Abstract
IdeS, a secreted cysteine protease of the important human pathogen Streptococcus pyogenes, interferes with phagocytic killing by specifically cleaving the heavy chain of immunoglobulin G (IgG). Two allelic variants of the enzyme have been described, the IgG-specific endopeptidase, IdeS (or Mac-1) and Mac-2, a protein with only weak IgG endopeptidase activity, which has been suggested to interfere with opsonophagocytosis by blocking Fcγ receptors of phagocytic cells. However, despite the fact that Mac-2 proteins interact with Fcγ receptors, no inhibition of reactive oxygen species (ROS) production, opsonophagocytosis, or streptococcal killing by Mac-2 has been reported. In the present study, Mac-2 proteins are shown to contain IgG endopeptidase activity indistinguishable from the enzymatic activity exhibited by IdeS/Mac-1 proteins. The earlier reported weak IgG endopeptidase activity appears to be unique to Mac-2 of M28 serotype strains (Mac-2M28) and is most likely due to the formation of a disulfide bond between the catalytic site cysteine and a cysteine residue in position 257 of Mac-2M28. Furthermore, Mac-2 proteins are shown to inhibit ROS production ex vivo, independently of the IgG endopeptidase activity of the proteins. Inhibition of ROS generation per se, however, was not sufficient to mediate streptococcal survival in bactericidal assays. Thus, in contrast to earlier studies, implicating separate functions for IdeS and Mac-2 protein variants, the current study suggests that Mac-2 and IdeS are bifunctional proteins, combining Fcγ receptor binding and IgG endopeptidase activity. This finding implies a unique role for Mac-2 proteins of the M28 serotype, since this serotype has evolved and retained a Mac-2 protein lacking IgG endopeptidase activity.
The gram-positive bacteria Streptococcus pyogenes is a common human bacterial pathogen and the causative agent of a variety of clinical conditions, including pharyngotonsillitis, impetigo, scarlet fever, septicemia, necrotizing fasciitis, and the streptococcal toxic shock syndrome (5-7). The survival of S. pyogenes in its human host relies on its ability to circumvent the various actions of the human immune response. S. pyogenes has evolved a specific enzyme to avoid recognition by opsonizing immunoglobulin G (IgG) antibodies and to interfere with Ig Fc-mediated phagocytosis. This enzyme, designated IdeS, or streptococcal Mac-1 (13, 18), is a secreted cysteine protease that specifically cleaves the heavy chain (Hc) of IgG (1, 16, 18). Based on differences in the amino acid sequence of the middle one-third of the protein, two protein variants of IdeS, complex I and complex II, have been described (14). Complex I variants, hereafter designated as IdeS (18), exert their inhibitory function through proteolytic cleavage of IgG (1, 16, 18). Complex II variants, hereafter designated as Mac-2 (1), were reported to contain only weak IgG endopeptidase activity (1, 14). Instead, Mac-2 variants were suggested to interfere with opsonophagocytosis through their interaction with Fcγ receptors of phagocytic cells (1, 14). However, in contrast to this suggested functional mechanism, Mac-2 failed to affect the production of reactive oxygen species (ROS) and inhibited neither opsonophagocytosis nor streptococcal killing by human polymorphonuclear leukocytes (PMNs) (14). The crystal structure of IdeS has been determined (2, 21), and properties of the Mac-2 protein have been inferred from the determined crystal structures (2). From these structures, it was suggested that a cysteine residue at position 257 of Mac-2 of M28 serotype strains (Mac-2M28) could interfere with substrate recognition through the formation of a disulfide bond with the catalytic cysteine of the active site (2). This suggestion has earlier been put forward to explain the weak IgG endopeptidase activity of S. pyogenes Mac-2M28 proteins (4). In the latter study, sequence analysis of the Mac-2 allele from several clinical isolates revealed that only strains of the M28 serotype express a Mac-2 variant carrying a cysteine residue in the flexible loop region, while Mac-2 variants of all other strains analyzed in the study had a tyrosine residue in the corresponding position (4). Therefore, it has been suggested that previous characterizations of Mac-2, which were performed using recombinant Mac-2M28 (1, 14) might not generally apply to Mac-2 proteins secreted by other serotypes (4). Supported by the finding that mac-2 clinical streptococcal isolates exhibit IgG endopeptidase activity (4), the goal of the present study was to clarify the role of the streptococcal Mac-2 protein in the inhibition of opsonophagocytosis and to try to shed light on the discrepancy of the reported Mac-2 binding to Fcγ receptors and its inability to inhibit phagocytic killing and ROS production of PMNs. We show that impaired enzymatic activity of Mac-2M28 is in fact due to the additional cysteine residue in the flexible loop of the protein and is thus unique to the M28 serotype. We demonstrate that Mac-2 proteins of the other streptococcal serotypes exhibit IgG endopeptidase activity indistinguishable from that of IdeS and that bacterial survival in bactericidal assays is significantly promoted in the presence of enzymatically active Mac-2 proteins, including the enzymatically active Mac-2M28 mutant protein, while native Mac-2M28 protein with weak enzymatic activity only has minor influence on bacterial survival. However, we also present support for the suggested function of Mac-2 to act through binding of Fcγ receptor in that we demonstrate that Mac-2M28 and Mac-2M8 inhibit ROS production ex vivo independently of their enzymatic activity. Thus, it appears that streptococcal M28 serotype strains express a Mac-2 protein mainly designed to target PMN cell receptors, while Mac-2 proteins of other serotypes also are efficient IgG endopeptidases. These results underline the importance of IgG endopeptidase activity for streptococcal survival in its human host, but more importantly also highlight the fact that ongoing allelic variation contributes to changes in streptococcal virulence and potentially affects the outcome of streptococcal disease.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The S. pyogenes strains used in this study have been described previously (4). Escherichia coli strain NovaBlue (Novagen) was used for gene cloning, and E. coli BL21 (Novagen) was used for recombinant protein expression.
S. pyogenes cells were routinely grown in Todd-Hewitt (TH) broth (BD Biosciences) at 37°C in 5% CO2 or in TH broth supplemented with 1% heparinized human plasma at 37°C in 5% CO2.
Igs.
Human polyclonal IgG and myeloma IgG1 (kappa) were purchased from Sigma. Myeloma IgG1 was labeled with 125I using the Bolton and Hunter reagent as described by the manufacturer (GE Amersham Bioscience). Free 125I was separated from labeled protein on a PD10 column (GE Amersham Bioscience). Antistreptococcal antibodies were purchased from Abcam, Cambridge, United Kingdom. Rabbit antiserum against IdeS/Mac-2 was generated by Agriserum, Vännäs, Sweden, using purified recombinant IdeS as the antigen. Polyclonal IgG antibodies from two different rabbits were affinity purified using protein G Sepharose 4 Fast Flow (GE Amersham Biosciences).
Western blot analysis.
Proteins secreted by S. pyogenes into TH growth medium were precipitated by adding trichloroacetic acid (TCA) to a final concentration of 5%. Precipitates were resuspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer, separated by 12% SDS-PAGE, and transferred to an Immobilon-P polyvinylidene difluoride membrane (Millipore) using a semidry electrophoretic transfer cell (Bio-Rad). Primary antibodies were diluted in phosphate-buffered saline (PBS) (1:2,000), and membranes were hybridized for 30 min at room temperature. Secondary peroxidase-labeled polyclonal goat anti-rabbit antibodies (Bio-Rad), diluted 1:3,000 in PBS, were used for detection. Immunoreactive proteins were detected using an ECL Plus Western blotting detection system (GE Amersham Biosciences) according to the manufacturer's instructions.
Expression and purification of recombinant Mac-2.
PCR amplification and cloning of Mac-2 alleles was performed as previously described (4). For PCR amplification of mac-2, primers Ide1 (5′-TCGGTAGATCGTGGGATCCTAGCAGATAGT-3′), introducing a BamHI site, and Ide2 (5′-CGGAATTCTTAATTGGTCTGATTCCAAC-3′), introducing an EcoRI, site were used. PCR fragments were digested with restriction enzymes BamHI and EcoRI (Roche Diagnostics) and cloned into the corresponding sites of plasmid pGEX-5X-3 (GE Amersham Biosciences). Protein expression was induced by addition of 0.1 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at an optical density at 620 nm (OD620) of ∼0.2. Fusion proteins were purified on glutathione-Sepharose (GE Amersham Biosciences) according to standard protocols, and the glutathione S-transferase (GST) tag protein was removed by Factor Xa cleavage (Novagen) according to standard protocols. The purity of the recombinant proteins was assessed by 12% SDS-PAGE.
Site-directed in vitro mutagenesis.
Plasmid pGEX-5X-3 carrying mac-2M28 and mac-2M8 was used as a template for site-directed in vitro mutagenesis using primer (C257Y) 5′-TCACACCCTACGCTAACGTACGC-3′ for mac-2M28 and primer (Y257C) 5′-TCACACCCGACGCTAACGTACGC-5′ for mac-2M8 together with the Transformer site-directed mutagenesis kit (Clontech) according to the supplier's instructions. Successful mutagenesis was confirmed by sequence analysis using an Applied Biosystems 3100 automated sequencer (Applied Biosystems).
IgG endopeptidase activity assays.
For standard IdeS activity assays in bacterial growth medium, bacteria were grown to an OD620 of ∼0.4 in TH broth, supplemented with 5% heparinized human plasma. Cleavage of IgG was determined by analyzing supernatant samples on 12% SDS-PAGE, and IdeS activity was determined by the presence or absence of a diagnostic 31-kDa IgG cleavage product (CP) (14, 16, 18). For determination of purified Mac-2 protein activity, proteins were incubated with 3 μg human myeloma IgG1 (Sigma-Aldrich) in 20 mM Tris (pH 6.8) at 37°C. IgG cleavage was determined by the presence or absence of a diagnostic 31-kDa IgG CP on 12% SDS-PAGE.
Quantification of IgG endopeptidase activity.
IgG endopeptidase activity was quantified as previously described (4, 10). Briefly, purified IgG endopeptidase was incubated with unlabeled myeloma IgG1 (human IgG1; Sigma-Aldrich) and 125I-IgG (∼105 cpm). IgG and IgG cleavage products were separated by 12% SDS-PAGE and visualized by staining with Coomassie brilliant blue (R-250) (USB). Bands on the SDS-PAGE corresponding to IgG Hc, IgG light chain (Lc), and IgG CP were excised from the gel for determination of radioactivity. Samples were counted in an LKB Wallac Compugamma counter, and Mac-2 activity was calculated as the ratio of radioactivity in the CP to total Hc radioactivity (CP + Hc) (4).
Measurement of ROS production.
The generation of ROS in whole-blood samples was induced by the addition of IgG-opsonized latex beads. Latex beads (Sigma) were incubated with polyclonal IgG (1 mg/ml) in 30 mM MES (morpholineethanesulfonic acid [pH 6.8]) buffer. After incubation, beads were washed in PBS prior to the addition to human blood. ROS generation was measured as chemiluminescence using an ABEL cell activation kit (Knight Scientific, Ltd., United Kingdom) according to the manufacturer's instructions. ROS generation was monitored continuously for 90 min using a Sirius luminometer (Berthold Detection Systems). The rate of ROS production was determined in 5-min intervals. For example, ROS production at time 30 represents the ratio of ROS production between 25 and 30 min compared to ROS generation between 20 and 25 min. Inhibition of ROS peak production was determined by calculating the average ROS production from 8 min to 45 min (peak production) in the presence of 5 μg Mac-2 or IdeS proteins, compared to peak production in the buffer control that arbitrarily has been set to 100. All measurements were repeated at least three times.
Bactericidal assays.
Purified Mac-2 or IdeS (21) protein variants (9 μg) were added to 1 ml of heparinized human blood from healthy volunteers, supplemented with antibodies against S. pyogenes (Abcam). Blood samples were incubated for 30 min at 37°C with rotation. Approximately 500 CFU of a mid-log culture of strain AP1 (OD620 of ∼0.15) were used to inoculate the pretreated blood. The tubes were incubated with rotation at 37°C for 30 min, and bacterial survival was monitored by plating dilutions on blood agar plates. Survival rates were calculated by dividing the number of CFU at time 30 by the number of CFU present at time zero h. Experiments were repeated at least three times with duplicate or triplicate samples.
RESULTS
IgG endopeptidase activity of streptococcal mac-2 strains.
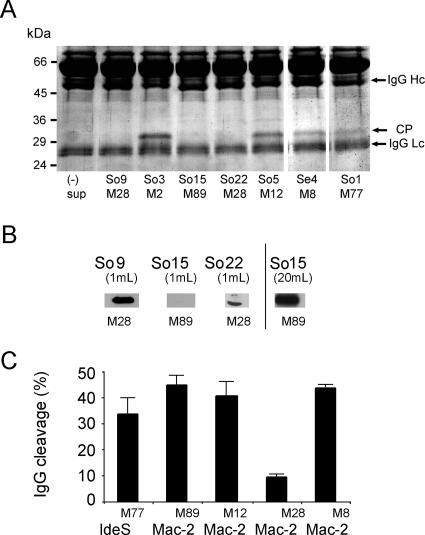
For analysis of Mac-2 enzymatic activity, streptococcal mac-2 strains (4) were grown in TH medium in the presence of 5% human plasma. Growth medium was analyzed by 12% SDS-PAGE, and IgG endopeptidase activity was determined by the presence of a diagnostic 31-kDa IgG CP (18). This CP was detected in the growth medium of four out of seven strains tested (Fig. 1A). Three strains, two of the M28 serotype and one of the M89 serotype, apparently lacked detectable IgG endopeptidase activity, as judged from the SDS-PAGE. Western blot analysis (Fig. 1B) revealed that strain So15 of the M89 serotype secreted less protein than the two M28 serotype strains, which explains the lack of IgG endopeptidase activity in standard growth medium under these experimental conditions. However, M28 serotype strains So9 and So22 were not deficient in Mac-2 secretion and the lack of detectable IgG endopeptidase activity appears to be due to an enzymatically inactive protein. These results indicate that Mac-2 proteins in general are not deficient in IgG endopeptidase activity and that the lack of enzymatic activity appears to be a property of Mac-2 proteins of M28 serotype streptococcal strains.
FIG. 1.
Analysis of Mac-2 protein expression and activity. (A) Analysis of IgG endopeptidase activity in culture supernatants of clinical S. pyogenes isolates. S. pyogenes strains were grown in TH broth supplemented with 5% human plasma. Aliquots of culture supernatant were analyzed by 12% SDS-PAGE. Cleavage of IgG is indicated by the presence of a 31-kDa CP. (B) Western immunoblot of TCA-precipitated proteins from bacterial cultures grown in TH broth. Mac-2 was identified with rabbit anti-IdeS antibodies and detected with peroxidase-conjugated goat anti-rabbit antibodies. Culture volumes used for TCA precipitation are indicated. (C) Quantification of IgG endopeptidase activity of purified recombinant Mac-2/IdeS proteins. Purified proteins were incubated with 125I-labeled myeloma IgG1 as the substrate, and enzyme activity was calculated as the ratio of radioactivity in the CP to total Hc radioactivity (CP + Hc). (3). All values are means ± standard deviations (n = 3).
Activity of recombinant Mac-2 proteins.
To confirm the indicated IgG endopeptidase activity of Mac-2 protein variants, mac-2 alleles of strains So5 (M12), So9 (M28), So15 (M89), and Se4 (M8) and the ideS allele of strain So1 (M77) were cloned into plasmid pGEX-5X-3 (GE Amersham Biosciences) and expressed in Escherichia coli strain BL21. Proteins were purified according to standard procedures, and equal amounts of recombinant proteins were assayed for IgG endopeptidase activity using 125I-radiolabeled myeloma IgG as the substrate (4). With the exception of the Mac-2 protein derived from the M28 serotype strain (So9), Mac-2 proteins of all strains showed IgG endopeptidase activity indistinguishable from that of IdeS (So1) (Fig. 1C). The Mac-2M28 protein showed only weak and significantly lower IgG-cleaving activity (Fig. 1C) compared to all other Mac-2 proteins, confirming the previously reported findings for Mac-2M28 (4, 14).
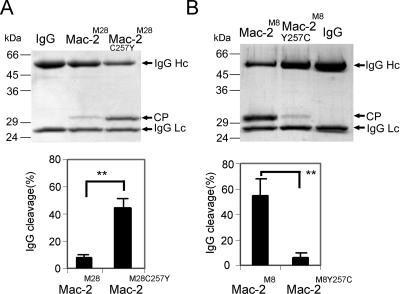
Cysteine 257 impairs Mac-2M28 IgG endopeptidase activity.
In order to identify a molecular explanation for the impaired enzymatic activity of the Mac-2M28 protein, we sought experimental support for the earlier notion (2, 4) implying that a disulfide bond between a cysteine residue in position 257 of the flexible loop structure of Mac-2M28 and the catalytic cysteine of the active site could interfere with substrate recognition. Thus, the cysteine residue in position 257 of Mac-2M28 was replaced with a tyrosine residue present in Mac-2 of other serotypes (4). Activity assays with purified recombinant proteins revealed a significant (P < 0.01) increase in enzymatic activity for the mutant Mac-2M28C257Y protein compared to the wild-type control Mac-2M28 (Fig. 2A), indicating that formation of a disulfide bond indeed interferes with IgG endopeptidase activity. To further confirm these results, the tyrosine residue in position 257 of the enzymatically active Mac-2 protein of the M8 serotype strain Se4 was replaced with a cysteine residue by site-directed mutagenesis (Mac-2M8Y257C). We predicted that the introduction of a cysteine in position 257 would lead to the formation of a disulfide bond between this cysteine and the cysteine of the catalytic site and thereby interfere with enzymatic activity. As predicted, activity assays revealed a dramatically decreased ability of the mutant Mac-2M8Y257C to cleave IgG, as demonstrated by an 87.5% reduction (P < 0.01) of IgG endopeptidase activity compared to wild-type Mac-2M8 (Fig. 2B). Thus, it is most likely that the cysteine residue present in the flexible loop structure of Mac-2 proteins of M28 serotype strains is part of a disulfide bond involving the catalytic site cysteine residue. Consequently, the formation of a disulfide bond in these proteins could interfere with the intrinsic IgG endopeptidase activity.
FIG. 2.
Role of position 257 and potential disulfide bond formation in IgG endopeptidase activity of Mac-2 and Mac-2 mutant proteins. (A) In the upper panel, purified Mac-2M28 and Mac-2M28C257Y were incubated with myeloma IgG1 and IgG cleavage activity was analyzed by SDS-PAGE. The lower panel shows quantification of Mac-2M28 and Mac-2M28C257Y enzymatic activity using 125I-IgG as the substrate. *, P < 0.01 by Student's t test. (B) In the upper panel, purified Mac-2M8 and Mac-2M8Y257C were incubated with myeloma IgG1 and IgG cleavage activity was analyzed by SDS-PAGE. The lower panel shows quantification of Mac-2M8 and Mac-2M8Y257C enzymatic activity using 125I-IgG as the substrate. **, P < 0.01 by Student's t test.
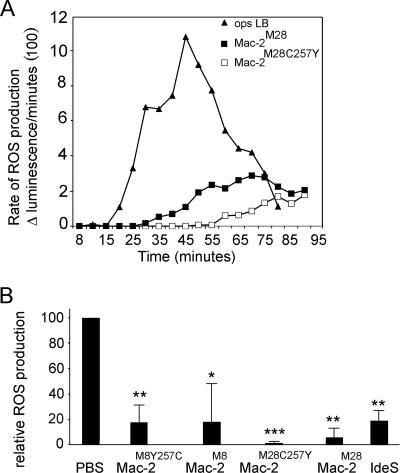
Mac-2M28 interferes with ROS production, but does not mediate streptococcal survival in human immune blood.
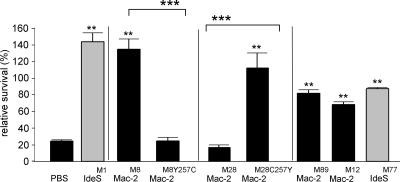
It has previously been reported that Mac-2M28 interacts with FcγRII and FcγRIII receptors and thereby blocks the recognition of IgG by these Fcγ receptors (1). A mechanism for a function of Mac-2M28 was suggested, implying that the protein could interfere with immune complex-mediated activation of PMNs (1). The generation of ROS during opsonophagocytosis has previously been used to monitor such activation (13, 14), but in contrast to the proposed functional mechanism of Mac-2M28, no effect on ROS production, opsonophagocytosis, or streptococcal killing was detected (14). The course of an immunocomplex-induced respiratory burst in whole blood in the presence of purified Mac-2M28 or the enzymatically active Mac-2M28C257Y variant was measured using a chemiluminescence-based assay (Fig. 3). Interestingly, in the presence of the Mac-2M28 protein, we observed a clear and sustained inhibition in opsonophagocytosis-induced ROS production (Fig. 3A). A similar inhibitory effect was observed when the enzymatically active Mac-2M28C257Y protein was used. In both cases, inhibition of ROS production was indistinguishable from IdeS-mediated inhibition (Fig. 3B) (13, 14). Similar inhibition of ROS production was achieved with enzymatically active or inactive Mac-2 protein of the M8 serotype (Fig. 3B), indicating that the ability to inhibit ROS generation is common to Mac-2 and IdeS proteins independently of IgG endopeptidase activity. Next, we investigated whether the observed inhibition of ROS generation would be sufficient to mediate streptococcal survival in a human immune blood bactericidal assay (18). Blood was incubated with either Mac-2M28, Mac-2M28C257Y, Mac-2M8, or Mac-2M8Y257C prior to inoculation with S. pyogenes. Clearly, while the number of surviving bacteria in blood supplemented with the enzymatically inactive Mac-2M28 or Mac-2M8Y257C protein variants was significantly reduced and indistinguishable from the number of bacteria surviving in the buffer control, bacterial survival was significantly increased (P < 0.01) when human immune blood was supplemented with the enzymatically active Mac-2M28C257Y or Mac-2M8 protein variants (Fig. 4). Similar results were obtained for Mac-2 IdeS proteins from streptococcal serotypes M12, M89, and M77 (Fig. 4). Thus, bacterial survival in these assays is mediated by IgG endopeptidase activity and inhibition of ROS production alone appears not to be sufficient to mediate streptococcal survival.
FIG. 3.
Effect of Mac-2 on ROS generation in human blood stimulated with opsonized latex beads (Ops LB). ROS generation was determined continuously over a 90-min period (A) in the presence of 5 μg/ml Mac-2M28 (▪) or Mac-2M28C257Y (□) or without antagonist (▴). Rates of ROS generation were determined for every 5 min as described above. (B) Inhibition of ROS peak production in the presence of Mac-2M8, Mac-2M8Y257C, Mac-2M28, Mac-2M28C257Y, or IdeSM1. ROS peak production is the average ROS production from 8 min to 45 min. All values are means ± standard deviations (n = 3). *, P < 0.05; **, P < 0.01; and ***, P < 0.001 (Student's t test).
FIG. 4.
Mac-2, but not enzymatically inactive Mac-2M28, confers resistance against phagocytic killing of S. pyogenes. Whole human immune blood was treated with either Mac-2M28, the enzymatically active Mac-2M28C257Y mutant protein, Mac-2M8, the enzymatically inactive Mac-2M8Y257C mutant protein, Mac-2M12, Mac-2M89, IdeSM77 (gray bar), IdeSM1 (gray bar), or a buffer control (PBS). Blood samples were inoculated with S. pyogenes and incubated at 37°C. Bacterial survival rates are shown as number of CFU at 30 min divided by the number of CFU at time zero. All values are means ± standard deviation. **, P < 0.01 compared to the buffer control; and ***, P < 0.001 between wild-type and mutant protein variants (Student's t test).
DISCUSSION
The survival of S. pyogenes in its human host is highly dependent on its ability to circumvent the actions of the innate and adapted immune response, in particular to avoid recognition by specific IgG. S. pyogenes has evolved an IgG-specific endopeptidase, IdeS, that specifically cleaves the heavy chain of IgG (1, 16, 18). Two allelic variants of the enzyme have been described, the IgG-specific endopeptidase, IdeS (or Mac-1) and Mac-2, a protein with only weak enzymatic activity toward IgG but with binding affinity for Fcγ receptors (1, 14). Based on these properties, Mac-2 proteins were suggested to interfere with opsonophagocytosis by blocking the FcγII and FcγIII receptors on phagocytic cells (1). However, the suggested role of Mac-2 was challenged by several findings. First, clinical streptococcal strains carrying a mac-2 allele exhibit IgG endopeptidase activity (4). Second, despite the fact that Mac-2 proteins interact with Fcγ receptors (1), no inhibition of ROS production, opsonophagocytosis, or streptococcal killing by Mac-2 has been observed (14). In this study, we show that Mac-2 proteins have IgG endopeptidase activity indistinguishable from the enzymatic activity exhibited by IdeS/Mac-1 proteins. The earlier reported weak enzymatic activity toward IgG is—so far—unique to Mac-2 proteins secreted by streptococcal strains of the M28 serotype and most likely due to a disulfide bond between the catalytic site cysteine in position 94 and a cysteine residue in position 257 of Mac-2M28. We also found that the interaction of Mac-2 with PMNs actually interferes with ROS production ex vivo, independently of the enzymatic capacity of the protein (Fig. 3B). However, bactericidal assays reveal that inhibition of ROS generation per se was not sufficient to mediate streptococcal survival (Fig. 4). The finding that IgG endopeptidase activity is common to Mac-2 proteins and, with the exception for Mac-2M28, indistinguishable from the IgG-cleaving activity of IdeS, underlines the importance of IgG endopeptidase activity in streptococcal virulence (19, 20). The role of IgG endopeptidase activity in streptococcal survival has previously been investigated (14, 19). One study demonstrated a decrease in phagocytosis of IgG-opsonized latex beads in the presence of an enzymatically inactive IdeS protein variant (14), while a second study demonstrated contradictory results in that an enzymatically inactive IdeS protein was unable to inhibit streptococcal killing in bactericidal assays (19), suggesting an essential role for IgG endopeptidase activity in inhibition of opsonophagocytosis. The hypothesis of an important role for IgG-cleaving activity was also supported by the finding that seroconversion toward IdeS resulted in the generation of neutralizing antibodies targeting IgG endopeptidase activity of IdeS (4). In addition, the results of the present study, in which enzymatically active Mac-2M28 inhibits streptococcal killing in whole blood, while native Mac-2M28 has no effect on streptococcal survival, further emphasize a decisive role for IgG endopeptidase activity. Although the findings of these studies appear to be contradictory, the results are consistent with a functional model in which IdeS/Mac-2 inhibits the recognition of immunocomplexes by Fcγ receptors, which will result in the reduction of phagocytosis rates, as reported in one study (14). However, to prevent bacterial killing a simultaneous blockage of at least the majority of Fcγ receptors would be required. Apparently, the remaining activation of Fcγ receptors is sufficient to induce innate immune responses, such as ROS generation, degranulation, and secretion of proteolytic enzymes, and phagocytosis, all of which affect bacterial survival, as scored in bactericidal assays using enzymatically inactive proteins (19) (Fig. 4). In contrast to the earlier hypothesis, implicating separate functions for IdeS and Mac-2 protein variants (1), the results of the current study suggest that Fcγ receptor binding activity and IgG endopeptidase activity are not exclusive, but instead complement each other to achieve an efficient protection of S. pyogenes against IgG-mediated phagocytosis.
Due to sequence similarities between Mac-2 proteins and the Streptococcus equi Mac homologue (14), also designated IdeE (12), it was suggested that IdeS variants evolved from Mac-2 variants (14). Thus, Mac-2 protein variants exhibit IgG endopeptidase activity, but have also preserved the evolutionarily older ability to bind to PMN receptor proteins and thereby to block ROS production. Since sequence similarities do not include the cysteine residue in position 257, these data imply that Mac-2M28 represents a unique Mac-2 variant favoring receptor binding properties before IgG endopeptidase activity. Although streptococcal strains of the M1 serotype are currently most frequently associated with invasive streptococcal disease (e.g., see references 3, 8, and 15), M28 serotype strains are repeatedly reported as the leading cause of invasive and noninvasive disease (3, 9, 11, 15, 17). It is tempting to speculate whether the evolution (or preservation) of MacM28 correlates with the reemergence of M28-associated pathogenesis: i.e., the prevalence of M28 serotypes in puerperal sepsis and/or neonatal infections (9). Although additional studies are needed to address this issue, it is clear that the occurrence of different functional variants of important virulence factors is of importance for our understanding of streptococcal pathogenesis (14).
Acknowledgments
This work was supported by grants from the Swedish Research Council (projects 3213 and 4522), the Foundation of Wiberg, the Medical Faculty at Umeå University, and Insamlingsstiftelsen at Umeå University.
Hansa Medical AB has filed a patent application for IdeS. U.v.P.R. is listed as the inventor in this application.
Editor: J. N. Weiser
Footnotes
Published ahead of print on 10 March 2008.
REFERENCES
- 1.Agniswamy, J., B. Lei, J. M. Musser, and P. D. Sun. 2004. Insight of host immune evasion mediated by two variants of group A streptococcus Mac protein. J. Biol. Chem. 27952789-52796. [DOI] [PubMed] [Google Scholar]
- 2.Agniswamy, J., M. J. Nagiec, M. Liu, P. Schuck, J. M. Musser, and P. D. Sun. 2006. Crystal structure of group A streptococcus Mac-1: insight into dimer-mediated specificity for recognition of human IgG. Structure 2225-235. [DOI] [PubMed] [Google Scholar]
- 3.Åkesson, P., M. Rasmussen, E. Mascini, U. von Pawel-Rammingen, R. Janulczyk, M. Collin, A. Olsén, E. Mattsson, M. L. Olsson, L. Björck, and B. Christensson. 2004. Low antibody titers to cell wall-attached proteins of Streptococcus pyogenes predispose for severe invasive disease. J. Infect. Dis. 189797-804. [DOI] [PubMed] [Google Scholar]
- 4.Åkesson, P., L. Moritz, M. Truedsson, B. Christensson, and U. von Pawel-Rammingen. 2006. IdeS, a highly specific immunoglobulin (IgG)-cleaving enzyme from Streptococcus pyogenes, is inhibited by specific IgG antibodies generated during infection. Infect. Immun. 74497-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bisno, A. L. 2001. Acute pharyngitis. N. Engl. J. Med. 344205-211. [DOI] [PubMed] [Google Scholar]
- 6.Carapetis, J. R., A. C. Steer, E. K. Mulholland, and M. Weber. 2005. The global burden of group A streptococcal diseases. Lancet Infect. Dis. 11685-694. [DOI] [PubMed] [Google Scholar]
- 7.Cunningham, M. W. 2000. Pathogenesis of group A streptococcal infections. Clin. Microbiol. Rev. 13470-511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ekelund, K., P. Skinhøj, J. Madsen, and H. Bossen Konradsen. 2005. Reemergence of emm1 and a changed superantigen profile for group A streptococci causing invasive infections: results from a nationwide study. J. Clin. Microbiol. 431789-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eriksson, B. K. G., M. Norgren, K. McGregor, B. G. Spratt, and B. Henriques Normark. 2003. Group A streptococcal infections in Sweden: a comparative study of invasive and noninvasive infections and analysis of dominant T28 emm28 isolates. Clin. Infect. Dis. 371189-1193. [DOI] [PubMed] [Google Scholar]
- 10.Gilbert, J. V., A. G. Plaut, B. Longmaid, and M. E. Lamm. 1983. Inhibition of microbial IgA proteases by human secretory IgA and serum. Mol. Immunol. 201039-1049. [DOI] [PubMed] [Google Scholar]
- 11.Green, N. M., S. B. Beres, E. A. Graviss, J. E. Allison, A. J. McGeer, J. Vuopio-Varkila, R. B. LeFebvre, and J. M. Musser. 2005. Genetic diversity among type emm28 group A Streptococcus strains causing invasive infections and pharyngitis. J. Clin. Microbiol. 434083-4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lannergård, J., and B. Guss. 2006. IdeE, an IgG-endopeptidase of Streptococcus equi ssp. equi. FEMS Microbiol. Lett. 262230-235. [DOI] [PubMed] [Google Scholar]
- 13.Lei, B., F. R. DeLeo, N. P. Hoe, M. R. Graham, S. M. Mackie, R. L. Cole, M. Liu, H. R. Hill, D. E. Low, M. J. Federle, J. R. Scott, and J. M. Musser. 2001. Evasion of human innate and acquired immunity by a bacterial homolog of CD11b that inhibits opsonophagocytosis. Nat. Med. 71298-1305. [DOI] [PubMed] [Google Scholar]
- 14.Lei, B., F. R. DeLeo, S. D. Reid, J. M. Voyich, L. Magoun, M. Liu, K. R. Braughton, S. Ricklefs, N. P. Hoe, R. L. Cole, J. M. Leong, and J. M. Musser. 2002. Opsonophagocytosis-inhibiting Mac protein of group A Streptococcus: identification and characteristics of two genetic complexes. Infect. Immun. 706880-6890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tyrrell, G. J., M. Lovgren, B. Forwick, N. P. Hoe, J. M. Musser, and J. A. Talbot. 2002. M types of group A streptococcal isolates submitted to the National Centre for Streptococcus (Canada) from 1993 to 1999. J. Clin. Microbiol. 404466-4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vincents, B., U. von Pawel-Rammingen, L. Björck, and M. Abrahamson. 2004. Biochemical characterization of IdeS, an IgG specific endopeptidase from Streptococcus pyogenes. Biochemistry 4315540-15549. [DOI] [PubMed] [Google Scholar]
- 17.Vlaminckx, B. J. M., F. H. J. Schuren, R. C. Montijn, M. P. M. Caspers, M. M. Beitsma, W. J. B. Wannet, L. M. Schouls, J. Verhoef, and W. T. M. Jansen. 2007. Dynamics in prophage content of invasive and noninvasive M1 and M28 Streptococcus pyogenes isolates in The Netherlands from 1959 to 1996. Infect. Immun. 753673-3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.von Pawel-Rammingen, U., B. P. Johansson, and L. Björck. 2002. IdeS, a novel streptococcal cysteine protease with unique specificity for immunoglobulin G. EMBO J. 211607-1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.von Pawel-Rammingen, U., B. P. Johansson, H. Tapper, and L. Björck. 2002. Streptococcus pyogenes and phagocytic killing. Nat. Med. 81044-1045. [DOI] [PubMed] [Google Scholar]
- 20.von Pawel-Rammingen, U., and L. Björck. 2003. IdeS and SpeB: immunoglobulin-degrading cysteine proteases of Streptococcus pyogenes. Curr. Opin. Microbiol. 650-55. [DOI] [PubMed] [Google Scholar]
- 21.Wenig, K., L. Chatwell, U. von Pawel-Rammingen, L. Björck, R. Huber, and P. Sondermann. 2004. Structure of the streptococcal endopeptidase IdeS, a cysteine protease with strict specificity for IgG. Proc. Natl. Acad. Sci. USA 10117371-17376. [DOI] [PMC free article] [PubMed] [Google Scholar]