Abstract
Buruli ulcer is a chronic skin disease caused by Mycobacterium ulcerans, which produces a toxic lipid mycolactone. Despite the extensive necrosis and tissue damage, the lesions are painless. This absence of pain prevents patients from seeking early treatment and, as a result, many patients experience severe sequelae, including limb amputation. We have reported that mice inoculated with M. ulcerans show loss of pain sensation and nerve degeneration. However, the molecules responsible for the nerve damage have not been identified. In order to clarify whether mycolactone alone can induce nerve damage, mycolactone A/B was injected to footpads of BALB/c mice. A total of 100 μg of mycolactone induced footpad swelling, redness, and erosion. The von Frey sensory test showed hyperesthesia on day 7, recovery on day 21, and hypoesthesia on day 28. Histologically, the footpads showed epidermal erosion, moderate stromal edema, and moderate neutrophilic infiltration up to day 14, which gradually resolved. Nerve bundles showed intraneural hemorrhage, neutrophilic infiltration, and loss of Schwann cell nuclei on days 7 and 14. Ultrastructurally, vacuolar change of myelin started on day 14 and gradually subsided by day 42, but the density of myelinated fibers remained low. This study demonstrated that initial hyperesthesia is followed by sensory recovery and final hypoesthesia. Our present study suggests that mycolactone directly damages nerves and is responsible for the absence of pain characteristic of Buruli ulcer. Furthermore, mice injected with 200 μg of mycolactone showed pulmonary hemorrhage. This is the first study to demonstrate the systemic effects of mycolactone.
Buruli ulcer is a chronic painless skin disease caused by Mycobacterium ulcerans (12, 14). M. ulcerans produces a toxic lipid, mycolactone, that induces apoptosis in guinea pig lesions, as well as in human patients (6, 13). Painlessness is a major characteristic of Buruli ulcer, and this phenomenon may often lead the patients to underestimate the disease, which can interfere with the early diagnosis and effective treatment, resulting in severe sequelae such as limb amputation. However, the mechanism of the painlessness was obscure. We have recently reported that in mice inoculated with M. ulcerans, nerve bundles are invaded and damaged by the bacilli (8). Whether the nerve damage was due to mechanical compression by the invaded bacilli or due to the chemical substance(s) produced by the bacilli remained unclear.
In this study, we examined whether mycolactone alone can induce lesions in mice footpads similar to those produced by M. ulcerans inoculation. Sensory changes, footpad swelling, and detailed histopathology, including the ultrastructure of the peripheral nerves, were studied. By comparing the kinetics of pain loss, we tried to reveal the characteristics of neurotoxicity of mycolactone.
MATERIALS AND METHODS
Mycolactone injection.
The animal experiment was approved by Kagoshima University Committee of Animal Experiments. Initial experiments were conducted to determine the optimal concentrations of mycolactone for studies of pain loss. Mycolactone A/B was isolated from M. ulcerans 1615 and purified initially using a 2:1 chloroform extract. Lipids in this extract were subsequently separated by a centrifugal thin-layer chromatograph (Chromatotron; Harrison Research, Palo Alto, CA). The purity of mycolactone preparation was confirmed by mass spectroscopy (6). One mg of mycolactone A/B isolated from M. ulcerans 1615 was dissolved in 25 μl of ethanol and further diluted by 7H9 broth. Mycolactone at 1, 3, 10, 30, 100, or 200 μg in 25 μl of final volume was used as the inoculum. 7H9 broth (25 μl) containing 5 μl of ethanol served as a control. Each solution was injected into the left footpad of 35 6-week-old female BALB/c mice (1 μg, n = 4; 3 μg, n = 4; 10 μg, n = 3; 30 μg, n = 5; 100 μg, n = 5; 200 μg, n = 5; control, n = 9). Morphological studies were done on day 7.
In the second series of experiment, 100 μg of mycolactone was injected into the left footpad of 30 6-week-old female BALB/c mice (100 μg, n = 25; control, n = 5). Sensory and morphological studies were done on days 7 to 42.
Sensory test and thickness of footpads.
In order to examine whether the lesions are painless or not, a behavioral test was performed (8). Briefly, mechanical force to the dorsum of the mouse footpads from the bottom of punched metal floor was measured by using von Frey monofilaments (Touch-Test Sensory Evaluator Instrument; North Coast Medical, Inc., Morgan Hill, CA). Each animal was stimulated three times with filaments using pressures of 0.02, 0.04, 0.07, 0.4, 1, 4, or 10 g. If the animal raised the stimulated foot, it was counted as positive response. The data of animals were summed up in each group. After each sensory test, the thickness of the foot was measured with a caliper.
Histology and electron microscopy of footpads.
In the initial study, 35 animals in total (1 μg, n = 4; 3 μg, n = 4; 10 μg, n = 3; 30 μg, n = 5; 100 μg, n = 5; 200 μg, n = 5; control, n = 9) were examined. After induction of deep anesthesia, perfusion fixation was performed with 4% paraformaldehyde and 0.1% glutaradehyde in phosphate buffer (1 μg, n = 4; 3 μg, n = 4; 10 μg, n = 3; 30 μg, n = 3; 100 μg, n = 3; 200 μg, n = 3; control, n = 7) and with 4% paraformaldehyde and 1% glutaradehyde in phosphate buffer (30 μg, n = 2; 100 μg, n = 2; 200 μg, n = 2; control, n = 2). For the histological study, hind limbs and general organs were embedded in paraffin, cut into 4-μm sections, and examined by hematoxylin and eosin (H&E;) staining. For the electron microscopic study, hind limbs were postfixed with osmium tetroxide and embedded into Epon. Toluidine blue-stained 1-μm sections were screened, and nerve bundles were ultrathin sectioned and stained with uranium and lead for electron microscopy.
A TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) assay was performed by using an Apoptag kit (S7110; Chemicon International, Inc., Temecula, CA). Paraffin sections were treated with 20 μg of proteinase K/ml and then with 3% hydrogen peroxide in phosphate-buffered saline (PBS) before being washed with distilled water. Equilibration buffer was added and the mixture was incubated with terminal deoxynucleotidyltransferase for 1 h at 37°C. Stop/wash buffer was then applied for 10 min. After a wash with PBS, anti-digoxigenin conjugate was added, and the mixture was incubated at room temperature for 30 min. After a wash with PBS and color development with a peroxidase substrate, the specimens were counterstained with methyl green.
In the second series of experiment, 30 animals in total (100 μg, n = 25; control, n = 5) were examined. Perfusion fixation was performed with 4% paraformaldehyde and 0.1% glutaradehyde for the histological study (100 μg on days 7, 14, 21, 28, and 42, n = 3 each; control on day 42, n = 3) and with 4% paraformaldehyde and 1% glutaradehyde for the electron microscopic study (100 μg on days 7, 14, 21, 28, and 42, n = 2 each; control on day 42, n = 2). Histological and electron microscopic procedures were performed in the same manner.
RESULTS
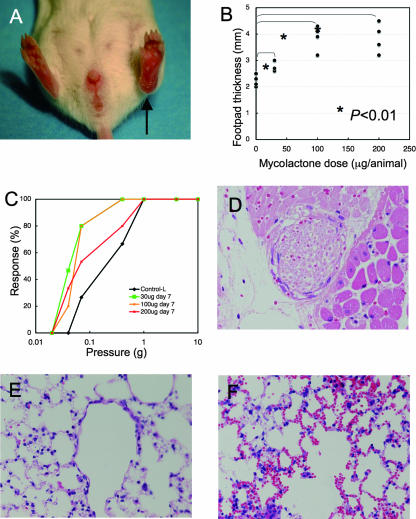
In the initial experiment, 1, 3, or 10 μg of mycolactone did not induce significant gross or histological changes. Significant changes were also not observed in Epon sections. However, 30, 100, or 200 μg of mycolactone induced local swelling (footpad thickness on day 7, control, 2.2 mm; 30 μg, 2.8 mm; 100 μg, 3.7 mm; 200 μg, 3.7 mm) (P < 0.01 between control and each dose), redness, and erosion at the injected site (Fig. 1A and B). A sensory test showed no significant changes on day 4, but hyperesthesia was noted on day 7 (Fig. 1C) (P = 0.022 between the control and the 30-μg injection, P = 0.024 between the control and all of the other injections).
FIG. 1.
Mycolactone injection into mouse footpad evoked swelling, hyperesthesia, nerve damage, and systemic pathology. Various amounts (30, 100, and 200 μg) of mycolactone A/B were injected into the left footpads of BALB/c mice. (A) Footpad swelling on day 7 after the injection of 100 μg (arrow). (B) Footpad thickness on day 7. Dose-dependent swelling was significant. (C) Sensory test showing hyperesthesia on day 7 (P = 0.02). (D) Nerve bundles showed a loss of Schwann cell nuclei and intraneural hemorrhage (H&E; magnification, ×168). (E) Lung without mycolactone injection (H&E, magnification, ×168). (F) Intra-alveolar hemorrhage in the lungs of mice injected with 200 μg of mycolactone into the footpad (H&E, magnification, ×168).
Histological examination of the footpads on day 7 after the injection of 30, 100, and 200 μg of mycolactone revealed focal epidermal erosion, moderate stromal edema, mild fibrinous exudates, and mild infiltration of neutrophils. Blood vessels showed high endothelial venule, and focal hemorrhage was observed. Nerve bundles showed a loss of Schwann cell nuclei (Fig. 1D) and/or intraneural hemorrhage beneath the erosion. Some Schwann cells showed nonspecific degeneration, but intracytoplasmic vacuoles observed in M. ulcerans inoculation was not yet identified. Nerve damage and the number of infiltrating neutrophils showed no significant differences between the low-dose mycolactone (30 μg) and high-dose mycolactone (100 and 200 μg) samples. However, the extent of fibrinous exudate and stromal edema was more prominent in high-dose injections than in low-dose injections. In the control animals, no histological change was observed.
Apoptosis was demonstrated by using the TUNEL method in some Schwann cells after injection of mycolactone (30 and 200 μg). However, similar apoptosis was observed in the Schwann cells of control animals, and there was no significant difference between the mice with mycolactone injection and those of negative controls.
After the injection of 200 μg of mycolactone into the footpad, significant nerve damage was observed in the back of the foot, but 30- and 100-μg injections produced no nerve damage in this region. Inflammation and edema in the back of foot was absent in the 30-μg injection animals, mild in the 100-μg injection animals, and marked in the 200 μg-injection animals. An ulcer was not observed in the back of foot at any of the doses used.
Systemic effects of mycolactone were also observed at the highest concentration. Injection of 200 μg of mycolactone into the foot produced multiple hemorrhages in the lungs (control, Fig. 1E; 200 μg of mycolactone, Fig. 1F), whereas injection of 100 or 200 μg yielded mild swelling in the spleen. No significant changes were observed in the thymus, liver, and small intestine after mycolactone injection.
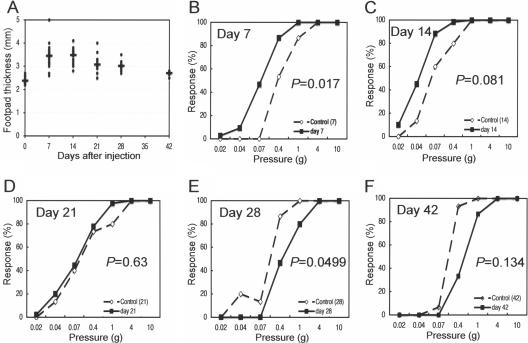
In the second experiment, we tried to evaluate chronological changes produced by mycolactone injection by using a sensory test and morphology in order to determine whether hyperesthesia observed in the first experiment is a transient phenomenon followed by hypoesthesia and whether mycolactone is sufficient to induce vacuolar changes of Schwann cell cytoplasm. Thickness after mycolactone injection (100 μg) showed peak swelling on day 14, with subsequent resolution (Fig. 2A). A sensory test showed significant hyperesthesia on day 7 (Fig. 2B, P = 0.017) and hyperesthetic tendency on day 14 (Fig. 2C, P = 0.081), normal sense on day 21 (Fig. 2D, P = 0.63), followed by significant hypoesthesia on day 28 (Fig. 2E, P = 0.0499) and hypoesthetic tendency on day 42 (Fig. 2F, P = 0.134).
FIG. 2.
Sequential analysis of footpad thickness and sensation after mycolactone injection (100 μg). (A) Footpad thickness after mycolactone injection showed the peak swelling on day 14, with subsequent resolution. Sensory test showed significant hyperesthesia on day 7 (B, P = 0.017) and hyperesthetic tendency on day 14 (C, P = 0.081), normal sense on day 21 (D, P = 0.63), followed by significant hypoesthesia on day 28 (E, P = 0.0499) and hypoesthetic tendency on day 42 (F, P = 0.13).
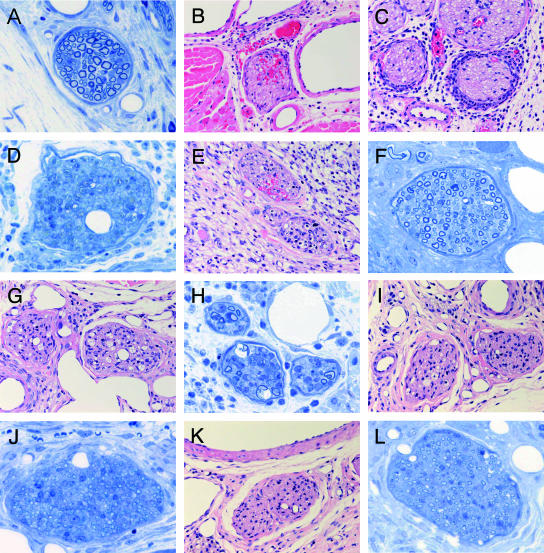
Nerves in the footpads of control mice (only ethanol and 7H9 broth injected, Epon section) showed no significant change (Fig. 3A). In the longitudinal morphological study of mycolactone-injected nerves, the nerves in the footpads of mycolactone-injected mice on day 7 showed intraneural hemorrhage (Fig. 3B) and massive neutrophilic infiltration to perineurium (Fig. 3C). Vacuolar changes of Schwann cells were not observed. Epon sections showed moderate loss of nerve fibers and myelin (Fig. 3D). Intraneural inflammatory cell infiltration and hemorrhage (Fig. 3E and F) were observed in nerves on day 14. On day 21, when the sensory test recovered to normal, nerves exhibited mild inflammatory cell infiltration and vacuolar changes (Fig. 3G). Vacuolar changes observed in Schwann cells induced by mycolactone injection on day 21 was similar to that found previously with M. ulcerans inoculation (Fig. 3H). On day 28, when sensory disturbance was detected by von Frey sensory test, histological damage remained was still evident in nerves (Fig. 3I). Thin myelin indicating remyelination was observed on day 28 (Fig. 3J). Nerves on day 42, when sensory disturbance lasted, histological damage remained (Fig. 3K). Mild fibrosis was observed on day 42 (Fig. 3L).
FIG. 3.
Sequential analysis of footpad histopathology after mycolactone injection (100 μg). (A) Nerve of control mouse (only ethanol and 7H9 broth injected) showed no significant change. (B to D) On day 7, nerves of mycolactone-injected mice showed intraneural hemorrhage (B) and massive neutrophilic infiltration to the perineurium (C). (D) Epon section showing moderate loss of nerve fibers and myelin. (E and F) Nerves on day 14 showed intraneural inflammatory cell infiltration and hemorrhage. (G) Nerves on day 21. Mild infiltration inflammatory cell infiltration and vacuolar change were observed. (H) Vacuolar change of Schwann cells induced by mycolactone injection on day 21 was similar to M. ulcerans inoculation. (I) In the nerves on day 28, histological damage remained. (J) Thin myelins indicating remyelination were observed on day 28. (K) In the nerves on day 42, when sensory disturbance lasted, histological damage remained. (L) Mild fibrosis was observed on day 42. (A, D, F, H, J, and L [Epon, 1-μm sections, toluidine blue staining], magnification, ×340; B, C, E, G, I, and K [paraffin sections, H&E staining], magnification, ×157).
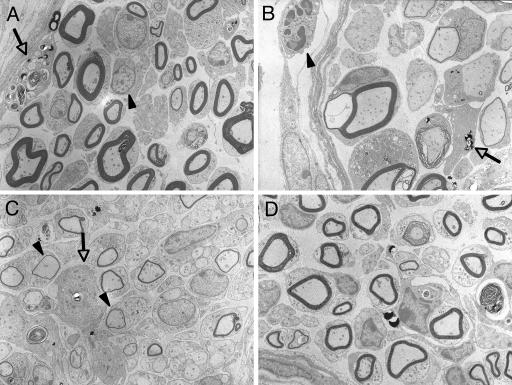
Electron micrographs showed intraneural infiltration of lymphocyte and macrophage containing myelin debris on day 14 after mycolactone injection (Fig. 4A). On day 21, perineural infiltration of neutrophil, vacuolar change of myelin, and macrophage were observed (Fig. 4B). On day 28, thin myelins indicating remyelination and infiltration of macrophage (arrow) were detected (Fig. 4C). On day 42, myelins of normal thickness were observed, a finding which indicates the completion of remyelination (Fig. 4D).
FIG. 4.
Ultrastructure of nerves after mycolactone injection. (A) Intraneural infiltration of lymphocytes (arrowhead) and macrophage (arrow) containing myelin debris on day 14 after mycolactone injection. (B) Vacuolar change of myelin (*) and macrophage (arrow) on day 21. (C) Thin myelins (arrows) indicate remyelination on day 28. D. Mild fibrosis observed on day 42. (Epon-embedded ultrathin sections stained by uranium and lead; magnification, ×1,960).
DISCUSSION
The lack of pain in Buruli ulcer has been one of the most puzzling and remarkable characteristics of this condition. However, until recently, the pathogenesis of painlessness in Buruli ulcer has not been studied. By a careful study of BALB/c mice inoculated by M. ulcerans, Addo et al. observed that mice failed to withdraw their limbs when pricked with a needle at the stage of foot-thigh edema or foot ulceration (1). These results suggested the presence of peripheral anesthesia. We have also demonstrated decreased response to the sensory test of mouse footpads accompanied by moderate swelling and erosion and demonstrated bacterial invasion into the nerves associated with nerve damage by using histopathological and ultrastructural studies (8). In these studies the ability of M. ulcerans to evoke nerve damage became clear; however, the exact molecule causing the nerve damage was not identified.
We report here that the M. ulcerans toxin, mycolactone, is sufficient to cause neurological damage. By studying the kinetics of sensory response after mycolactone injection, we have shown that the response shifted from hypersensitivity (days 7 and 14) to normal (day 21) to hyposensitivity (days 28 and 42), while morphological changes advanced up to day 42. Hypersensitivity seems to be induced by acute inflammation, as demonstrated by the dense neutrophilic infiltration into the perineurium and intraneural hemorrhage. Oliveira et al. (10) and Coutanceau et al. (4) also demonstrated a persistent neutrophilic infiltrate in M. ulcerans-injected mice, which resembles the findings in our mycolactone study. We suggest that the later hyposensitive phase is due to the resolution of the inflammatory reaction, associated with the loss of axons and fibrosis. Another interesting finding in our study is the remyelination observed on day 28. This was not observed after the injection of M. ulcerans (8). Among the clinical forms of Buruli ulcer, the edematous lesion is usually painful (3). This may be reflected in our early stage of mycolactone injection. As a negative control, control extract from mycolactone-negative M. ulcerans strains was not available. Thus, we used the same amount of mycolactone solvent (ethanol) as that used for mycolactone fluid preparation. This might have somewhat interfered with our results.
By injecting 100 μg of mycolactone into the backs of guinea pigs, George et al. (7) could induce pathological lesions characteristic of Buruli ulcer at the injection sites. These researchers also observed slight erythema near the injection site of 10 μg but no gross pathology caused by 1 μg (6). Since BALB/c mice have smaller bodies than guinea pigs, we initially expected that lesions could be induced by 1, 3, or 10 μg. However, no lesion was evoked by 1, 3, or 10 μg of mycolactone, and thus we had to increase the amount to 30, 100, and 200 μg in order to produce pathological findings. Injections of 100 and 200 μg induced similar lesions, but 30 μg produced milder lesions. Based on these data, we used 100 μg of mycolactone for the sequential analysis of footpad lesions. Mycolactone can induce fat necrosis (6); however, murine skin contains considerably less fat than is present in human or guinea pig skin. This may partially explain the higher amount of mycolactone required to produce pathology in murine skin.
In this study, we could not demonstrate significant apoptosis by mycolactone injection. Early work demonstrated that mycolactone can cause apoptosis on murine fibroblasts at very low concentrations of 2 pg/ml (6, 7). Dobos et al. suggested that M. ulcerans might secrete a proteinenous toxin which caused necrosis (5), but in that study the protein fractions may have been contaminated with mycolactone (2). A recent study reports that concentrations of mycolactone greater than 15 μg/ml causes necrosis of L929 fibroblasts as measured by lactate dehydrogenase release (2).A total of 100 μg of mycolactone in our experiment is thought to be a much higher dose than the minimal dose required for causing apoptosis, and necrosis seems to have been the predominant pathology produced in our experiment.
Our study is the first to show that the nerve damage and painlessness associated with Buruli ulcer can be induced by mycolactone alone. Mycolactone injection induced hyperesthesia in the early stage, followed by sensory loss. Morphologically, various nerve lesions, including neutrophilic infiltration, hemorrhage, necrosis, vacuolation of Schwann cell cytoplasm, remyelination, and fibrosis, were observed. This study demonstrated that reversible nerve damage can be induced by a single injection of mycolactone to mouse footpad and suggests that nerve damage induced by mycolactone is responsible for the abnormal sensation, especially painlessness, associated with Buruli ulcer.
Although Buruli ulcer is usually a focal disease, severe forms of the disease occur, including osteomyelitis and disseminated disease (11). Histopathology was found in lymph nodes adjacent to a lesion, suggesting that mycolactone-induced pathology might occur distant from the site of ulceration (9). The persistence of mycolactone in tissue is unknown, although it is likely that the molecule would be degraded by host esterases. In our study it is clear that mycolactone alone can induce pathology at sites distant from the site of inoculation.
In human lesions microhemorrhage is a characteristic finding. Our results suggest that mycolactone is sufficient to induce microhemorrhage. In summary, the present study shows for the first time that mycolactone produces nerve damage leading to painlessness and can also act at sites distant from the site of inoculation. To better understand mycolactone-mediated pathology further studies are needed to determine the kinetics of mycolactone in vivo as well as to determine how the molecule may be transported to distant tissues.
Acknowledgments
This research was supported by grants from the U.S.-Japan Cooperative Medical Science Program, Ministry of Health, Labor, and Welfare (2007-IM [assigned]-002), and the International Medical Centre, Japan (International Cooperation Research Grant, topic code 18C4).
We are grateful to T. Hatanaka, K. Sakazume, Y. Nishimura, and Y. Arimura for technical assistance.
Editor: F. C. Fang
Footnotes
Published ahead of print on 3 March 2008.
REFERENCES
- 1.Addo, P., E. Owusu, B. Adu-Addai, M. Quartey, M. Abbas, A. Doddo, and D. Ofori-Adjei. 2005. Findings from a Buruli ulcer mouse model study. Ghana Med. J. 3986-93. [PMC free article] [PubMed] [Google Scholar]
- 2.Adusumilli, S., A. Mve-Obiang, T. Sparer, W. Meyers, J. Hayman, and P. L. Small. 2005. Mycobacterium ulcerans toxic macrolide, mycolactone modulates the host immune response and cellular location of M. ulcerans in vitro and in vivo. Cell Microbiol. 71295-1304. [DOI] [PubMed] [Google Scholar]
- 3.Asiedu, K., W. Meyers, and P. Agbenorku. 2000. Clinical features and treatment, p. 37-38. In K. Asiedu, R. Scherpbier, and M. Raviglione (ed.), Buruli ulcer Mycobacterium ulcerans infection. World Health Organization, Geneva, Switzerland.
- 4.Coutanceau, E., P. Legras, L. Marsollier, G. Reysset, S. T. Cole, and C. Demangel. 2006. Immunogenicity of Mycobacterium ulcerans Hsp65 and protective efficacy of a Mycobacterium leprae Hsp65-based DNA vaccine against Buruli ulcer. Microbes Infect. 82075-2081. [DOI] [PubMed] [Google Scholar]
- 5.Dobos, K. M., P. L. Small, M. Deslauriers, F. D. Quinn, and C. H. King. 2001. Mycobacterium ulcerans cytotoxicity in an adipose cell model. Infect. Immun. 697182-7186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George, K. M., D. Chatterjee, G. Gunawardana, D. Welty, J. Hayman, R. Lee, and P. L. Small. 1999. Mycolactone: a polyketide toxin from Mycobacterium ulcerans required for virulence. Science 283854-857. [DOI] [PubMed] [Google Scholar]
- 7.George, K. M., L. Pascopella, D. M. Welty, and P. L. Small. 2000. A Mycobacterium ulcerans toxin, mycolactone, causes apoptosis in guinea pig ulcers and tissue culture cells. Infect. Immun. 68877-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goto, M., K. Nakanaga, T. Aung, T. Hamada, N. Yamada, M. Nomoto, S. Kitajima, N. Ishii, S. Yonezawa, and H. Saito. 2006. Nerve damage in Mycobacterium ulcerans-infected mice: probable cause of painlessness in Buruli ulcer. Am. J. Pathol. 168805-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyers, W. M. 2001. Histopathological methods, p. 37-46. In F. Portaels, P. Johnson, and W. M. Meyers (ed.), Diagnosis of Mycobacterium ulcerans disease (Buruli ulcer), World Health Organization, Geneva, Switzerland.
- 10.Oliveira, M. S., A. G. Fraga, E. Torrado, A. G. Castro, J. P. Pereira, A. L. Filho, F. Milanezi, F. C. Schmitt, W. M. Meyers, F. Portaels, M. T. Silva, and J. Pedrosa. 2005. Infection with Mycobacterium ulcerans induces persistent inflammatory responses in mice. Infect. Immun. 736299-6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pszolla, N., M. R. Sarkar, W. Strecker, P. Kern, L. Kinzl, W. M. Meyers, and F. Portaels. 2003. Buruli ulcer: a systemic disease. Clin. Infect. Dis. 37e78-e82. [DOI] [PubMed] [Google Scholar]
- 12.Sizaire, V., F. Nackers, E. Comte, and F. Portaels. 2006. Mycobacterium ulcerans infection: control, diagnosis, and treatment. Lancet Infect. Dis. 6288-296. [DOI] [PubMed] [Google Scholar]
- 13.Walsh, D. S., W. M. Meyers, F. Portaels, J. E. Lane, D. Mongkolsirichaikul, K. Hussem, P. Gosi, and K. S. Myint. 2005. High rates of apoptosis in human Mycobacterium ulcerans culture-positive Buruli ulcer skin lesions. Am. J. Trop. Med. Hyg. 73410-415. [PubMed] [Google Scholar]
- 14.Wansbrough-Jones, M., and R. Phillips. 2006. Buruli ulcer: emerging from obscurity. Lancet 3671849-1858. [DOI] [PubMed] [Google Scholar]