Abstract
The extracellular adhesion protein (Eap) secreted by the major human pathogen Staphylococcus aureus is known to have several effects on human immunity. We have recently added to knowledge of these roles by demonstrating that Eap enhances interactions between major histocompatibility complex molecules and human leukocytes. Several studies have indicated that Eap can induce cytokine production by human peripheral blood mononuclear cells (PBMCs). To date, there has been no rigorous attempt to identify the breadth of cytokines produced by Eap stimulation or to identify the cell subsets that respond. Here, we demonstrate that Eap induces the secretion of the proinflammatory cytokines interleukin 6 (IL-6) and tumor necrosis factor alpha (TNF-α) by CD14+ leukocytes (monocytes and macrophages) within direct ex vivo PBMC populations (note that granulocytes are also CD14+ but are largely depleted from PBMC preparations). Anti-intercellular adhesion molecule 1 (CD54) antibodies inhibited this induction and implicated a role for this known Eap binding protein in cellular activation. IL-6 and TNF-α secretion by murine cells exposed to Eap was also observed. The activation of CD14+ cells by Eap suggests that it could play a significant role in both septic shock and fever, two of the major pathological features of S. aureus infections.
Staphylococcus aureus is a major human pathogen, with strains that are resistant to antibiotics emerging worldwide (e.g., the methicillin- and vancomycin-resistant S. aureus strains) (13, 15). While it is largely a commensal organism living asymptomatically in the nasal cavities of a large proportion of the human population (17), it also causes infections that range widely in both body site and severity. Skin infections such as impetigo, folliculitis, and boils can be caused by S. aureus, as can life-threatening illnesses such as endocarditis, osteomyelitis, and septicemia (13). Despite its largely commensal lifestyle, this organism expresses a large number of factors such as toxins, adhesins, invasins, and modulins that contribute to its pathogenicity. These factors allow the bacteria to attach to and invade host tissues, causing widespread damage, and they also facilitate evasion of the host's protective immune responses (5, 19).
S. aureus secretes a multirepeat protein known as the extracellular adhesion protein (Eap) (7) or the major histocompatibility complex (MHC) analog protein (Map) (9). This is a member of the secreted expanded repertoire adhesive molecule (SERAM) family (3), and a recent study has shown that at least 98% of S. aureus strains secrete a form of this protein (1). With some strain-to-strain variation, the Eap consists of four to six repeats of approximately 110 amino acids (1). Structural studies have revealed homology between the individual repeats of Eap and the C-terminal half of superantigens such as toxic shock syndrome toxin 1 (TSST-1) and staphylococcal enterotoxin B, suggesting this protein may have superantigenic activity (6). A recent study comparing the activity of Eap to that of a known superantigen (TSST-1) showed that while similarities exist between the two proteins in relation to their ability to cross-link molecules, Eap's activity was not as specific as that of TSST-1 (14).
The ability of Eap to bind many host factors is well established (7), and the potential downstream effects of these interactions, which include T-cell modulation (11) and the prevention of leukocyte extravasation (2), have suggested that Eap may act as an anti-inflammatory factor. This anti-inflammatory activity has led to the proposition that this protein may be used to treat diseases such as multiple sclerosis (20). To determine what effects Eap's cross-linking activity has on inflammatory cytokine expression by human cells, we incubated Eap with human peripheral blood mononuclear cells (PBMCs) and found that it resulted in the induction of proinflammatory cytokine expression (interleukin 6 [IL-6] and tumor necrosis factor alpha [TNF-α]). Despite its structural similarity to superantigens, Eap's proinflammatory activity was not dependent upon its cross-linking activity where a single repeat was active. We observed no cytokine secretion by T cells; instead, CD14+ cells were responsible for IL-6 and TNF-α secretion. Production of these proinflammatory cytokines was reduced by the preincubation of cells with anti-intercellular adhesion molecule 1 (ICAM-1; CD54) antibodies, suggesting that this ligand is involved in Eap-induced activation. The effects of Eap on murine cells were tested with three different cell populations, and the degree of proinflammatory activation was found to vary depending on the anatomical site from which the cells were harvested.
MATERIALS AND METHODS
Isolation of human PBMCs.
Blood was collected from healthy volunteers. PBMCs were isolated from heparinized blood by density-gradient centrifugation over Lymphoprep (Axis Shield). PBMCs were washed in RPMI medium supplemented with 100 units/ml penicillin, 100 units/ml streptomycin, and 2 mM l-glutamine (Sigma-Aldrich).
Antibodies.
Anti-CD14-PerCP (BD Biosciences), anti-IL-6-phycoerythrin (PE), and anti-TNF-α-PE antibodies (Caltag-Medsystems Ltd.) were used in this study. Anti-CD14, anti-CD19, anti-CD54, and anti-CD66 antibodies were purchased from Serotec and used at a concentration of 50 μg/ml.
Native and recombinant Eap purification.
Native and pseudo-Eap were purified from the Newman, mAH12, and mAH12(pCXEap) strains as described previously (14). As such, protein preparations from Newman and mAH12(pCXEap) contain full-length Eap protein and the preparation from mAH12 contains no Eap (the negative control, referred to as pseudo-Eap). Recombinant forms of Eap repeat subunits (Eap19 and Eap10, also known as Map19 and Map10) were purified as described previously (12). Eap19 consisted of amino acids Gln20 to Gln240 and Eap10 of amino acids Gln20 to Gln130 of the mature peptide. All protein preparations were tested for the presence of contaminating endotoxins by using an E-TOXATE kit from Sigma in accordance with the manufacturer's instructions and found to contain undetectable levels of endotoxin.
Cytokine secretion assay.
Cells were resuspended in RPMI medium containing 10% fetal calf serum (FCS). Cells (2 × 105 per 96-well U-bottom plate well) were incubated in the presence or absence of the indicated concentration of Eap protein preparations for 6 h at 37°C with 5% CO2. Phytohemagglutinin (PHA; 5 μg/ml; Sigma-Aldrich) was used as a positive control. Assays were performed in triplicate and repeated three times with PBMCs isolated from different donors. Following its incubation, the supernatant was assayed for IFN-γ, TNF-α, IL-2, IL-4, IL-6, and IL-10, using a Th1/2 Cytometric Bead array kit (BD Biosciences) according to the manufacturer's instructions. The results were averaged and are presented as either the mean concentration in the supernatant (± standard error of the mean [SEM]) or as the mean induction (n-fold) of the level of cytokine expressed by the cells in the absence of Eap (±SEM). Student's t test was used to determine P values. Antibody blocking was performed by incubating the PBMCs in 50 μg/ml of the indicated antibody for 30 min prior to the addition of 5 μg/ml Eap19.
Intracellular cytokine staining assay.
Fresh PBMCs (1 × 106) were washed and resuspended in RPMI medium supplemented with 100 units/ml penicillin, 100 units/ml streptomycin, and 2 mM l-glutamine and 10% FCS. Cells were incubated in the presence or absence of Eap10 protein (10 μg/ml) (this preparation of Eap was used as the others aggregated the cells, making the study of individual cells and their fluorescence intensities difficult) for 4 h at 37°C with 5% CO2. Brefeldin A (10 μg/ml) was added after 1 h, and following the incubation, the cells were washed in cold phosphate-buffered saline (PBS), stained with surface marker antibodies, washed again, permeabilized with Cytofix/Cytoperm (BD Biosciences), and stained with antibodies to intracellular proteins. Experiments were performed in triplicate and repeated three times with PBMCs isolated from different donors.
Isolation and activation of murine cells.
Mice were bred and maintained in a conventional pathogen-free animal facility. All experiments were performed using age-matched female BALB/c mice and with the permission of the Home Office, according to the Animals Scientific Procedures Act of 1986. Spleens were harvested from naïve BALB/c mice, and single-cell suspensions were prepared. Blood was collected in heparin, and red blood cells were lysed by using ACK lysing buffer (Invitrogen). To obtain peritoneal macrophages, mice were sacrificed and peritoneal lavage was performed with 10 ml of sterile isotonic saline (PBS), and total cell numbers were counted. All cells were resuspended in RPMI medium supplemented with 100 units/ml penicillin, 100 units/ml streptomycin, and 2 mM l-glutamine (Sigma-Aldrich). For the assay, 1 × 106 splenocytes, 2 × 105 blood cells, and 2 × 105 peritoneal macrophages were incubated with Eap, pseudo-Eap, medium only, or lipopolysaccharide (LPS; as the positive control) for 6 h. The cell supernatant was then collected and assayed for IL-12p70, TNF-α, IFN-γ, MCP-1, IL-10, and IL-6, using a mouse inflammation bead array kit (BD Biosciences).
RESULTS
Eap induces the expression of proinflammatory cytokines by human PBMCs.
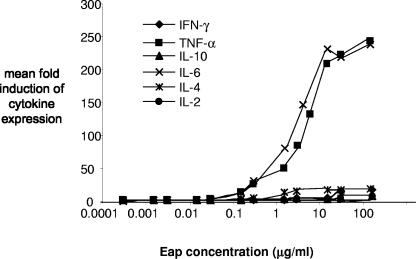
Previous studies have found that the Eap protein can induce the expression of IL-4 by PBMCs (8). Based on these and other studies, it has been suggested that Eap skews the immune response toward a Th2 rather than a Th1 immune response (7, 11). To confirm this, we tested the supernatant of human PBMCs exposed to a range of native Eap protein concentrations (Fig. 1) for the presence of cytokines, using a Th1/Th2 cytometric bead array. A dose-dependent effect of Eap on the secretion of several cytokines was observed (Fig. 1). In agreement with a previous study (8), we saw Eap-induced induction of IL-4 (P = 0.02); however, substantially larger amounts of IL-6 and TNF-α were induced (P < 0.01). These findings do not support the assertion that Eap induces a Th2-type response.
FIG. 1.
Eap induces the expression of proinflammatory cytokines by PBMCs. Cytokine production was measured with the Th1/Th2 cytometric bead array in PBMC supernatant and is expressed as the increased (fold) expression relative to the PBMCs incubated in medium alone. The native Eap protein from the Newman strain induces the expression of TNF-α and IL-6 (proinflammatory cytokines) by PBMCs at high levels.
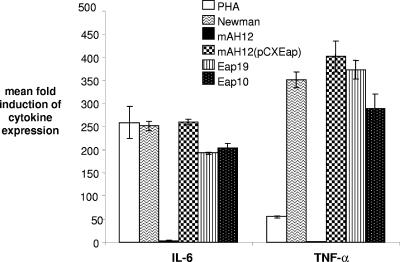
To verify that IL-6 and TNF-α secretion induction was Eap specific, cytokine production was assayed after incubation with equimolar concentrations of full-length native Eap (from the wild-type Newman strain [10 μg/ml]), pseudo-Eap (from mAH12, a Newman mutant lacking Eap), full-length Eap isolated from the complemented mutant strain [mAH12(pCXEap); 10 μg/ml]; and a recombinant form of the first two repeats of Eap (Eap19 [3 μg/ml] and a recombinant form of the first repeat of Eap [Eap10; 1.5 μg/ml]). All preparations containing Eap protein induced the secretion of high levels of IL-6 and TNF-α (Fig. 2), while pseudo-Eap from the knockout strain did not (P < 0.01). Eap10-induced (a single repeat of Eap) expression suggests a direct effect of Eap binding rather than an effect resulting from the cross-linking of molecules or cells, as two or more repeats of Eap are required for this (14). These data demonstrate that Eap can act as a proinflammatory mediator.
FIG. 2.
Eap alone is responsible for the induction of cytokine expression, and a single repeat is sufficient. PHA, control mitogen; Eap purified from Newman (the wild-type S. aureus strain); pseudo-Eap purified from the mAH12 strain lacking eap; Eap purified from the complemented mAH12(pCXEap) strain; Eap19, recombinant double repeat of Eap; and Eap10, recombinant single repeat of Eap.
Eap activates the CD14+ leukocytes.
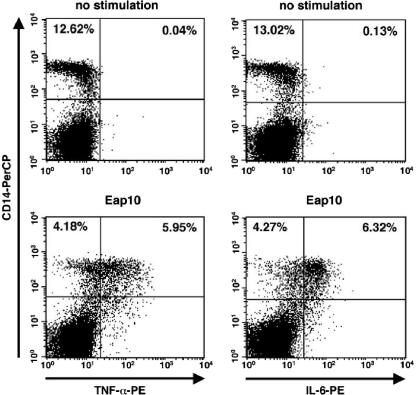
To determine which cells are activated by Eap, intracellular cytokine staining was performed with PBMCs following their incubation with Eap10 (1 μg/ml). It was necessary to use the single repeat of Eap for these experiments as cell aggregation was often observed when multiple-repeat forms of Eap were used. Figure 3 shows the expression levels of TNF-α and IL-6 in PBMCs with and without Eap stimulation. Costaining cells with fluorochrome-labeled anti-CD14 antibodies showed that CD14+ cells (i.e., monocytes; note that granulocytes are also CD14+ but are largely depleted from PBMC preparations) express IL-6 and TNF-α after Eap stimulation. CD14− cells, such as T cells, were not expressing these cytokines.
FIG. 3.
CD14+ cells express IL-6 and TNF-α following stimulation by Eap. Intracytoplasmic staining was performed to determine which cells were being activated by Eap. The panels on the left show TNF-α expression in unstimulated (upper plot) and Eap10-stimulated (lower plot) PBMCs. The panels on the right show IL-6 expression in unstimulated (upper plot) and Eap10-stimulated (lower plot) PBMCs. Only CD14+ cells produce proinflammatory cytokines in response to Eap.
Anti-ICAM-1 antibodies block cell activation by Eap.
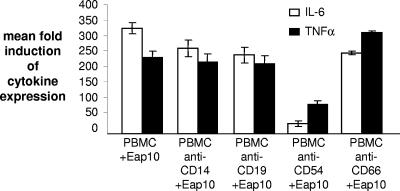
A previous study demonstrated that Eap binds to ICAM-1 (CD54) on cell surfaces (2). We used antibodies to this protein to determine whether this ligand played a role in the induction of CD14+ cells to produce IL-6 and TNF-α by Eap10. PBMCs were incubated with Eap10 (1 μg/ml) following incubation in the presence of different antibodies. Blocking antibodies (the same isotype as our anti-CD54 antibody) to CD14, CD19, and CD66 had a small effect on IL-6 expression but no effect on TNF-α expression (P = 0.8), but incubation with anti-ICAM-1 antibody significantly reduced IL-6 and TNF-α expression (P < 0.01) (Fig. 4). The anti-CD54 antibody had no effect on the proinflammatory activity of PHA or LPS, suggesting that the effect is specific. This suggests that the ICAM-1 molecule is involved in Eap-mediated induction of proinflammatory cytokine expression by CD14+ cells.
FIG. 4.
Anti-ICAM-1 (CD54) antibodies block Eap-induced IL-6 and TNF-α secretion. PBMCs were preincubated with a range of antibodies to identify the ligand used by Eap to induce cytokine expression. Only anti-CD54 (ICAM-1) antibodies significantly reduce cytokine expression.
Eap can induce IL-6 and TNF-α expression by murine cells.
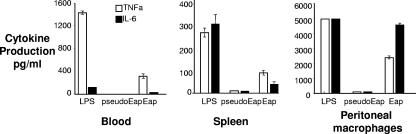
To determine whether the induction of proinflammatory cytokines by Eap was specific to humans, the ability of Eap to stimulate the production of IL-6 and TNF-α by three different murine cell populations was tested (Fig. 5). Cells harvested from blood and spleens were exposed to 5 μg/ml Eap. The level of cytokine production by the peritoneal macrophages following exposure to 5 μg/ml Eap was beyond the levels of detection of our assay, and so the data from the exposure of these cells to 1 μg/ml of Eap are shown. IL-6 and TNF-α were detected in all samples incubated with Eap, with the peritoneal macrophages showing the highest level of cytokine production. It should be noted that of the three cell preparations, the peritoneal macrophages contained the highest proportion of CD14+ cells.
FIG. 5.
Eap induced TNF-α and IL-6 secretion by murine cells. Murine cells were harvested from blood, from the peritoneal cavity, and from the spleens of mice. All three populations were tested for the ability to secrete IL-6 and TNF-α upon exposure to native Eap protein relative to that of the negative pseudo-Eap control. LPS was used as a positive control.
DISCUSSION
Inflammation is a local tissue response to infection or damage. It is a complex process that leads to an elevated blood supply and an increase in vascular permeability in the blood vessels surrounding the affected area. Included in the series of events leading to inflammation is an increased ICAM-1 expression in the surrounding vascular endothelium, resulting in increased adhesion and migration of leukocytes to the affected area. The expression of proinflammatory cytokines is also increased, resulting in a cascade of events that culminate in inflammation. Previous studies of the activity of the Eap protein suggest that it is anti-inflammatory due to its ability to bind ICAM-1 and as a consequence to block leukocyte recruitment to an inflamed area (2). It has also been reported that Eap induces the expression of the anti-inflammatory cytokine IL-4 by human cells (8).
We have previously shown that Eap has the ability to increase the binding of MHC molecules to human leukocytes (14), and here we examined what effects this might have on cytokine expression by human cells. Using a Th1/Th2 cytokine bead array, we also noted an increase in IL-4; however, the increase in expression of the proinflammatory cytokines IL-6 and TNF-α was substantially higher. Technology has advanced such that the expression levels of multiple cytokines can be simultaneously assayed in a single experiment. The former study reports only on the examination of expression of IL-4 and IFN-γ and so would not have detected any increase in IL-6 or TNF-α expression.
Immunomodulatory proteins expressed by S. aureus can have human-specific activity (4), and we initially believed that the explanation for the apparent conflict between our findings of proinflammatory activity and the anti-inflammatory activity reported in murine studies (2, 20) could be explained by such host specificity. We found some body site specificity in murine cellular responses to Eap, a likely reflection of the proportion of CD14+ cells present, but conclude that Eap also appears to be proinflammatory in mice. It is likely that the expression of proinflammatory cytokines and the downstream effects of this, such as the induction of fever, were beyond the remit of the murine studies that established the anti-inflammatory activity for this protein. The findings of this study in combination with the previous murine reports show that through interaction with ICAM-1, the Eap protein can induce an inflammatory response through the induction of IL-6 and TNF-α and, at the same time, prevent leukocyte extravasation.
Our previous findings (14) led us to believe that Eap is not a superantigen, and as further evidence for this, we observed no cytokine secretion by naive T cells. Activation of T cells by superantigens requires the cross-linking of MHC class II molecules with T-cell receptors on the T-cell surface (12). We have demonstrated that the single-repeat unit present in the Eap10 protein is insufficient to induce such Eap “cross-linking” (14). In contrast, the Eap10 molecule was sufficient to induce cytokine production by CD14+ cells (Fig. 3), suggesting different requirements for the “cross-linking” and CD14+ cell-activating activities of the Eap protein. Antibody inhibition experiments (Fig. 4) suggest that ICAM-1 plays a role in the activation of CD14+ cells. However, ICAM-1 is also expressed on some CD14− cells (10, 16). These cells do not respond to Eap (Fig. 3), suggesting that additional factors may also be involved in the Eap-induced activation of cytokine expression by CD14+ cells.
In summary, our findings extend those of previous studies by showing that the Eap protein can induce both human and murine cells to produce copious amounts of IL-6 and TNF-α. This potential proinflammatory role of Eap has not been appreciated previously and may be significant in vivo. We believe that the effects of Eap are likely to be highly variable depending on both the body site and the levels of expression of both ICAM-1 and Eap. A recent study has found that relative to the antibodies in healthy individuals, those of Eap were significantly higher in patients suffering from infective endocarditis caused by S. aureus infection (18). This suggests that significant amounts of Eap must be present in the bloodstream to induce an antibody response. Although the exact concentration reached is as yet unknown, we believe that this and other studies suggest Eap could reach levels sufficient to induce septic shock and fever. Further in vivo work is required to examine this and to establish the concentrations of Eap protein in the bloodstream during infections. This study opens up the intriguing possibility that Eap-induced activation of CD14+ cells in vivo plays a significant role in disease and that strategies aimed at preventing such activation may be helpful in treating patients infected with this increasingly problematic human pathogen.
Editor: J. L. Flynn
Footnotes
Published ahead of print on 10 March 2008.
REFERENCES
- 1.Buckling, A., J. Neilson, J. Lindsay, R. Ffrench-Constant, M. Enright, N. Day, and R. C. Massey. 2005. Clonal distribution and phase-variable expression of a major histocompatibility complex analogue protein in Staphylococcus aureus. J. Bacteriol. 1872917-2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chavakis, T., M. Hussain, S. M. Kanse, G. Peters, R. G. Bretzel, J. I. Flock, M. Herrmann, and K. T. Preissner. 2002. Staphylococcus aureus extracellular adherence protein serves as anti-inflammatory factor by inhibiting the recruitment of host leukocytes. Nat. Med. 8687-693. [DOI] [PubMed] [Google Scholar]
- 3.Chavakis, T., K. Wiechmann, K. T. Preissner, and M. Herrmann. 2005. Staphylococcus aureus interactions with the endothelium: the role of bacterial “secretable expanded repertoire adhesive molecules” (SERAM) in disturbing host defense systems. Thromb. Haemost. 94278-285. [DOI] [PubMed] [Google Scholar]
- 4.de Haas, C. J., K. E. Veldkamp, A. Peschel, F. Weerkamp, W. J. Van Wamel, E. C. Heezius, M. J. Poppelier, K. P. Van Kessel, and J. A. van Strijp. 2004. Chemotaxis inhibitory protein of Staphylococcus aureus, a bacterial antiinflammatory agent. J. Exp. Med. 199687-695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foster, T. J. 2005. Immune evasion by staphylococci. Nat. Rev. Microbiol. 3948-958. [DOI] [PubMed] [Google Scholar]
- 6.Geisbrecht, B. V., B. Y. Hamaoka, B. Perman, A. Zemla, and D. J. Leahy. 2005. The crystal structures of EAP domains from Staphylococcus aureus reveal an unexpected homology to bacterial superantigens. J. Biol. Chem. 28017243-17250. [DOI] [PubMed] [Google Scholar]
- 7.Harraghy, N., M. Hussain, A. Haggar, T. Chavakis, B. Sinha, M. Herrmann, and J. I. Flock. 2003. The adhesive and immunomodulating properties of the multifunctional Staphylococcus aureus protein Eap. Microbiology 1492701-2707. [DOI] [PubMed] [Google Scholar]
- 8.Jahreis, A., P. Beckheinrich, and U. F. Haustein. 2000. Effects of two novel cationic staphylococcal proteins (NP-tase and p70) and enterotoxin B on IgE synthesis and interleukin-4 and interferon-gamma production in patients with atopic dermatitis. Br. J. Dermatol. 142680-687. [DOI] [PubMed] [Google Scholar]
- 9.Jonsson, K., D. McDevitt, M. H. McGavin, J. M. Patti, and M. Höök. 1995. Staphylococcus aureus expresses a major histocompatibility complex class II analog. J. Biol. Chem. 27021457-21460. [DOI] [PubMed] [Google Scholar]
- 10.Lebedeva, T., M. L. Dustin, and Y. Sykulev. 2002. ICAM-1 co-stimulates target cells to facilitate antigen presentation. Curr. Opin. Immunol. 17251-258. [DOI] [PubMed] [Google Scholar]
- 11.Lee, L. Y., Y. J. Miyamoto, B. W. McIntyre, M. Höök, K. W. McCrea, D. McDevitt, and E. L. Brown. 2002. The Staphylococcus aureus Map protein is an immunomodulator that interferes with T cell-mediated responses. J. Clin. Investig. 1101461-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li, H., A. Llera, E. L. Malchiodi, and R. A. Mariuzza. 1999. The structural basis of T cell activation by superantigens. Annu. Rev. Immunol. 17435-466. [DOI] [PubMed] [Google Scholar]
- 13.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339520-532. [DOI] [PubMed] [Google Scholar]
- 14.Massey, R. C., T. J. Scriba, E. L. Brown, R. E. Phillips, and A. K. Sewell. 2007. Use of peptide-major histocompatibility complex tetramer technology to study interactions between Staphylococcus aureus proteins and human cells. Infect. Immun. 755711-5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menichetti, F. 2005. Current and emerging serious Gram-positive infections. Clin. Microbiol. Infect. 322-28. [DOI] [PubMed] [Google Scholar]
- 16.Milićević, N. M., Z. Milićević, and J. Westermann. 2002. Lymphocyte function-associated antigen-1 and intercellular adhesion molecule-1 expression on B-cell subsets and the effects of splenectomy-experimental studies. Leuk. Lymphoma 432071-2074. [DOI] [PubMed] [Google Scholar]
- 17.Peacock, S. J., I. de Silva, and F. D. Lowy. 2001. What determines nasal carriage of Staphylococcus aureus? Trends Microbiol. 9605-610. [DOI] [PubMed] [Google Scholar]
- 18.Rindi, S., S. Cicalini, G. Pietrocola, M. Venditti, A. Festa, T. J. Foster, N. Petrosillo, and P. Speziale. 2006. Antibody response in patients with endocarditis caused by Staphylococcus aureus. Eur. J. Clin. Investig. 36536-543. [DOI] [PubMed] [Google Scholar]
- 19.Rooijakkers, S. H., K. P. van Kessel, and J. A. van Strijp. 2005. Staphylococcal innate immune evasion. Trends Microbiol. 13596-601. [DOI] [PubMed] [Google Scholar]
- 20.Xie, C., P. Alcaide, B. V. Geisbrecht, D. Schneider, M. Herrmann, K. T. Preissner, F. W. Luscinskas, and T. Chavakis. 2006. Suppression of experimental autoimmune encephalomyelitis by extracellular adherence protein of Staphylococcus aureus. J. Exp. Med. 203985-994. [DOI] [PMC free article] [PubMed] [Google Scholar]