that were found to be valuable in assessing P. vivax transmission-blocking antibodies in both membrane feeding assays and in vitro ookinete development assays (16). The availability of an animal model would circumvent the need for an artificial MFA and permit direct evaluation of the potency of malaria transmission-blocking vaccine formulations based on the Pfs25 antigen in preclinical studies and functional assessments of malaria transmission from vaccinated hosts to mosquitoes. This model could also simplify assessment of transmission-blocking antibodies of sera during malaria vaccine trials, especially in areas where it is difficult or challenging to routinely maintain infectious P. falciparum gametocytes in culture.
MATERIALS AND METHODS
Plasmids, P. berghei transfection, and cloning by limiting dilution.
To generate P. berghei transgenic parasites expressing Pfs25, the pB3D plasmid (courtesy of Andy Waters) was digested with KpnI and HindIII, and a cassette containing the pb25- 5′ untranslated region (UTR; 649 bp), full-length pfs25 (654 bp; P. falciparum 3D7), and pb25- 3′ UTR (600 bp) was ligated upstream of the 5′ end of the Tg.dhfr probe. The primers used for amplifying the different fragments were as follows (restriction enzyme sites are shown in lowercase letters): pb25-5′ UTR (Pb25-5′ UTR sense, 5′-ggtaccAGTTACATAAGCATTGTAAGAATTATTCA-3′, nucleotides (nt) −649 to −620 upstream of pb25; Pb25-5′ UTR antisense, 5′-atcgatTTTTAAATAAATTTAAACGAATAAC-3′, nt −25 to −1 upstream of pb25); pfs25 (Pfs25F sense, atcgatATGAATAAACTTTACAGTTTGTTTCT, nt +1 to +26; Pfs25R antisense, 5′-gaattcTTACATTATAAAAAAGCATACTC-3′, nt +631 to +654); pb25-3′ UTR (Pb25-3′ UTR sense, 5′-gaattcTTAAATAAACAAATATACCTGG-3′, nt 1 to 22 downstream from the pb25 stop codon; Pb25-3′ UTR antisense, 5′-aagcttTTTCCTTATGCGCAG-3′, nt 585 to 600 downstream from the pb25 stop codon). PCR-generated fragments were ligated through introduced restriction sites (Fig. 1A). The resulting plasmid was digested with NotI and BamHI to allow ligation of 708 bp of pb28-3′ UTR (Pb28-3′ UTR sense, 5′-ggatccTATATTCAATTGTTATCGC-3′, nt 1 to 19 downstream from the pb28 stop codon; Pb28-3′ UTR antisense, 5′-gcggccgcATTCGTATAAACACATTTC-3′, nt 689 to 708 downstream from the pb28 stop codon) (Fig. 1A). The 3′ UTR (pb25 and pb28) and 5′ UTR (pb25) were amplified by PCR from P. berghei (ANKA strain 2.34) genomic DNA used as a template. Plasmid DNA was purified using the Qiagen plasmid mini kit, and sequences were verified for all the cloned DNA fragments.
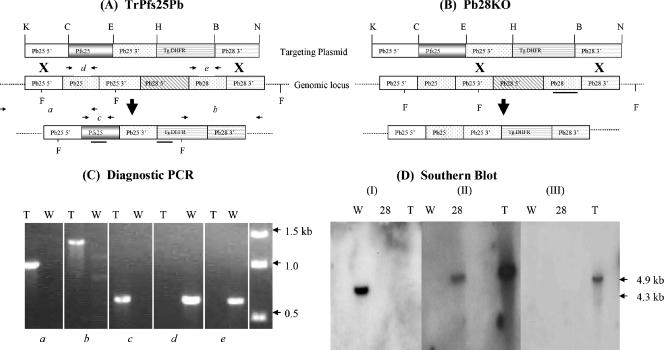
FIG. 1.
DNA plasmid constructs, PCR, and Southern blot analysis. (A) Schematic representation (not drawn to scale) of the targeting DNA vector plasmid used for transfection and the resultant locus after vector integration. The vector construct contained a drug-selectable marker (Tg.DHFR), 5′ and 3′ UTR sequences of pb25, pfs25 (full-length coding sequence), and 3′ UTR of pb28 (ligated through introduced restriction sites [K, KpnI; C, ClaI; E, EcoRI; H, HindIII; B, BamHI; N, NotI]) for targeted integration. Two homologous recombination events (indicated by the X) occurred, generating pb25/28 knockout parasites designated TrPfs25Pb (A) and Pb28KO (B) parasites. The regions to which the different probes hybridized are indicated by bold horizontal lines in panels A and B. (C) PCR analysis of genomic DNA to confirm correct integration of the vector construct into the transgenic (T) parasite's genome. The primer pairs used in gels a to e are identified in the legend for panel A, and DNA from wild-type (W) P. berghei parasites was used as a control. (D) Southern analysis of FokI (F)-digested genomic DNA from wild-type (W), Pb28KO (28), and TrPfs25Pb (T) to confirm proper integration of the targeting plasmid in the genomic DNA and to rule out the possible presence of untransformed wild-type DNA or episomal plasmid DNA. The pb28 probe hybridized to a 4.3-kb fragment in the W parasites (I), the dhfr probe hybridized to a 4.9-kb fragment in the DHFR backbone (II) in 28 and T parasites, and the pfs25 probe hybridized to a 4.9-kb fragment in T parasites (III).
P. berghei (ANKA 2.34) was maintained in Swiss-Webster mice, and when the parasitemia reached ∼3%, blood was drawn and cultured overnight at 37°C in RPMI 1640. The targeting plasmid construct was linearized by KpnI and NotI restriction digestion, and 10 μg of linearized plasmid was used for transfection using the Amaxa human T-cell nucleofactor kit and the T01 setting. Parasites were immediately injected intravenously (i.v.) into mice; 24 h later mice were given water containing 0.07 mg/ml pyrimethamine, and the percent parasitemia was monitored by Giemsa staining of blood smears. Drug-resistant parasites after diagnostic PCR verification were cloned by the limiting dilution method. Briefly, mice (4 to 12 mice per group) were injected i.v. with (0.2 ml) infected mouse blood serially diluted twofold to 5, 2.5, 1.25, and 0.6 parasites/ml. Pb28 knockout (Pb28KO) parasite clones were obtained from mice that received 1.25 parasites/ml, resulting in a 91% probability of clonality. In transgenic parasites (TrPfs25Pb) in which both pb25 and pb28 were replaced by pfs25, a clonal population was obtained from mice that received 2.5 parasites/ml.
Diagnostic PCR and characterization by Southern blot analysis of P. berghei transgenic parasites.
Drug-resistant parasites were characterized for crossover at the pb25 5′ UTR, pb25 3′ UTR, and pb28 3′ UTR by diagnostic PCR using primers for various specific regions and then cloned by limiting dilution. Diagnostic PCR revealed two different crossover events, namely, parasites with a crossover involving the pb25 5′ UTR and pb28 3′ UTR (designated TrPfs25Pb) and parasites with a crossover involving the pb25 3′ UTR and pb28 3′ UTR (designated Pb28KO).
Integration of the targeting construct at the appropriate locus was assessed by PCR and by Southern blotting of genomic DNA digested with FokI and probing with Tg.dhfr probe, full-length pb28, and full-length pfs25. Briefly, 5 μg DNA (wild type [WT], Pb28KO, and TrPfs25Pb) was digested with FokI, run on an agarose gel, and transferred onto a Hybond N+ nylon membrane (Amersham, Piscataway, NJ) by capillary action using 10× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). DNA was cross-linked to the membrane by UV cross-linking and incubated at 65°C for 2 h in the prehybridization buffer (4× SSC, 5× Denhardt's reagent, 0.1% sodium dodecyl sulfate, 100 μg/ml denatured salmon sperm DNA). Radioactively [32P[dCTP-labeled probes were added to the blots and allowed to hybridize overnight at 65°C. Following hybridization, blots were washed in wash buffer (0.2% SSC, 0.1% sodium dodecyl sulfate) twice at room temperature and once at 65°C. The blots were wrapped in plastic wrap and exposed to Hyperfilm ECL at −80°C.
All probes were generated by PCR, gel purified, and radiolabeled with 50 μCi [32P]dCTP (Amersham, Piscataway, NJ) using the High Prime DNA labeling kit (Roche, Indianapolis, IN). Nonincorporated radioactivity was removed using Sephadex MicroSpin G-25 columns (Amersham), and approximately 200,000 cpm were used for each probe for hybridization.
RNA extraction and Pfs25 gene-specific RT-PCR.
Approximately 100 μl blood was drawn from Swiss-Webster mice infected with transgenic parasites (∼10% parasitemia), and 500 μl of RNAlater (Qiagen) was added to the sample. Total RNA from parasite blood stages was extracted using the RNeasy mini kit (Qiagen) following the manufacturer's instructions, and RNA was suspended in 30 μl RNase-free water. Three microliters of RNA was added into a master mix containing 500 μM of each deoxynucleoside triphosphate, 10 units RNase inhibitor, 1× reverse transcription (RT) buffer, 0.5 μM of antisense primer Pfs25R, and 4 units of Omniscript reverse transcriptase (Qiagen) in a total volume of 20 μl. The mixture was incubated at 37°C for 45 min to synthesize cDNA. Two microliters from the RT step was added to a 23-μl master mix containing 100 μM of each deoxynucleoside triphosphate, buffer (50 mM KCl, 10 mM Tris-HCl pH 8.3, 1.5 mM MgCl2), a 0.4 μM concentration of sense primer Pfs25F and antisense primer Pfs25R, and 1.25 U of enzyme Taq polymerase. The PCR cycling conditions were as follows: initial denaturation at 94°C for 2 min and 25 cycles of denaturation at 94°C for 30 s, annealing at 50°C for 35 s, and extension at 68°C for 2 min 30 s. Two microliters of the product of the first PCR was used as template for nested PCR using a set of internal primers (sense, 25-1, 5′-TAATGCGAAAGTTACCGTGG-3′; antisense, 25-2, 5′-TCCATCAACAGCTTTACAGG-3′) (2). The PCR cycling conditions were as described above, with 30 cycles instead of 25. The PCR products were resolved on a 1% agarose gel stained with ethidium bromide and visualized with UV illumination.
Asexual growth kinetics and mosquito transmission.
Transgenic parasites (TrPfs25Pb) were compared to Pb28KO and WT parasites with respect to their asexual growth kinetics and infectivity to mosquitoes. Five- to 8-week-old Swiss-Webster mice (five per group) were inoculated i.v. with approximately 105 parasites (WT, Pb28KO, or TrPfs25Pb), and mice were monitored daily by examining parasite smears stained with Giemsa. For comparison of mosquito infectivity, Swiss-Webster mice were infected with 106 parasites (WT, Pb28KO, or TrPfs25Pb), and 4 days postinoculation, starved Anopheles stephensi mosquitoes were allowed to feed on infected mice. In all the mosquito feeding experiments, unfed mosquitoes were removed from the cages, blood-fed mosquitoes were maintained at 20°C at 70 to 80% relative humidity, and oocysts were counted 10 days post-blood feeding.
Immunofluorescence assays.
Surface expression of Pfs25 was tested by performing immunofluorescence assays (IFAs) on methanol-fixed blood-stage parasites and ookinetes derived either from cultures (in vitro) as previously described (17) or from mosquitoes 24 h after infected blood meal (in vivo) (25). Ookinete preparations were spotted on multiwell slides, and IFAs were performed using monoclonal anti-Pfs25 (4B7) antibody (1:200 dilution) and monoclonal anti-Pb28 (1:500). The secondary anti-mouse antibody conjugated to fluorescein isothiocyanate (FITC) was used at a 1:200 dilution. Slides were examined using an upright fluorescent Nikon E800 microscope (Japan) at 100× magnification.
Assessment of transmission-blocking activity (passive immunization).
Swiss-Webster female mice (5 to 8 weeks old) were infected with 106 TrPfs25Pb parasites i.v. Four days after inoculation, 4- to 6-day-old A. stephensi mosquitoes that had been starved 6 to 8 h were allowed to feed on mice to determine infectivity of parasites. After mosquito feeding, mice were randomly divided into two groups (three per group): one group of mice received 200 μl i.v. of preimmune monkey sera (control), and the other group received immune sera from monkeys previously immunized with DNA encoding Pfs25 and boosted with recombinant Pfs25 (5). The injected serum was allowed to equilibrate in the circulation for 15 min (1, 23), and another batch of starved A. stephensi mosquitoes was allowed to feed on mice again. In addition to the use of preimmune sera as a control, each mouse also acted as its own control, i.e., transmission was examined before and after receiving monkey sera. Ten days postfeeding, mosquitoes were dissected and midguts were stained with 0.1% mercurochrome and scored for the number of oocysts. The number of oocysts/mosquito was expressed as the mean ± standard deviation (SD) for each group.
Assessment of transmission-blocking activity (active immunization).
Female BALB/c mice (5 to 8 weeks old) were immunized (intramuscularly) with 50 μg of DNA vaccine plasmid DV1020 encoding Pfs25 followed by two boosts given 4 weeks apart. The Pfs25 coding sequence was synthesized after optimization for preferred codons in Homo sapiens. Control mice were immunized with 50 μg control (wild-type empty) DV1020 plasmid DNA. Three weeks after the second boost, mice were bled and titers of antibodies were determined by enzyme-linked immunosorbent assay (ELISA) with recombinant Pfs25 (obtained from the Malaria Vaccine Development Branch, NIAID, NIH) as the ELISA plate-coating antigen. After determinations of titers, mice that had received DV1020/Pfs25 plasmid DNA were divided into three groups (three mice per group) and each group was infected with 106 parasites i.v. of WT, Pb28KO, or TrPfs25Pb. Likewise, control plasmid-immunized mice were divided into three groups and infected with WT, Pb28KO, or TrPfs25Pb. Four days postinfection, A. stephensi mosquitoes were fed on the mice and oocysts were counted as described above.
All animal studies were done as per the protocol approved by the Institutional Animal Use and Care Committee of The Johns Hopkins University.
Statistical analysis.
The statistical significance of differences in oocyst numbers in mosquitoes fed on different groups of mice was analyzed by the Wilcoxon test, and a P value of <0.05 was considered statistically significant.
RESULTS
Plasmids, P. berghei transfection, and characterization of transformed parasites.
The targeting plasmid used to create various genetically modified parasites is shown in Fig. 1A. Two possible crossover events that would result in drug-resistant parasites are also shown in Fig. 1A and B, one that would replace both pb25 and pb28 with pfs25 and the other one that would knock out only pb28 while maintaining native pb25. PCR diagnostic tests were performed on drug-resistant parasites, and integration (Fig. 1C, gel a and b) of the targeting plasmid was demonstrated as shown by the presence of pfs25 (Fig. 1C, gel c) and the absence of both pb25 (Fig. 1C, gel d) and pb28 (Fig. 1C, gel e) in TrPfs25Pb transgenic parasites. The Pb28KO parasites demonstrated the presence of pb25 and absence of pb28 (data not shown). We next examined integration of the targeting plasmid at the appropriate genomic locus to rule out the possibility of episomal plasmid DNA or the presence of untransformed WT P. berghei parasites. Additional evidence for proper genomic integration was obtained by Southern blotting (Fig. 1D). DNA from WT parasites hybridized to the pb28 probe, Pb28KO parasite DNA hybridized to the Tg.dhfr probe, and TrPfs25Pb parasite DNA hybridized to the Tg.dhfr probe and to the pfs25 probe; these results are consistent with diagnostic PCR results. Taken together, these findings indicate pfs25 replaced pb25 and pb28 in TrPfs25Pb and also confirm the absence of untransformed WT parasites or episomal plasmid DNA.
Growth kinetics of transgenic compared to wild-type parasites.
After demonstrating proper integration of the targeting plasmid and the presence of pfs25 in transgenic parasites (TrPfs25Pb), additional experiments were conducted to characterize the phenotype of TrPfs25Pb parasites compared to either WT or Pb28KO parasites. To compare asexual growth kinetics, mice were infected with 105 WT, Pb28KO, or TrPfs25Pb parasites, and parasitemia was monitored. Results in Table 1 show that the growth of TrPfs25Pb was comparable to that of WT parasites during the first 5 days of infection. We next assessed the comparative infectivities of TrPfs25Pb and WT parasites by conducting A. stephensi mosquito infection studies. The infectivity (number of oocysts per mosquito) and the rate of infectivity (percent infected mosquitoes) were comparable between these WT and TrPfs25Pb parasites (60 ± 34 oocysts/midgut [WT] versus 54 ± 10 [TrPfs25Pb]; 87.5% [WT] versus 82.8% [TrPfs25Pb]infectivity; 80 and 64 mosquitoes were infected with WT and TrPsf25Pb parasites, respectively). These findings suggest that pfs25 was able to functionally complement the combined functions of pb25 and pb28 without compromising parasite growth and infectivity to A. stephensi mosquitoes.
TABLE 1.
Asexual growth kinetics of WT, Pb28KO, and TrPfs25Pb parasites following inoculation of 105 parasites i.v. in mice
| Day p.i.a | % Parasitemiab
|
||
|---|---|---|---|
| WT | Pb28KO | TrPfs25Pb | |
| 3 | 0.03 ± 0.1 | 0.02 ± 0.1 | 0.27 ± 0.1 |
| 4 | 1.33 ± 0.4 | 1.15 ± 0.3 | 1.15 ± 0.4 |
| 5 | 6.80 ± 1.5 | 14.00 ± 2.9 | 8.48 ± 2.1 |
| 6 | 9.71 ± 3.3 | 20.20 ± 7.2 | 25.12 ± 4.6 |
| 7 | 12.40 ± 5.9 | 23.40 ± 7.4 | 40.00 ± 12.6 |
p.i., postinoculation.
Values are means ± SD for five mice per group.
Expression of Pfs25 in transgenic parasites.
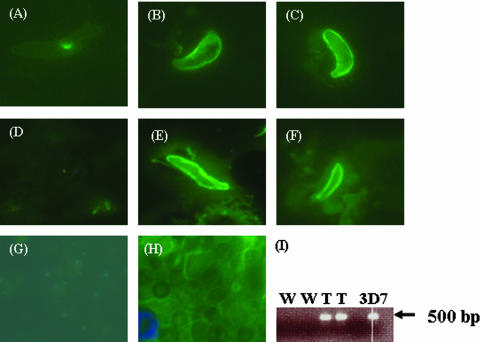
To determine if Pfs25 in TrPfs25Pb localized on the surface of zygotes and ookinetes, IFAs were performed using culture-produced ookinetes and anti-Pfs25 (10) or anti-Pb28 (20). As expected, WT parasites fluoresced (Fig. 2) when labeled with anti-Pb28 (Fig. 2B) but not with anti-Pfs25 (Fig. 2A). Conversely, TrPfs25Pb parasites fluoresced when labeled with anti-Pfs25 (Fig. 2C), while there was no fluorescence signal with the anti-Pb28 antibody (not shown), consistent with the loss of the pb28 genomic locus after recombination. As expected, Pb28KO parasites were negative when labeled with either anti-Pb28 or anti-Pfs25 (data not shown). Similar IFA results were obtained with in vivo-derived ookinetes (Fig. 2D to F). To examine whether expression of Pfs25 was stage specific, IFAs were also performed on erythrocytic stages of TrPfs25Pb parasites. Transgenic erythrocytic parasites examined by IFA showed no expression of Pfs25 (Fig. 2H) despite the presence of Pfs25 transcripts (Fig. 2I).
FIG. 2.
Surface localization of Pfs25 in the ookinetes of TrPfs25Pb. In vitro-derived (A to C) and in vivo-derived (D to F) ookinetes were fixed with methanol and reacted with antibodies for IFA. (A and D) Ookinetes of WT P. berghei labeled with anti-Pfs25; (B and E) WT ookinetes labeled with anti-Pb28 antibody; (C and F) ookinetes of TrPfs25Pb labeled with anti-Pfs25. (G and H) WT parasites (merged image from FITC and 4′,6′-diamidino-2-phenylindole labels) (G) and transgenic parasites (FITC merged with 4′,6′-diamidino-2-phenylindole labels) (H) show IFA results with blood-stage parasites allowed to react with anti-Pfs25 antibodies. (I) RT-PCR results demonstrating expression of Pfs25 transcripts in blood stages of transgenic parasites (T, duplicate lanes) compared to wild-type parasites (W, duplicate lanes). Genomic DNA from P. falciparum 3D7 was used as a positive control.
Evaluation of transmission-blocking activity of anti-Pfs25 antibodies by passive and active immunization.
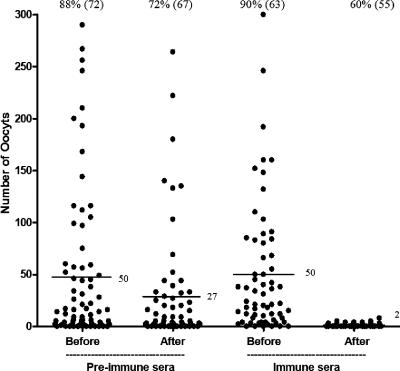
Having shown that the TrPfs25Pb parasites displayed Pfs25 on their surface and were transmission competent, we addressed the question whether antibodies against Pfs25 would block transmission of TrPfs25Pb parasites. We first employed a passive immunization scheme involving preimmune and immune sera from nonhuman primates immunized with DNA vaccine encoding Pfs25 and boosted with recombinant Pfs25. These immune primate sera had previously shown >95% reduction of malaria transmission by MFA (5). Mosquitoes that fed on TrPfs25Pb-infected mice passively immunized with immune sera revealed a robust transmission-blocking activity with greater than 90% inhibition of oocysts (P < 0.001) (Fig. 3). After assessing transmission to mosquitoes, mice (above) were bled, the blood diluted 1:5 in ookinete culture medium (RPMI 1640 supplemented with 25 mM HEPES, neomycin at 5 mg/liter, 10% fetal bovine serum; pH 8.4) and incubated at 19°C for 24 h as described in reference 17. Ookinete numbers were lower (9/60, ookinetes in 60 microscope fields) in mice receiving immune sera compared to mice receiving preimmune sera (40/60), suggesting that inhibition of transmission was primarily at the level of ookinete-stage parasite development.
FIG. 3.
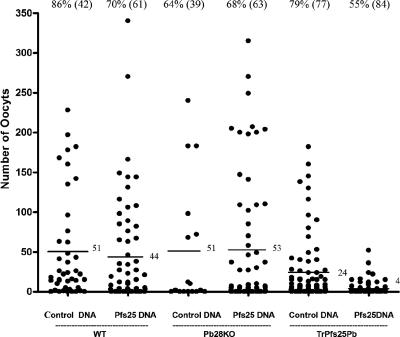
Evaluation of transmission-blocking activity by passive immunization. Mice were infected with TrPfs25Pb and used to assess transmission to mosquitoes before and after administration of rhesus preimmune or anti-Pfs25 immune sera. Mosquito midguts were examined 10 days postfeeding. Data points represent the number of oocysts found in individual mosquitoes that fed on three mice/group. Numbers on top of each set of data points indicate the percent mosquitoes infected (total number of mosquitoes dissected), and those next to horizontal lines indicate the mean number of oocysts. The reduction in the number of oocysts/mosquito by immune sera was significant (P < 0.001; Wilcoxon test).
Finally, we immunized mice with a DNA vaccine encoding Pfs25 prior to infection and evaluation of transmission reduction. These mice after three vaccinations contained Pfs25 antibody titers of 1:128,000 as revealed by ELISA. When these vaccinated mice were infected with TrPfs25Pb parasites, transmission of parasites to mosquitoes was reduced by >80% compared to mice vaccinated with control DNA and infected with TrPfs25Pb in parallel (Fig. 4). Sera from these actively immunized mice when tested in a standard MFA revealed a >50% oocyst reduction in two independent experiments (Table 2). On the other hand, anti-Pfs25 antibodies had no such inhibitory effect on transmission of either WT or Pb28KO parasites used for infection (Fig. 4). Our findings from passive and active immunization experiments thus demonstrate that TrPfs25Pb parasites can be used in a mouse model to evaluate transmission-blocking activities of antibodies induced by Pfs25 vaccines. Various vaccine formulations to optimize potency of Pfs25 vaccines can be tested in mice infected with TrPfs25Pb parasites and malaria transmission to mosquitoes can be evaluated. Likewise, the same murine model can be employed to evaluate sera from nonhuman primates or humans vaccinated with various Pfs25 formulations developed to optimize vaccine potency.
FIG. 4.
Evaluation of transmission-blocking activity by active immunization. Mice were immunized (prime and two boosts 4 weeks apart) with Pfs25-encoding plasmid or control (wild-type) plasmid. Mice were divided into three groups and infected with WT P. berghei, Pb28KO, or TrPfs25Pb and used to assess transmission to mosquitoes. Mosquito midguts were examined 10 days postfeeding. Data points represent mosquitoes that fed on three mice (Pfs25 DNA plasmid) or two mice (control DNA plasmid). Numbers on top of each data point indicate the percentage of mosquitoes infected (total number of mosquitoes dissected), and those next to horizontal lines indicate the mean number of oocysts. The reduction in oocysts of TrPfs25Pb parasites by vaccine-induced immunity was significant (P < 0.001; Wilcoxon test).
TABLE 2.
Comparison of transmission blocking assayed using an MFA versus the P. berghei transgenic in vivo model
| Assay and sample | Expt. I
|
Expt. II
|
||
|---|---|---|---|---|
| No. of oocysts/mosquito (n)a | % Blockingb | No. of oocysts/mosquito (n) | % Blockingb | |
| MFA | ||||
| Control | 158.3 ± 13.56 (82) | 25.4 ± 3.57 (49) | ||
| Immune serum | 75.32 ± 5.99 (185) | 52.4* | 12.7 ± 2.20 (59) | 50* |
| In vivo murine model (three mice/group)c | ||||
| Control | 24 ± 41 (84) | |||
| Immune serum | 4 ± 8 (77) | 83 | ||
Results are means ± SD, with the total number of mosquitoes indicated in parentheses.
Control versus immune serum. *, P < 0.01, Wilcoxon test.
Data are from Fig. 4, for comparison.
DISCUSSION
Antibodies against zygote or ookinete surface proteins can block transmission of malaria by preventing further development of the parasite inside the mosquito vector. Such surface antigens (P25 and P28) have been considered as candidates for the development of TBV, which are expected to play a significant role in reducing malaria transmission (4, 12). TBV, in addition to reducing malaria transmission, can also help to restrict spread of mutant parasites that have become resistant to vaccines targeting other stages or parasites that have become resistant to antimalarial drugs. Other advantages of malaria transmission-blocking vaccines include less immune selection pressure on the parasite in the human host, since antibodies act on the sexual stages inside the mosquito host. Pfs25, among other antigens, represents one such vaccine candidate, and antibodies against Pfs25 induced by experimental immunization in animals, including nonhuman primates, have been shown to block transmission as assessed by MFA (3, 5, 11, 12, 15, 24).
In order to assess the immunological efficacy of a vaccine, it is absolutely critical to employ a robust and reliable assay to assess the functional activity of elicited immune responses. Although there is a lack of a convenient animal model for P. falciparum malaria, MFA has provided valuable insights into functional transmission-blocking activities of sera from immunized animals. However, MFA is an in vitro method, and we addressed the possibility of developing a transgenic murine malaria model to allow immunogenicity evaluations of vaccines based on Pfs25, a strong target of TBV under development. We hypothesized that since P25 and P28 are partially functionally redundant molecules, replacing one of these or both in a rodent malaria parasite (P. berghei) with Pfs25 would not severely compromise their transmission competence. Moreover, if expressed on the surface in the appropriate stage (zygote or ookinete), parasite transmission may be halted by Pfs25-specific antibodies induced by a vaccine. We replaced native pb25 and pb28 with pfs25 to construct a transgenic P. berghei parasite. Our findings were consistent with those for Pvs25 transgenic P. berghei parasites (16) and suggest functional conservation of P25 between the human malaria parasites (P. falciparum and P. vivax) and the murine parasite (P. berghei), since Pfs25 and Pvs25 (16) successfully complemented the functions of both Pb25 and Pb28 during mosquito-stage development. At the amino acid level, Pfs25 and Pb25 share 44% sequence identity, including the presence of N-terminal signal peptides, a C-terminal glycosylphosphatidyinositol anchor signal, and four epidermal growth factor-like domains (6, 10). Interestingly, similar to P. falciparum infections in humans, transcripts of pfs25 were also detected during blood-stage infection with transgenic parasites, though the protein was not expressed in blood-stage parasites, suggesting that regulatory elements required for P25 expression are likely to be functionally conserved between these species.
Assaying P. falciparum transmission-blocking antibodies in mice is an alternate approach that would circumvent the use of the artificial in vitro MFAs. Mice are well-adapted laboratory animals that are inexpensive, easy to handle, and are used in almost every vaccine preclinical evaluation study. More importantly, since there is no P. falciparum animal challenge model to test transmission-blocking antibodies, a mouse model employing TrPfs25Pb parasites would closely mimic vaccination and direct human-to-mosquito transmission reduction. Furthermore, the use of mice could be more informative than the in vitro MFA, since murine blood, like human blood, may have additional immune factors (cytokines and complement) that may synergize the blocking activity of antibodies compared to the fractionated sera or plasma used in a standard MFA. The data presented thus suggest an alternate surrogate assay for transmission-blocking immunity, especially in places where resources and availability of the normal human serum and normal human red blood cells needed to continuously culture and produce infective gametocytes may be limiting. The animal model could be an important tool in preclinical optimization of the Pfs25 vaccine as well as in future testing of sera from people vaccinated with the Pfs25 vaccine, without requiring P. falciparum gametocyte cultures in the vaccination field sites in areas of endemicity. We anticipate that deploying mice to laboratories near vaccine testing sites, although challenging, could provide an alternative for MFAs, which require successful maintenance of infectious P. falciparum gametocytes in culture.
Acknowledgments
We thank Alan Scott for critical reading of the manuscript. We thank Yessika Vasquez and the JHMRI core facilities for P. falciparum parasites and mosquitoes used for these studies.
These studies were supported by NIH grant RO1AI047089 and support from grant RR00052 for the human red blood cells used for parasite culture.
Editor: J. F. Urban, Jr.
Footnotes
Published ahead of print on 3 March 2008.
REFERENCES
- 1.Abraham, E. G., S. Islam, P. Srinivasan, A. K. Ghosh, J. G. Valenzuela, J. M. C. Ribeiro, F. C. Kafatos, G. Dimopoulos, and M. Jacobs-Lorena. 2004. Analysis of the Plasmodium and Anopheles transcriptional repertoire during ookinete development and midgut invasion. J. Biol. Chem. 2795573-5580. [DOI] [PubMed] [Google Scholar]
- 2.Babiker, H. A., A. Abdel-Wahab, S. Ahmed, S. Suleiman, L. Ranford-Cartwright, R. Carter, and D. Walliker. 1999. Detection of low level Plasmodium falciparum gametocytes using reverse transcriptase polymerase chain reaction. Mol. Biochem. Parasitol. 99143-148. [DOI] [PubMed] [Google Scholar]
- 3.Barr, P. J. 1991. Recombinant Pfs25 protein of Plasmodium falciparum elicits malaria transmission-blocking immunity in experimental animals. J. Exp. Med. 1741203-1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter, R. 2001. Transmission blocking malaria vaccines. Vaccine 192309-2314. [DOI] [PubMed] [Google Scholar]
- 5.Coban, C., M. T. Philipp, J. E. Purcell, D. B. Keister, M. Okulate, D. S. Martin, and N. Kumar. 2004. Induction of Plasmodium falciparum transmission-blocking antibodies in nonhuman primates by a combination of DNA and protein immunizations. Infect. Immun. 72253-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.del Carmen Rodriguez, M., P. Gerold, J. Dessens, K. Kurtenbach, R. T. Schwartz, R. E. Sinden, and G. Margos. 2000. Characterisation and expression of pbs25, a sexual and sporogonic stage specific protein of Plasmodium berghei. Mol. Biochem. Parasitol. 110147-159. [DOI] [PubMed] [Google Scholar]
- 7.Duffy, P. E., and D. C. Kaslow. 1997. A novel malaria protein, Pfs28, and Pfs25 are genetically linked and synergistic as falciparum malaria transmission-blocking vaccines. Infect. Immun. 651109-1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greenwood, B. M., K. Bojang, C. J. M. Whitty, and G. A. T. Targett. 2005. Malaria. Lancet 3651487-1498. [DOI] [PubMed] [Google Scholar]
- 9.Kaslow, D. C., I. C. Bathurst, and P. J. Barr. 1992. Malaria transmission-blocking vaccines. Trends Biotechnol. 10388-391. [DOI] [PubMed] [Google Scholar]
- 10.Kaslow, D. C., I. A. Quakyi, C. Syin, M. G. Raum, D. B. Keister, J. E. Coligan, T. F. McCutchan, and L. H. Miller. 1988. A vaccine candidate from the sexual stage of human malaria that contains EGF-like domains. Nature 33374-76. [DOI] [PubMed] [Google Scholar]
- 11.Kaslow, D. C., and J. Shiloach. 1994. Production, purification and immunogenicity of a malaria transmission-blocking vaccine candidate: TBV 25 H expressed in yeast and purified using nickel-NTA agarose. Biotechnology 12494-499. [DOI] [PubMed] [Google Scholar]
- 12.Kumar, N. 2007. A vaccine to prevent transmission of human malaria: a long way to travel on a dusty and often bumpy road. Curr. Sci. 921535-1544. [Google Scholar]
- 13.Kumar, N., and R. Carter. 1985. Biosynthesis of two stage-specific membrane proteins during transformation of Plasmodium gallinaceum zygotes into ookinetes. Mol. Biochem. Parasitol. 14127-139. [DOI] [PubMed] [Google Scholar]
- 14.Malkin, E. M., A. P. Durbin, D. J. Diemert, J. Sattabongkot, Y. Wu, K. Miura, C. A. Long, L. Lambert, A. P. Miles, and J. Wang. 2005. Phase 1 vaccine trial of Pvs25H: a transmission blocking vaccine for Plasmodium vivax malaria. Vaccine 233131-3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petri, W. A., A. W. Stowers, D. B. Keister, O. Muratova, and D. C. Kaslow. 2000. A region of Plasmodium falciparum antigen Pfs25 that is the target of highly potent transmission-blocking antibodies. Infect. Immun. 685530-5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramjanee, S., J. S. Robertson, B. Franke-Fayard, R. Sinha, A. P. Waters, C. J. Janse, Y. Wu, A. M. Blagborough, A. Saul, and R. E. Sinden. 2006. The use of transgenic Plasmodium berghei expressing the Plasmodium vivax antigen P25 to determine the transmission-blocking activity of sera from malaria vaccine trials. Vaccine 25886-894. [DOI] [PubMed] [Google Scholar]
- 17.Rodríguez, M. C., G. Margos, H. Compton, M. Ku, H. Lanz, M. H. Rodríguez, and R. E. Sinden. 2002. Plasmodium berghei: routine production of pure gametocytes, extracellular gametes, zygotes, and ookinetes. Exp. Parasitol. 10173-76. [DOI] [PubMed] [Google Scholar]
- 18.Snow, R. W., C. A. Guerra, A. M. Noor, H. Y. Myint, and S. I. Hay. 2005. The global distribution of clinical episodes of Plasmodium falciparum malaria. Nature 434214-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stowers, A., and R. Carter. 2001. Current developments in malaria transmission-blocking vaccines. Expert. Opin. Biol. Ther. 1619-628. [DOI] [PubMed] [Google Scholar]
- 20.Tirawanchai, N., and R. E. Sinden. 1990. Three non-repeated transmission blocking epitopes recognized in the 21 kDa surface antigen of zygotes-ookinetes of Plasmodium berghei. Parasite Immunol. 12435-446. [DOI] [PubMed] [Google Scholar]
- 21.Tomas, A. M., G. Margos, G. Dimopoulos, L. H. M. van Lin, T. F. de Koning-Ward, R. Sinha, P. Lupetti, A. L. Beetsma, M. C. Rodriguez, and M. Karras. 2001. P25 and P28 proteins of the malaria ookinete surface have multiple and partially redundant functions. EMBO. J. 203975-3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Toure, Y. T. 1998. Gametocyte infectivity by direct mosquito feeds in an area of seasonal malaria transmission: implications for Bancoumana, Mali as a transmission-blocking vaccine site. Am. J. Trop. Med. Hyg. 59481-486. [DOI] [PubMed] [Google Scholar]
- 23.Tsuboi, T., Y. M. Cao, Y. Hitsumoto, T. Yanagi, H. Kanbara, and M. Torii. 1997. Two antigens on zygotes and ookinetes of Plasmodium yoelii and Plasmodium berghei that are distinct targets of transmission-blocking immunity. Infect. Immun. 652260-2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu, Y., C. Przysiecki, E. Flanagan, S. N. Bello-Irizarry, R. Ionescu, O. Muratova, G. Dobrescu, L. Lambert, D. Keister, and Y. Rippeon. 2006. Sustained high-titer antibody responses induced by conjugating a malarial vaccine candidate to outer-membrane protein complex. Proc. Natl. Acad. Sci. USA 10318243-18248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zollner, G. E., N. Ponsa, R. E. Coleman, J. Sattabongkot, and J. A. Vaughan. 2005. Evaluation of procedures to determine absolute density of P. vivax ookinetes. J. Parasitol. 91453-457. [DOI] [PubMed] [Google Scholar]