Abstract
Wall teichoic acids (WTAs) and membrane lipoteichoic acids (LTAs) are the major polyanionic polymers in the envelope of Staphylococcus aureus. WTAs in S. aureus play an important role in bacteriophage attachment and bacterial adherence to certain host cells, suggesting that WTAs are exposed on the cell surface and could also provide necessary binding sites for cationic antimicrobial peptides and proteins (CAMPs). Highly cationic mammalian group IIA phospholipase A2 (gIIA PLA2) kills S. aureus at nanomolar concentrations by an action(s) that depends on initial electrostatic interactions, cell wall penetration, membrane phospholipid (PL) degradation, and activation of autolysins. A tagO mutant of S. aureus that lacks WTA is up to 100-fold more resistant to PL degradation and killing by gIIA PLA2 and CAMP human β-defensin 3 (HBD-3) but has the sensitivity of the wild type (wt) to other CAMPs, such as Magainin II amide, hNP1-3, LL-37, and lactoferrin. In contrast, there is little or no difference in either gIIA PLA2 activity toward cell wall-depleted protoplasts of the wt and tagO strains of S. aureus or in binding of gIIA PLA2 to wt and tagO strains. Scanning and transmission electron microscopy reveal increased surface protrusions in the S. aureus tagO mutant that might account for reduced activity of bound gIIA PLA2 and HBD-3 toward the tagO mutant. In summary, the absence of WTA in S. aureus causes a selective increase in bacterial resistance to gIIA PLA2 and HBD-3, the former apparently by reducing access and/or activity of bound antibacterial enzyme to the bacterial membrane.
Staphylococcus aureus is part of the normal bacterial flora residing within the anterior nares of 30 to 50% of humans usually without significant pathological consequences (30, 35, 42). However, S. aureus can cause a variety of localized and more invasive infections, when the skin or a mucosal barrier is breached (30). The mobilization and action of specific innate immune systems, including professional phagocytes and soluble antimicrobial agents, are crucial for limitation of infection (9, 20, 29, 46). Possibly the most potent among the soluble antimicrobial agents is the 14-kDa secretory group IIA phospholipase A2 (gIIA PLA2) (48). This enzyme is produced in many different cell types, including phagocytic cells, platelets, Paneth cells, and lacrimal glands (18, 25, 32, 34, 37, 40, 46). Extracellular levels of gIIA PLA2 sufficient for significant antibacterial action are constitutively high in tears and seminal fluid (34, 37, 40). In plasma and tissue fluid, however, the resting levels of gIIA PLA2 are low but increase markedly (100- to 1,000-fold) during inflammation (33, 46). These gIIA PLA2-rich cell-free fluids display potent antibacterial activity against S. aureus and many other gram-positive bacteria that is largely due to the presence of the gIIA PLA2 (46-48). In addition, transgenic mice expressing human gIIA PLA2 show greater host resistance to Escherichia coli and S. aureus infection than their nontransgenic littermates, implying a protective role of gIIA PLA2 versus these bacterial infections in vivo (27, 28).
The ability of gIIA PLA2 to attack S. aureus and other gram-positive bacteria reflects the abilities of the enzyme to bind to the cell wall and to penetrate the cell wall to gain access to phospholipids (PL) in the cell membrane (11). At least four steps are involved: binding of the enzyme to the bacterial cell surface, penetration of the enzyme through peptidoglycan layers, degradation of PL in the cell membrane, and activation of bacterial autolysins (11). The phospholipolytic activity of gIIA PLA2 and, hence, ultimate bacterial killing requires calcium as a cofactor, but initial gIIA PLA2 binding to the cell surface does not (47).
Initial binding of gIIA PLA2 to the cell surface of S. aureus involves electrostatic interactions between gIIA PLA2 and the bacterial cell surface. Among the more than 100 structurally related low-molecular-mass (∼14-kDa) PLA2 that have been characterized so far, the mammalian gIIA PLA2 are unique in their very high net positive charge ranging from +12 to +17. This very high net basicity is essential for the enzyme's potent bactericidal activity toward gram-positive bacteria, principally by promoting initial interactions and penetration of the cell wall (1, 49). In contrast, the highly cationic properties of gIIA PLA2 are not essential for calcium-dependent catalytic activity (49) or for its ability and that of other structurally related 14-kDa PLA2 to degrade PL in cell wall-depleted bacterial protoplasts (1, 21). Specific bacterial sites for gIIA PLA2 binding are not known but probably involve anionic cell envelope moieties. Major cell envelope-associated polyanions in S. aureus include wall teichoic acid (WTA) and lipoteichoic acid (LTA) (10, 31).
WTA and LTA contain repeating units of ribitol or glycerol phosphate that can be modified by glycosyl substituents or d-alanine esters (31). While LTA is anchored to the lipid membrane, WTA is covalently linked to the peptidoglycan (31, 41). The role of WTA in adsorption of certain bacteriophages to S. aureus (4, 5, 44) and in adherence of S. aureus to host cells (44, 45) is consistent with exposure of WTA beyond the cell wall layer (41).
We therefore speculated that WTA, by virtue of its location and polyanionic properties, might be an important target of initial gIIA PLA2 interactions with S. aureus. The recent construction of a viable WTA-deficient mutant (S. aureus tagO strain [44]) made it possible to address this hypothesis. We show that in the absence of WTA, S. aureus is ∼100-fold more resistant to gIIA PLA2. Surprisingly, however, this resistance is not due to reduced binding of gIIA PLA2 to the S. aureus tagO mutant but rather decreased penetration and/or activity of bound gIIA PLA2. The S. aureus tagO mutant also shows increased resistance to human β-defensin 3 (HBD-3) but not against human neutrophil α-defensins, Magainin II amide, and several other antimicrobial proteins (44, 45). These findings suggest an important and apparently selective role of WTA in the antistaphylococcal actions of gIIA PLA2 and HBD-3, the two most cationic and potent human antibacterial polypeptides active against S. aureus.
MATERIALS AND METHODS
Materials.
(Ala8,13,18) Magainin II amide, lysostaphin (3,000 U/mg of protein), DNase I (2,500 Kunitz units/mg of protein), and horseradish peroxidase-conjugated monoclonal mouse anti-goat antibodies were purchased from Sigma Chemical Co. (St. Louis, MO). HBD-3 was a generous gift from Paul McCray (Department of Pediatrics, The University of Iowa, Iowa City). [1-14C]oleic acid was purchased from DuPont New England Nuclear (Boston, MA). High-performance thin-layer chromatography (TLC) plates were purchased from Merck (Germany), and bovine serum albumin (BSA) was purchased from Boehringer Mannheim (Indianapolis, IN). RPMI was obtained from GibcoBRL (Rockville, MD). Precise protein gels and SuperSignal west chemiluminescence substrates were purchased from Pierce (Rockford, IL). Recombinant wild-type (wt) and mutant (D49S) human secretory gIIA PLA2 were expressed in E. coli and purified as described previously (49). The purity of gIIA PLA2 was confirmed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and by reversed-phase high-performance liquid chromatography (49). The enzymatic activity of D49S gIIA PLA2 against autoclaved E. coli was approximately 0.001% of wt gIIA PLA2. Polyclonal goat anti-gIIA PLA2 antibodies were isolated from goat serum by affinity purification using affinity gel resin (Hi-Trap; GE Healthcare, Piscataway, NJ) containing coupled gIIA PLA2.
Bacterial strains and growth conditions.
The strains of S. aureus used were the parent S. aureus 113 strain (19) and the isogenic S. aureus tagO and S. aureus pRBtagO complemented strains (44). Bacteria were grown overnight at 37°C in BM broth (1% tryptone, 0.5% yeast extract, 0.5% NaCl, 0.1% K2HPO4, 0.1% glucose), washed, and resuspended in fresh medium with a starting optical density at 550 nm (OD550) of 0.05 and subcultured for 2 to 2.5 h at 37°C with shaking until mid-logarithmic phase.
Assay of bacterial viability.
The effects of various antimicrobial proteins and peptides on bacterial viability were determined by measuring the ability of the treated bacteria to form colonies on tryptic soy agar (TSA). Typical incubation mixtures contained 106 or 107 bacteria/ml in RPMI supplemented with 10 mM HEPES (pH 7.4), 1% (wt/vol) BSA, and 1 mM CaCl2 in the presence or absence of the indicated protein/peptide. In assays with (Ala8,13,18)-Magainin II amide and HBD-3, CaCl2 was omitted, since these peptides are more sensitive to inhibition by added divalent cations than gIIA PLA2 is. Incubations were carried out at 37°C for up to 2 h. At each time point, aliquots of bacterial suspensions were serially diluted in sterile physiological saline and plated in 5 ml of molten (50°C) TSA. Bacterial viability was measured by counting bacterial colonies (i.e., CFU) after 18 to 24 h of incubation at 37°C.
Radiolabeling of S. aureus lipids during bacterial growth.
Bacterial PL were radiolabeled during growth in subculture as previously described (11, 24). Briefly, bacteria were subcultured at 37°C to mid-logarithmic phase in BM medium supplemented with 1 μCi of [1-14C]oleic acid per ml and 0.01% BSA, washed, resuspended in half of the volume of fresh BM medium without [1-14C]oleic acid and incubated for another 20 min at 37°C. BSA was then added to the medium to a final concentration of 0.5% (wt/vol), and bacteria were washed to remove remaining free oleic acid. Washed bacteria were resuspended to the desired concentration in incubation medium and used promptly.
Assay of bacterial PL degradation.
Bacteria prelabeled with [1-14C]oleic acid, as described above, were incubated in the presence or absence of gIIA PLA2 as indicated. PL degradation products generated during gIIA PLA2 treatment were quantitatively recovered in the extracellular medium as complexes with BSA (11, 24) and measured by liquid scintillation spectroscopy. PL degradation (radioactivity recovered in the extracellular medium) was calculated as a percentage of total sample radioactivity.
Production and assay of S. aureus protoplasts.
Cell wall-depleted protoplasts were prepared from metabolically labeled S. aureus by incubation with lysostaphin and DNase I (final concentration of 250 μg/ml and 500 μg/ml, respectively) in Tris-buffered saline osmotically stabilized with 30% (wt/vol) raffinose as described before (24). Greater than 80% of [1-14C]oleate-labeled bacterial PL of intact bacteria were present in the recovered protoplasts. The virtual absence of intact/viable bacteria in the recovered protoplast samples was confirmed by Gram staining and assay of CFU without raffinose. Cell wall-depleted protoplasts were gently resuspended (1 × 107/ml) in RPMI containing 1% BSA, 10 mM HEPES (pH 7.4), 1 mM CaCl2, and 30% raffinose and incubated with or without gIIA PLA2 for 60 min. 1-14C-labeled lipids were extracted using the method of Bligh and Dyer (3, 47) and resolved by TLC in a chloroform-methanol-water-acetic acid (65:25:4:1 [vol/vol]) solvent system. Lipids were identified by comparison to migration positions of lipid standards and quantified by phosphorimage analysis using PhosphorImager and ImageQuant software (Amersham Pharmacia Biotech Inc.-Molecular Dynamics Division) and expressed as a percentage of total 1-14C-labeled lipid detected in the sample.
Assay of gIIA binding to S. aureus.
Initial binding of gIIA PLA2 was assayed using catalytically inactive D49S gIIA PLA2 that retains wt gIIA PLA2 binding properties to S. aureus but is unable to degrade PL (24). This mutant gIIA PLA2 D49S (1 μg/ml) was added to mid-log bacteria (1 × 108/ml) in RPMI containing 1% BSA, 10 mM HEPES (pH 7.4), and 1 mM CaCl2 and incubated for 15 min at 37°C with slow shaking. After 15 min, bacteria were pelleted and washed once with phosphate-buffered saline (PBS) to remove unbound gIIA PLA2. Bacterial number and recovery were monitored by studying OD550, CFU, 1-14C-labeled lipids, and total protein (Bio-Rad). To dissociate gIIA PLA2 bound to bacteria by electrostatic interactions, bacterial pellets were resuspended in 1 M NaCl and incubated for 15 min at 37°C with slow shaking. Bacteria were pelleted and washed once with 1 M NaCl. To concentrate the 1 M NaCl eluate for immunoblot analysis, this sample was precipitated on ice with trichloroacetic acid (final concentration of 20%). Bacterial pellets and/or trichloroacetic acid precipitates of the 1 M NaCl eluate were resuspended in sample buffer containing 2% (wt/vol) SDS, 36% (wt/vol) urea, and 6% (wt/vol) dithiothreitol and boiled for 10 min. Insoluble material was removed by centrifugation at 14,000 rpm for 5 min, and recovered supernatants, corresponding to 2 × 107 bacterial equivalents, were resolved in a 4 to 20% polyacrylamide gel. Samples were transferred to a nitrocellulose membrane, and then the membrane was incubated with blocking buffer (PBS supplemented with 4% BSA and 0.05% Tween 20) and then with primary antibody diluted in blocking buffer for 1 h at room temperature. After the blot was washed in PBS containing 0.05% Tween 20, it was incubated with horseradish peroxidase-conjugated mouse anti-goat immunoglobulin G (1:20,000) for 1 h at room temperature in blocking buffer. After the blots were washed extensively, they were developed using the Pierce SuperSignal substrate system.
TEM and SEM.
The wt and tagO strains of S. aureus were grown until mid-logarithmic phase in BM, then harvested by centrifugation, and washed extensively with PBS. Bacteria were processed as described previously (16). Briefly, bacteria were processed in 1.5-ml Eppendorf tubes for transmission electron microscopy (TEM) or applied to coverslips precoated with poly-l-lysine for scanning electron microscopy (SEM). Cells were first fixed with 2.5% glutaraldehyde and 0.1 M cacodylate for 10 min and then with 1% OsO4 buffer for 1 h. Samples for SEM were sequentially dehydrated in ethanol and chemically dried with hexamethyldisilizane, mounted on stubs sputter coated with gold-palladium mixture, and examined under the Hitachi S-4800 SEM at the University of Iowa Central Microscopy Research Facility. Samples for TEM were, after fixation, stained with uranyl acetate, sequentially dehydrated in acetone, and embedded in Spurrs's medium. Microtome sections were further stained with uranyl acetate and lead citrate and examined with a Hitachi H-7000 TEM in the University of Iowa Central Microscopy Research Facility.
Statistical methods.
Statistical analyses were performed with the Prism 4.0 package (GraphPad Software, San Diego, CA), and differences between the paired groups (wt and tagO strains and wt and complemented strains) were analyzed for significance with Student's t test.
RESULTS
Effects of WTA deficiency on the sensitivity of S. aureus to gIIA PLA2 and HBD-3.
Prior evidence suggests little or no effect of WTA deficiency on S. aureus growth or on sensitivity to a variety of cationic antimicrobial peptides (hNP1-3 and LL-37) or proteins (lysozyme, lactoferrin, and lysostaphin) (CAMPs) (2, 23, 44). However, the effect of d-alanylation of LTA has a much greater effect on gIIA PLA2 than on these other antimicrobial agents, suggesting different molecular determinants of antistaphylococcal action of the highly cationic gIIA PLA2 versus those of these other CAMPs and proteins. To test the effects of the absence of WTA on the sensitivity of S. aureus to gIIA PLA2, we compared the bactericidal potency of gIIA PLA2 against wt and tagO strains of S. aureus. The S. aureus tagO mutant exhibited at least 100-fold-higher resistance to gIIA PLA2 than the isogenic wt S. aureus strain (Fig. 1A). Complementation of the S. aureus tagO mutant with a plasmid encoding tagO (44) restored the sensitivity of S. aureus against gIIA PLA2 (Fig. 1A).
FIG. 1.
Sensitivities of wt, tagO, and tagO complemented strains to gIIA PLA2, HBD-3, and Magainin II amide. S. aureus (wt, tagO, and complemented strains) at 1 × 106/ml were incubated for 2 h as described in Materials and Methods in the presence of increasing concentrations of gIIA PLA2 (A), HBD-3 (B), and Magainin II amide (C), as indicated. Bacterial viability was measured as CFU in TSA and is expressed as a percentage of the initial inoculum. The lowest doses tested of gIIA PLA2, HBD-3, and Magainin II amide were 10 ng/ml, 1.25 μg/ml, and 0.4 μg/ml, respectively. Growth of all three bacterial strains during 2 h of incubation without gIIA PLA2, HBD-3, and Magainin II amide was similar, resulting in >100% CFU. The results shown represent the means of three experiments ± standard errors of the means (error bars). Where indicated, the asterisks denote statistically significant (P < 0.05) greater resistance of the S. aureus tagO mutant to killing by gIIA PLA2 and by HBD-3 versus that of the wt and S. aureus tagO complemented strains.
Because the marked increase in resistance of the S. aureus tagO mutant to gIIA PLA2 contrasted greatly with previous studies showing similar sensitivities of S. aureus tagO mutant to other CAMPs, we extended our experiments to additional CAMPs, i.e., Magainin II amide and HBD-3. Magainin II amide is a α-helical CAMP derived from frog skin, while HBD-3 is a member of the β-defensin family (13). The S. aureus tagO mutant was as sensitive to Magainin II amide as the wt strain was (Fig. 1C). In contrast, the S. aureus tagO mutant was at least 10-fold more resistant to HBD-3 than the wt or complemented strain (Fig. 1B). Our findings indicate that the absence of WTA in S. aureus results in a dramatic and apparently selective increase in resistance to gIIA PLA2 and HBD-3.
Differences in killing of the wt and tagO strains of S. aureus parallel differences in bacterial PL hydrolysis.
As indicated in the introduction, killing of S. aureus by gIIA PLA2 depends on initial protein binding, penetration of the cell wall, degradation of membrane PL, and activation of bacterial autolysins (11, 24). Each of these steps can be assayed independently, thus making it possible to study in greater detail the mechanism of bacterial sensitivity and resistance to gIIA PLA2 (24). To test whether differences in killing by gIIA PLA2 of the wt versus tagO strains of S. aureus paralleled differences in PL hydrolysis, bacterial lipids were prelabeled during growth by [1-14C]oleic acid, and extracellular accumulation of radioactive lipid degradation products during gIIA PLA2 treatment was measured. Figure 2 shows that the difference in sensitivities of wt and tagO strains to killing by gIIA PLA2 was paralleled by a similar difference in sensitivity to gIIA PLA2-induced bacterial PL degradation (i.e., release of 1-14C-labeled lipids). To produce a similar effect on bacterial lipids, a 100-fold-higher dose of gIIA PLA2 was required for the tagO mutant strain than for the wt strain (Fig. 2, compare panels A and B). Complementation of the S. aureus tagO mutant with a plasmid encoding tagO fully restored bacterial sensitivity to gIIA PLA2 phospholipid degradation and killing to wt levels (Fig. 2C and F, respectively). As shown before, bacterial killing required nearly complete PL degradation within 30 min (11, 12, 24), which was not achieved in the S. aureus tagO mutant even at the highest dose (1 μg/ml) of gIIA PLA2 tested (Fig. 2).
FIG. 2.
Effects of gIIA PLA2 on lipid release and killing of wt and tagO strains of S. aureus. S. aureus wt strain (A and D), S. aureus tagO strain (B and E), and S. aureus tagO complemented strain (C and F) lipids were prelabeled with [1-14C]oleic acid as described in Materials and Methods before incubation with increasing concentrations of gIIA PLA2. Samples were taken at 1 and 2 h to measure accumulation of 1-14C-labeled lipid breakdown products in the extracellular medium and CFU in TSA. Increasing size of symbols corresponds to increasing gIIA PLA2 concentrations (0, 10, 100, and 1,000 ng/ml). The results shown represent the means of three experiments ± standard errors of the means (error bars). Where indicated, the asterisks denote statistically significant (P < 0.05) greater resistance of the S. aureus tagO mutant to the phospholipolytic and bactericidal activities of gIIA PLA2 versus those of the wt and S. aureus tagO complemented strains.
Similar sensitivities of cell wall-depleted membrane protoplasts from wt and tagO strains of S. aureus to gIIA PLA2.
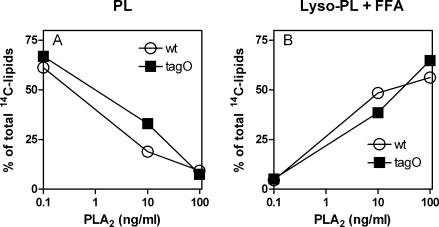
Differences in PL degradation by gIIA PLA2 could reflect reduced access of the enzyme to the membrane of the more resistant S. aureus tagO mutant or lower sensitivity of PL within the membrane of these more resistant bacteria. To distinguish between these two possibilities, we prepared bacterial protoplasts from wt and tagO strains of S. aureus by removal of the bacterial cell wall using lysostaphin and tested their susceptibility to gIIA PLA2-mediated PL hydrolysis. Recovery of bacterial membranes in the form of protoplasts was ∼80%, as deduced from recovery of [1-14C]oleate-labeled material. The contamination with intact bacteria, as assessed by plating on TSA without 30% raffinose was ≤0.001% for both the wt and tagO strains. 1-14C-labeled lipids in recovered membrane protoplasts were analyzed by TLC. The radiolabeled lipid pattern of protoplasts resembled that of intact bacteria and did not differ between the wt and tagO strains (not shown), further suggesting similarities between the two strains. In contrast to intact bacteria, protoplasts of both wt and tagO strains were virtually equally sensitive to gIIA PLA2-dependent PL degradation with dose-dependent loss of 1-14C-labeled PL (Fig. 3A) accompanied by accumulation of 1-14C-labeled lysophospholipid plus 1-14C-labeled free fatty acid (Fig. 3B). These data indicate that the absence of WTA does not affect the sensitivity of membrane PL to gIIA PLA2 when the cell wall is removed.
FIG. 3.
Sensitivity of protoplasts from wt and tagO S. aureus strains to gIIA PLA2. Membrane protoplasts derived from 107 bacteria prelabeled with [1-14C]oleic acid were incubated with increasing concentrations of gIIA PLA2 (10 and 100 ng/ml) in RPMI supplemented with 10 mM HEPES, 1 mM CaCl2, and 1% BSA and osmotically stabilized with 30% raffinose. After 60 min of incubation at 37°C in the presence or absence of gIIA PLA2, lipids were extracted from the protoplasts and separated by TLC as described in Materials and Methods. (A) Loss of [1-14C]PL; (B) accumulation of gIIA PLA2-mediated PL degradation products ([1-14C]lysophospholipid [lyso-PL] and 1-14C-labeled free fatty acid [FFA]), as quantified by densitometric analysis. The results shown are from one experiment, representative of two similar experiments.
The absence of WTA does not diminish initial binding of gIIA PLA2 to S. aureus.
The greater resistance of intact S. aureus tagO mutant but not cell wall-depleted protoplasts of this strain to gIIA PLA2 suggested that binding and/or cell wall penetration of bound gIIA PLA2 is markedly reduced in the tagO mutant. Since initial binding of gIIA PLA2 depends on electrostatic interactions between the positively charged gIIA PLA2 and negatively charged moieties on the bacterial surface, we speculated that the absence of WTA, an abundant polyanionic polymer that extends through the cell wall, would result in reduced gIIA PLA2 binding, hence accounting for reduced antimicrobial activity of gIIA PLA2 toward the S. aureus tagO mutant. We therefore compared binding of gIIA PLA2 to wt and tagO strains of S. aureus, monitoring gIIA PLA2 binding by SDS-PAGE and immunoblotting of washed bacterial pellets using affinity-purified goat anti-gIIA PLA2 antibodies. Because membrane PL degradation creates additional binding sites in S. aureus for gIIA PLA2 (24), we used a catalytically inactive mutant D49S of gIIA PLA2 that retains the binding properties of wt gIIA PLA2 to compare initial surface binding of gIIA PLA2 to wt and tagO S. aureus strains. Sample loads were normalized by bacterial number as measured by OD550, CFU, [1-14C]oleate-labeled lipids, and total protein. Densitometric analysis of the immunoreactive gIIA PLA2 bands revealed that the binding of gIIA PLA2 to the S. aureus tagO mutant was essentially equal to the binding of gIIA PLA2 to the wt S. aureus (the ratio of PLA2 bound to the wt versus that bound to the tagO S. aureus strain was 0.95 ± 0.19; n = 3, P > 0.05; Fig. 4A). Moreover, virtually all of gIIA PLA2 bound to both wt and tagO strains was eluted by treatment of gIIA PLA2-associated bacteria with 1 M NaCl for 15 min at 37°C (Fig. 4B), suggesting that gIIA PLA2 binding to S. aureus was mainly driven by electrostatic interactions even in the absence of WTA. These findings indicate that the marked increase in resistance of the S. aureus tagO mutant to gIIA PLA2 is not due to a difference in the amount of gIIA PLA2 initially bound to the bacteria.
FIG. 4.
Initial binding of gIIA PLA2 to S. aureus (wt and tagO strains). S. aureus (1 × 108 bacteria/ml) was incubated with catalytically inactive D49S gIIA PLA2 (1 μg/ml) for 15 min at 37°C as described in Materials and Methods. Unbound gIIA PLA2 was separated from bacterium-associated PLA2 by sedimentation of bacteria. Bound gIIA PLA2 was analyzed by SDS-PAGE and immunoblotting before and after treatment with 1 M NaCl as described in Materials and Methods. (A) Immunoblot of samples containing 2 × 107, 1 × 107, and 5 × 106 (from left to right) bacterial equivalents of wt and tagO strains of S. aureus. (B) Immunoblot of samples containing 2 × 107 bacterial equivalents before and after treatment with 1 M NaCl. gIIA PLA2 recovered in the 1 M NaCl eluate and, for comparison, purified gIIA PLA2 standard (20 ng) are shown in the three rightmost lanes. The results shown are representative of three similar experiments.
Effects of WTA deficiency on detergent sensitivity and morphology of S. aureus.
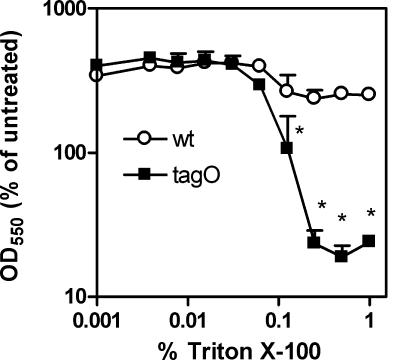
The findings described above indicate dramatically greater resistance of the S. aureus tagO mutant to bound gIIA PLA2 that is dependent on the integrity of the cell walls of these bacteria. To probe other possible differences in the properties of the cell envelopes of wt and tagO strains of S. aureus that may arise secondary to WTA deficiency, we compared the sensitivities of these strains to Triton X-100 and examined their appearance by electron microscopy. In comparison to the wt strain, the mutant strain was much more sensitive to Triton X-100, with marked effects on bacterial growth and structural integrity seen at concentrations above 0.1% of Triton X-100 in the growth medium (Fig. 5). These data indicate that in marked contrast to the increased resistance of the S. aureus tagO mutant to the cytotoxic effects of bound gIIA PLA2, the absence of WTA markedly increases envelope sensitivity to Triton X-100-mediated bacterial lysis.
FIG. 5.
Sensitivity of wt and tagO S. aureus strains to Triton X-100. Bacteria (wt and tagO strains) were grown until the mid-logarithmic growth phase and then exposed to increasing concentrations of Triton X-100 (twofold dilutions ranging from 1% to 0.001% [vol/vol]). Bacterial absorbance (OD550) was measured after 2 h of incubation at 37°C. The values represent the percentages of OD550 of initial inoculum after 2 h of incubation of bacteria with or without Triton X-100. The results shown are the means of two independent experiments ± standard errors of the means (error bars), each done in duplicate samples. Where indicated, the asterisks denote statistically significant (P < 0.05) differences in sensitivity of wt and tagO S. aureus strains to Triton X-100.
The surface morphology of the two strains as assessed by electron microscopy was also notably different. The surface of the S. aureus tagO strain appeared less smooth, with hairy-like or knot-like protrusions, apparent by both TEM and SEM (Fig. 6) in all cocci observed.
FIG. 6.
Morphology of wt and tagO strains of S. aureus as examined by electron microscopy. (Top) TEM; (bottom) SEM. Note that wt cocci are round and have a smooth surface, whereas S. aureus tagO cocci have a rough surface with many surface protrusions seen in all cocci.
DISCUSSION
We have shown dramatic effects of the absence of WTA on the sensitivity of S. aureus to gIIA PLA2 and to HBD-3 (Fig. 1 and 2). Detailed studies of gIIA PLA2 action against wt and tagO strains of S. aureus have revealed that the effects of WTA deficiency are not on either initial binding of the gIIA PLA2 (Fig. 4) or on the susceptibility of membrane PL to the enzyme once the bacterial cell wall is stripped away (Fig. 3). Rather, the effect of WTA deficiency is on the ability of the bound enzyme to access and attack membrane phospholipids in intact S. aureus tagO bacteria. The fact that initial binding of gIIA PLA2 to wt and tagO strains of S. aureus is indistinguishable and virtually completely reversible by treatment with 1 M NaCl (Fig. 4B) strongly suggests that the initial interactions of gIIA PLA2 with either the wt or tagO strain are mediated by electrostatic interactions between the polycationic enzyme and anionic sites in the bacterial envelope. These may include but, as indicated by the binding of gIIA PLA2 to the S. aureus tagO mutant, are not limited to the polyribitol phosphate chain of WTA.
Equally striking as the increase in bacterial resistance to gIIA PLA2 and HBD-3 in the tagO mutant bacteria is the apparently selective effect of this bacterial envelope alteration on bacterial sensitivity/resistance to certain cell wall or cell membrane active antibacterial agents. Thus, while the absence of WTA increases bacterial resistance to HBD-3 greater than 10-fold (Fig. 1) and to gIIA PLA2 approximately 100-fold (Fig. 1 and 2), the sensitivity or resistance of S. aureus to several other cell wall- and/or cell membrane-directed antibacterial agents, including both CAMPs (44) and larger proteins (2, 23, 44), is essentially unchanged by the absence of WTA. Moreover, the sensitivity to the nonionic detergent Triton X-100 is actually increased in the S. aureus tagO mutant strain relative to that of the wt parent strain (Fig. 5). These findings suggest mechanistic requisites shared by gIIA PLA2 and HBD-3 in their antibacterial actions that are not shared by Magainin II amide, hNP1-3, LL-37, lysozyme, lactoferrin, lysostaphin, or Triton X-100. Although the precise natures of these determinants are still unknown, comparison of the structural and functional properties of these agents suggest that distinguishing characteristics of the gIIA PLA2 and HBD-3 are their extremely high net positive charge and polycationic charge density and their cytotoxic action within the cytoplasmic bacterial membrane (Table 1) (22, 24, 38, 39, 49). It is possible that the charge properties of gIIA PLA2 and HBD-3 confer special requirements for their penetration of the cell wall to the bacterial cytoplasmic membrane where their lethal action is exerted. It has been proposed that the combination of WTA, extending outside the bacterial cell beyond the peptidoglycan layers, and of LTA, extending from the cytoplasmic membrane into the peptidoglycan lattice, can provide a polyanionic ladder through which polycationic macromolecules could traverse from the outside to the cytoplasmic membrane (6, 7, 10, 31). Adducts (e.g., d-alanine) of the polyglycerol phosphate chain of LTA can be transferred nonenzymatically from LTA to WTA, implying close juxtaposition of the polyglycerol phosphate and polyribitol phosphate chains of LTA and WTA, respectively (15). Thus, in the absence of WTA, gIIA PLA2 (and HBD-3) may be bound electrostatically at sites in the cell wall from which movement to LTA and further penetration to the cell membrane may be much less facile. The much greater abundance of surface protrusions seen in the S. aureus tagO mutant (Fig. 6) may also provide initial binding sites for gIIA PLA2 and HBD-3 from which transfer to LTA is less likely. Such a scenario predicts that a deficiency in LTA would also confer hyperresistance to gIIA PLA2 and HBD-3. Unfortunately, in contrast to WTA, a mutant fully deficient in LTA is not viable (14), precluding at this time such direct testing.
TABLE 1.
Summary of physical and antimicrobial properties of cationic peptides and proteins
| Antimicrobial agent | Molecular mass (kDa) | Net chargea | Site of actionb | LD90 of wtc | MIC of wtd | Lysozyme sensitivity of wte | tagO/wt ratiof | Reference(s) |
|---|---|---|---|---|---|---|---|---|
| hNP1-3 | 3.5 | +2/+3 | CM | 100 | 1 | 36, 44 | ||
| Magainin II amide | 2.5 | +4 | CM | 10-40 | 10-40 | 1 | 24, 36; this study | |
| LL-37 | 4.5 | +6 | CM | 10 | 1 | 44, 50 | ||
| HBD-3 | ∼10g | +11 | CM | 10 | 10 | ∼10 | 24, 39; this study | |
| gIIA PLA2 | 14 | +15 | CM | 0.1 | 0.1 | ∼100 | 24; this study | |
| Lysozyme | 14.7 | +9 | CW | >5 × 104 | 1 | 2 | ||
| Lysostaphin | 25 | +10.5 | CW | 0.004 | 1 | 26, 43 | ||
| Lactoferrin | 80 | +11 | CMh | 500 | 1 | 44 |
(Lys + Arg) − (Asp + Glu).
CM, cell membrane; CW, cell wall.
The 90% lethal dose (LD90) (in micrograms per milliliter) was determined as described in the legend for Fig. 1.
MIC (in grams per milliliter) determined by Kusuma and Kokai-Kun (26).
Lysozyme sensitivity was determined by agar diffusion assay. Note that the growth of both strains was unaffected by 50 mg/ml of lysozyme.
Estimated difference in the resistance of wt and tagO strains of S. aureus to the indicated antimicrobial agent. This difference was calculated as the ratio of the estimated MIC, LD90, or lysozyme sensitivity of the indicated agent toward the tagO strain versus that of the wt parent strain.
HBD-3 is primarily found as a dimer (39).
The principal antistaphylococcal action of lactoferrin is likely mediated by sequestration of iron.
In several infectious and inflammatory settings, gIIA PLA2 appears to be the dominant extracellular antistaphylococcal agent (27, 28, 46-48). Thus, increased bacterial resistance by reduced WTA could be a useful adaptive measure to increase S. aureus survival in settings where gIIA PLA2 is mobilized and bacteria would otherwise be sensitive to its antibacterial action. Thus, while WTA may be essential for establishing nasal colonization and also adherence to certain endothelial sites (44, 45), a reduction in WTA content could facilitate bacterial invasion and promote the survival of invading bacteria that induce host inflammatory responses, including the mobilization of gIIA PLA2. Regulation of WTA expression and/or envelope levels has not yet been reported. However, recent studies of the two-component WalKR system on cell wall metabolism showed direct interaction of WalR with the tagA or tagD promoter (8, 17), suggesting a possible involvement of the two-component WalKR system in expression of tag genes. Therefore, future studies directed at examination of WTA levels in vitro and in vivo could reveal more new insights about the roles and functions of WTA in S. aureus interaction with the human host.
Acknowledgments
We thank Paul McCray for his generous gift of HBD-3, Catherine Miller-Hunt for performing the initial experiments with the S. aureus tagO strain and gIIA PLA2, Randy Nessler and Kathy Walters of the University of Iowa Central Microscopy Research Facility for assistance with electron microscopy, and Polonca Prohinar for helpful suggestions and critique.
This work was supported in part by United States Public Health Service grant AI-18571 and American Heart Association Postdoctoral fellowship 0725702Z.
Editor: J. B. Bliska
Footnotes
Published ahead of print on 17 March 2008.
REFERENCES
- 1.Beers, S. A., A. G. Buckland, R. S. Koduri, W. Cho, M. H. Gelb, and D. C. Wilton. 2002. The antibacterial properties of secreted phospholipases A2: a major physiological role for the group IIA enzyme that depends on the very high pI of the enzyme to allow penetration of the bacterial cell wall. J. Biol. Chem. 2771788-1793. [DOI] [PubMed] [Google Scholar]
- 2.Bera, A., R. Biswas, S. Herbert, E. Kulauzovic, C. Weidenmaier, A. Peschel, and F. Götz. 2007. Influence of wall teichoic acid on lysozyme resistance in Staphylococcus aureus. J. Bacteriol. 189280-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bligh, E. G., and W. J. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37911-917. [DOI] [PubMed] [Google Scholar]
- 4.Chatterjee, A. N. 1969. Use of bacteriophage-resistant mutants to study the nature of the bacteriophage receptor site of Staphylococcus aureus. J. Bacteriol. 98519-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chatterjee, A. N., D. Mirelman, H. J. Singer, and J. T. Park. 1969. Properties of a novel pleiotropic bacteriophage-resistant mutant of Staphylococcus aureus H. J. Bacteriol. 100846-853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doyle, R. J., M. L. McDannel, J. R. Helman, and U. N. Streips. 1975. Distribution of teichoic acid in the cell wall of Bacillus subtilis. J. Bacteriol. 122152-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doyle, R. J., M. L. McDannel, U. N. Streips, D. C. Birdsell, and F. E. Young. 1974. Polyelectrolyte nature of bacterial teichoic acids. J. Bacteriol. 118606-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubrac, S., I. G. Boneca, O. Poupel, and T. Msadek. 2007. New insights into the WalK/WalR (YycG/YycF) essential signal transduction pathway reveal a major role in controlling cell wall metabolism and biofilm formation in Staphylococcus aureus. J. Bacteriol. 1898257-8269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elsbach, P., and J. Weiss. 1988. Phagocytosis of bacteria and phospholipid degradation. Biochim. Biophys. Acta 94729-52. [DOI] [PubMed] [Google Scholar]
- 10.Fischer, W. 1994. Lipoteichoic acid and lipids in the membrane of Staphylococcus aureus. Med. Microbiol. Immunol. (Berlin) 18361-76. [DOI] [PubMed] [Google Scholar]
- 11.Foreman-Wykert, A. K., Y. Weinrauch, P. Elsbach, and J. Weiss. 1999. Cell-wall determinants of the bactericidal action of group IIA phospholipase A2 against Gram-positive bacteria. J. Clin. Investig. 103715-721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Foreman-Wykert, A. K., J. Weiss, and P. Elsbach. 2000. Phospholipid synthesis by Staphylococcus aureus during (sub)lethal attack by mammalian 14-kilodalton group IIA phospholipase A2. Infect. Immun. 681259-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ganz, T. 2003. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3710-720. [DOI] [PubMed] [Google Scholar]
- 14.Grundling, A., and O. Schneewind. 2007. Synthesis of glycerol phosphate lipoteichoic acid in Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 1048478-8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Haas, R., H. U. Koch, and W. Fischer. 1984. Alanyl turnover from lipoteichoic acid to teichoic acid in Staphylococcus aureus. FEMS Microbiol. Lett. 2127-31. [Google Scholar]
- 16.Hayat, M. A. 2000. Principles and techniques of electron microscopy: biological applications, 4th ed. Cambridge University Press, New York, NY.
- 17.Howell, A., S. Dubrac, K. K. Andersen, D. Noone, J. Fert, T. Msadek, and K. Devine. 2003. Genes controlled by the essential YycG/YycF two-component system of Bacillus subtilis revealed through a novel hybrid regulator approach. Mol. Microbiol. 491639-1655. [DOI] [PubMed] [Google Scholar]
- 18.Inada, M., H. Tojo, S. Kawata, S. Tarui, and M. Okamoto. 1991. Preferential distribution of group-II-like phospholipase A2 in mononuclear phagocytic cells in rat spleen and liver. Eur. J. Biochem. 197323-329. [DOI] [PubMed] [Google Scholar]
- 19.Iordanescu, S., and M. Surdeanu. 1976. Two restriction and modification systems in Staphylococcus aureus NCTC8325. J. Gen. Microbiol. 96277-281. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi, S. D., J. M. Voyich, and F. R. DeLeo. 2003. Regulation of the neutrophil-mediated inflammatory response to infection. Microbes Infect. 51337-1344. [DOI] [PubMed] [Google Scholar]
- 21.Koduri, R. S., S. F. Baker, Y. Snitko, S. K. Han, W. Cho, D. C. Wilton, and M. H. Gelb. 1998. Action of human group IIa secreted phospholipase A2 on cell membranes. Vesicle but not heparinoid binding determines rate of fatty acid release by exogenously added enzyme. J. Biol. Chem. 27332142-32153. [DOI] [PubMed] [Google Scholar]
- 22.Koduri, R. S., J. O. Gronroos, V. J. O. Laine, C. Le Calvez, G. Lambeau, T. J. Nevalainen, and M. H. Gelb. 2002. Bactericidal properties of human and murine groups I, II, V, X, and XII secreted phospholipases A2. J. Biol. Chem. 2775849-5857. [DOI] [PubMed] [Google Scholar]
- 23.Kokai-Kun, J. F., T. Chanturiya, and J. J. Mond. 2007. Lysostaphin as a treatment for systemic Staphylococcus aureus infection in a mouse model. J. Antimicrob. Chemother. 601051-1059. [DOI] [PubMed] [Google Scholar]
- 24.Koprivnjak, T., A. Peschel, M. H. Gelb, N. S. Liang, and J. P. Weiss. 2002. Role of charge properties of bacterial envelope in bactericidal action of human group IIA phospholipase A2 against Staphylococcus aureus. J. Biol. Chem. 27747636-47644. [DOI] [PubMed] [Google Scholar]
- 25.Kramer, R. M., C. Hession, B. Johansen, G. Hayes, P. McGray, E. P. Chow, R. Tizard, and R. B. Pepinsky. 1989. Structure and properties of a human non-pancreatic phospholipase A2. J. Biol. Chem. 2645768-5775. [PubMed] [Google Scholar]
- 26.Kusuma, C. M., and J. F. Kokai-Kun. 2005. Comparison of four methods for determining lysostaphin susceptibility of various strains of Staphylococcus aureus. Antimicrob. Agents Chemother. 493256-3263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laine, V. J., D. S. Grass, and T. J. Nevalainen. 1999. Protection by group II phospholipase A2 against Staphylococcus aureus. J. Immunol. 1627402-7408. [PubMed] [Google Scholar]
- 28.Laine, V. J., D. S. Grass, and T. J. Nevalainen. 2000. Resistance of transgenic mice expressing human group II phospholipase A2 to Escherichia coli infection. Infect. Immun. 6887-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lekstrom-Himes, J. A., and J. I. Gallin. 2000. Immunodeficiency diseases caused by defects in phagocytes. N. Engl. J. Med. 3431703-1714. [DOI] [PubMed] [Google Scholar]
- 30.Lowy, F. D. 1998. Staphylococcus aureus infections. N. Engl. J. Med. 339520-532. [DOI] [PubMed] [Google Scholar]
- 31.Neuhaus, F. C., and J. Baddiley. 2003. A continuum of anionic charge: structures and functions of d-alanyl-teichoic acids in gram-positive bacteria. Microbiol. Mol. Biol. Rev. 67686-723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nevalainen, T. J., H. J. Aho, and H. Peuravuori. 1994. Secretion of group 2 phospholipase A2 by lacrimal glands. Investig. Ophthalmol. Vis. Sci. 35417-421. [PubMed] [Google Scholar]
- 33.Nevalainen, T. J., M. M. Haapamaki, and J. M. Gronroos. 2000. Roles of secretory phospholipases A2 in inflammatory diseases and trauma. Biochim. Biophys. Acta 148883-90. [DOI] [PubMed] [Google Scholar]
- 34.Nevalainen, T. J., K. M. Meri, and M. Niemi. 1993. Synovial-type (group II) phospholipase A2 human seminal plasma. Andrologia 25355-358. [DOI] [PubMed] [Google Scholar]
- 35.Peacock, S. J., I. de Silva, and F. D. Lowy. 2001. What determines nasal carriage of Staphylococcus aureus? Trends Microbiol. 9605-610. [DOI] [PubMed] [Google Scholar]
- 36.Peschel, A., M. Otto, R. W. Jack, H. Kalbacher, G. Jung, and F. Gotz. 1999. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J. Biol. Chem. 2748405-8410. [DOI] [PubMed] [Google Scholar]
- 37.Qu, X. D., and R. I. Lehrer. 1998. Secretory phospholipase A2 is the principal bactericide for staphylococci and other gram-positive bacteria in human tears. Infect. Immun. 662791-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schaloske, R. H., and E. A. Dennis. 2006. The phospholipase A2 superfamily and its group numbering system. Biochim. Biophys. Acta 17611246-1259. [DOI] [PubMed] [Google Scholar]
- 39.Schibli, D. J., H. N. Hunter, V. Aseyev, T. D. Starner, J. M. Wiencek, P. B. McCray, Jr., B. F. Tack, and H. J. Vogel. 2002. The solution structures of the human beta-defensins lead to a better understanding of the potent bactericidal activity of HBD3 against Staphylococcus aureus. J. Biol. Chem. 2778279-8289. [DOI] [PubMed] [Google Scholar]
- 40.Takayama, K., S. Hara, I. Kudo, and K. Inoue. 1991. Detection of 14-kDa group II phospholipase A2 in human seminal plasma. Biochem. Biophys. Res. Commun. 1781505-1511. [DOI] [PubMed] [Google Scholar]
- 41.Umeda, A., S. Yokoyama, T. Arizono, and K. Amako. 1992. Location of peptidoglycan and teichoic acid on the cell wall surface of Staphylococcus aureus as determined by immunoelectron microscopy. J. Electron Microsc. (Tokyo) 4146-52. [PubMed] [Google Scholar]
- 42.von Eiff, C., K. Becker, K. Machka, H. Stammer, and G. Peters. 2001. Nasal carriage as a source of Staphylococcus aureus bacteremia. N. Engl. J. Med. 34411-16. [DOI] [PubMed] [Google Scholar]
- 43.Walsh, S., A. Shah, and J. Mond. 2003. Improved pharmacokinetics and reduced antibody reactivity of lysostaphin conjugated to polyethylene glycol. Antimicrob. Agents Chemother. 47554-558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Weidenmaier, C., J. F. Kokai-Kun, S. A. Kristian, T. Chanturiya, H. Kalbacher, M. Gross, G. Nicholson, B. Neumeister, J. J. Mond, and A. Peschel. 2004. Role of teichoic acids in Staphylococcus aureus nasal colonization, a major risk factor in nosocomial infections. Nat. Med. 10243-245. [DOI] [PubMed] [Google Scholar]
- 45.Weidenmaier, C., A. Peschel, Y. Q. Xiong, S. A. Kristian, K. Dietz, M. R. Yeaman, and A. S. Bayer. 2005. Lack of wall teichoic acids in Staphylococcus aureus leads to reduced interactions with endothelial cells and to attenuated virulence in a rabbit model of endocarditis. J. Infect. Dis. 1911771-1777. [DOI] [PubMed] [Google Scholar]
- 46.Weinrauch, Y., C. Abad, N. S. Liang, S. F. Lowry, and J. Weiss. 1998. Mobilization of potent plasma bactericidal activity during systemic bacterial challenge. Role of group IIA phospholipase A2. J. Clin. Investig. 102633-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weinrauch, Y., P. Elsbach, L. M. Madsen, A. Foreman, and J. Weiss. 1996. The potent anti-Staphylococcus aureus activity of a sterile rabbit inflammatory fluid is due to a 14-kD phospholipase A2. J. Clin. Investig. 97250-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weiss, J., A. S. Bayer, and M. Yeaman. 2006. Cellular and extracellular defenses against staphylococcal infections, p. 544-549. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens, 2nd ed. ASM Press, Washington, DC.
- 49.Weiss, J., M. Inada, P. Elsbach, and R. M. Crowl. 1994. Structural determinants of the action against Escherichia coli of a human inflammatory fluid phospholipase A2 in concert with polymorphonuclear leukocytes. J. Biol. Chem. 26926331-26337. [PubMed] [Google Scholar]
- 50.Zhao, C., T. Nguyen, L. M. Boo, T. Hong, C. Espiritu, D. Orlov, W. Wang, A. Waring, and R. I. Lehrer. 2001. RL-37, an alpha-helical antimicrobial peptide of the rhesus monkey. Antimicrob. Agents Chemother. 452695-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]