Abstract
Little is known about the role of the cytokine interleukin-12 (IL-12) in Pneumocystis pneumonia or its potential use as immunotherapy. We asked whether release of IL-12 is part of the normal host response to this infection and whether local treatment with IL-12 or gene transfer of IL-12 could accelerate clearance of infection. IL-12 was assayed by enzyme-linked immunosorbent assay in normal mice and in mice deficient in IL-12 after inoculation of Pneumocystis carinii. P. carinii-infected mice were treated with local instillation of IL-12 and gene transfer of the IL-12 gene. Inoculation of P. carinii into normal mice evoked a brisk release of IL-12 into lung tissue, and IL-12 P35-deficient mice showed delayed clearance of infection measured by PCR for P. carinii rRNA. In control mice, intranasal recombinant IL-12 accelerated clearance of infection, and this was associated with increased recruitment of inflammatory cells into lavage fluid and increased release of tumor necrosis factor alpha, IL-12, and gamma interferon. Similar results were observed in infected mice depleted of CD4+ lymphocytes by using in vivo transfer of the IL-12 gene in a replication-deficient adenoviral vector. IL-12 is part of the normal host response to infection with P. carinii. IL-12 therapy can enhance host resistance to infection in both normal mice and mice depleted of CD4+ T lymphocytes. A treatment effect of IL-12 is mediated through enhanced inflammatory cell recruitment into lung tissue and increased tissue concentrations of proinflammatory cytokines.
Pulmonary infection with the fungal pathogen Pneumocystis jirovecii was one of the first recognized complications of human immunodeficiency virus (HIV) infection (35). With antimicrobial prophylaxis and the advent of highly active antiretroviral therapy, the incidence of Pneumocystis pneumonia has declined (40). However, infection with Pneumocystis remains a serious clinical problem among HIV-infected persons and is often the first manifestation of otherwise unrecognized HIV infection (32). Furthermore, recent epidemiologic data indicate an increasing prevalence of Pneumocystis isolates with mutations in genes associated with sulfa antibiotic resistance (25, 38). The clinical relevance of these mutations is unclear, but they correlate with an increased need for mechanical ventilation and mortality from Pneumocystis pneumonia (10). The emergence of Pneumocystis strains potentially resistant to commonly used antibiotics highlights the limitations of conventional antibiotic therapy of infection and underscores the need for new modes of therapy.
Our laboratory has a long-standing interest in strategies to augment host defense mechanisms naturally operative against Pneumocystis infection. In a murine model of infection with Pneumocystis carinii f. sp. muris (hereafter referred to as Pneumocystis carinii), we have shown that aerosol delivery of the cytokine and gamma interferon (IFN-γ) (2) or pulmonary delivery of the IFN-γ gene in vivo (30) can restore host defenses against this infection, even in immunosuppressed mice depleted of CD4+ T lymphocytes. The therapeutic effect of cytokine therapy with IFN-γ is associated with increased recruitment into lung tissue of inflammatory cells, particularly CD8+ T lymphocytes (30).
Production of IFN-γ is critically dependent upon another cytokine, interleukin-12 (IL-12). IL-12 has been shown to stimulate the development of lymphocytes of the Th1 class (34) and to induce the production of IFN-γ from mouse Th1 clones (21) and from natural killer (NK) cells (31). IL-12 synergizes with IL-18 to enhance production of IFN-γ, in part through increased expression of the IL-18 receptor (41). There is little information available regarding the role of IL-12 in host defense against Pneumocystis. The link between IL-12 and IFN-γ also stimulated our interest in IL-12 as a possible therapy for Pneumocystis infection. Treatment with IL-12 has been employed by other investigators to enhance immune responses to a variety of infectious pathogens (7, 17, 23, 24, 26, 28), not including P. carinii.
In this paper, we investigate IL-12 as part of the host response to murine Pneumocystis pneumonia and as a potential immunoadjuvant therapy. Specifically, we asked whether release of IL-12 in lung tissue is part of the normal host response to this infection, whether local treatment with IL-12 can accelerate clearance of infection in normal mice, and whether gene transfer of IL-12 can restore clearance of infection in immunosuppressed mice.
(Data were presented in part at the International Conference of the American Thoracic Society, Seattle, WA, May 2003.)
MATERIALS AND METHODS
Animals.
Specific-pathogen-free female BALB/c, IL-12 P40 knockout (KO), IL-12 P35 KO, and scid/NCr (BALB/c background) mice were purchased at 5 to 6 weeks of age from Jackson Laboratory, Bar Harbor, ME. All animals were housed in filter-topped cages in an isolation room at the LSUHSC animal care facility. All caging procedures and surgical manipulations were done under a laminar flow hood. Mice were fed autoclaved chow and water ad libitum. The animals were kept in the facility for at least 2 days before any treatment was begun. These experiments were approved by the Institutional Animal Care and Use Committee for LSUHSC (protocol no. 2536).
Recombinant IL-12.
Recombinant mouse IL-12 was purchased from R&D Systems, Minneapolis, MN (catalog no. 419ML/CF).
Adenoviral vectors.
An adenoviral vector containing both the p35 and p40 subunit cDNA of murine IL-12 was generously provided by Zhou Xing of McMaster University. This vector has been shown to produce functional heterodimeric IL-12 when administered to mice (6). A control adenoviral vector encoding luciferase was prepared by the LSU Health Sciences Center Vector Core.
P. carinii inoculation.
P. carinii organisms for inoculation were isolated from lung homogenates from chronically infected scid/NCr (BALB/c background) mice as previously described (49). In brief, lungs were aseptically removed and frozen in 1 ml phosphate-buffered saline (PBS) at −70°C. Frozen lungs were homogenized in 10 ml of PBS, forced through a sterile cell strainer, and pelleted at 500 g for 10 min at 4°C. The pellet was then diluted 1:4 with PBS, and the number of Giemsa-stained P. carinii cyst forms quantified microscopically. Recipient mice were anesthetized with ketamine-xylazine and injected intratracheally (IT) with 2 × 105 organisms.
Bronchoalveolar lavage (BAL).
Mice were sacrificed at serial intervals after challenge with P. carinii. After a lethal dose of ketamine-xylazine, mice were exsanguinated by aortic transection. The trachea was exposed through a midline incision and cannulated with a polyethylene catheter. The lungs were lavaged with 0.5-ml aliquots of sterile, calcium- and magnesium-free PBS (Gibco/BRL, Gaithersburg, MD) containing 0.6 mM EDTA up to a total of 10 ml. Recovered lavage fluid was centrifuged at 500 × g for 10 min. Supernatants from the first 1 ml of recovered lavage fluid were collected and stored at −80°F for cytokine assay. Cellular assays were performed on cells recovered from the entire 10-ml lavage effluent.
Lavage cell total and differential cell counts.
Lavaged cells were collected by centrifugation at 500 × g for 10 min. The cells were then washed with PBS, counted in a hemocytometer, and then centrifuged onto glass slides at 500 rpm for 5 min and stained with Diff-Quik (Dade Behring, Inc.) for cell differential counting.
Flow cytometry analysis of T lymphocytes from lavage fluid.
Lymphocyte phenotypes within lavaged cells were identified by flow cytometry. Lavaged cells were suspended at 1 × 107 to 2 × 107 cells/ml in Dulbecco's modified Eagle's medium (Sigma) containing 0.1% sodium azide (Malinckrodt Chemicals, St. Louis, MO) and 25 mM HEPES (Sigma). Aliquots of 25 μl were stained with 50 μl of monoclonal antibody (all from BD Biosciences, San Jose, CA) against CD4 or CD8 (CyChrome conjugated), CD45RB (fluorescein isothiocyanate conjugated), CD44 (phycoerythrin conjugated), or CD62L (antigen-presenting cell conjugated) for 45 min at 4°C. Isotype control antibody staining was used to assist in gating. After cells were washed three times with PBS-sodium azide, cells were fixed with 0.05% paraformaldehyde in PBS-sodium azide. The surface expression of these molecules was determined using a FACSAria cytofluorometer (BD Biosciences). We identified lymphocytes among BAL cells by comparing forward- and side-scatter characteristics to those of splenic lymphocytes analyzed in the same experiment. Flow cytometry data are expressed as absolute numbers of lymphocyte phenotypes per mouse in lavage samples. We determined these numbers as the product of the total cell count (by hemocytometer), the percentage of lymphocytes (by differential cell counts of cytocentrifuged preparations), and the percentage of each lymphocyte subpopulation (by flow cytometry). Effector memory lymphocytes were defined as CD4+ or CD8+ cells that were CD44 (hi), CD45RB (lo), and CD62L (negative) (33).
Preparation of lung homogenates.
In some experiments, the left lung was placed in 1 ml of PBS, homogenized, and then stored at −80°F for a cytokine assay. Our previous experience has shown no differences in inflammatory responses between the right and left lungs in this experimental model.
Cytokine assays.
The first 1 ml of cell-free lung lavage fluids was assayed for macrophage inflammatory protein 2 (MIP-2), tumor necrosis factor alpha (TNF-α), IFN-γ, and IL-12 by using a sandwich-type enzyme-linked immunosorbent assay (ELISA) with two immunological steps. Standard ELISA kits for murine MIP-2, TNF-α, IFN-γ, and IL-12 were purchased from R&D Systems, Minneapolis, MN. Cytokines in lung homogenates were assayed by multiplex cytokine analysis (Bio-Plex; Bio-Rad, Hercules, CA).
RNA isolation and real-time RT-PCR for P. carinii rRNA.
At animal sacrifice, total RNA was isolated from the right lung using TRIzol reagent (Invitrogen, Carlsbad, CA). cDNAs were synthesized from total lung RNA. As a standard for the assay, a portion of P. carinii rRNA (GenBank accession no. AF257179) was cloned into PCR 2.1 vector (Invitrogen, Carlsbad, CA), and PC rRNA was produced by in vitro transcription using T7 TNA polymerase (Promega, Madison, WI). TaqMan PCR primers for mouse P. carinii rRNA were 5′-ATG AGG TGA AAA GTC GAAAGG G-3′ and 5′-TGA TTG TCT CAG ATG AAA AAC CTC TT-3′. The probe was labeled with a reporter fluorescent dye, 6-carboxyfluorescein (FAM), and the sequence was FAM-AACAGCCCAGAATAATGAATAAAGTTCCTCAATTGTTAC-TAMRA (6-carboxytetramethylrhodamine). Real-time reverse transcription (RT)-PCR was done using a two-step method. RT reactions were done in a volume of 25 μl containing 5 μl RNA sample, 1× TaqMan RT buffer, 5.5 mM magnesium chloride, 500 μM of each deoxynucleoside triphosphate (dNTP), 2.5 μM random hexamer, 0.4 U/μl RNase inhibitor, 1.25 U/μl MultiScribe reverse transcriptase (no. 808-0234; PE Biosystems, Foster City, CA). Samples were incubated at 25°C for 10 min, reverse transcribed at 48°C for 30 min, and reverse transcriptase inactivated at 95°C for 5 min. PCRs were done in a volume of 50 μl containing 5 μl cDNA, 1× TaqMan universal PCR master mix (PE Biosystems, Foster City, CA), a primer, and a probe. An initial 2-min incubation was done at 50°C for uracil-N-glycosylase activity to prevent carryover reaction. The reaction was terminated by heating at 95°C for 5 min. The PCR amplification was performed for 40 cycles, with each cycle at 94°C for 20 s and 60°C for 1 min. Data were converted to rRNA copy number using a standard curve of known copy P. carinii rRNA and expressed as copy number per lung.
Treatment with rmIL-12.
Mice were administered intranasal (IN) IL-12 according to the method of Arulanandam et al. (1). Mice received 0.4 μg recombinant murine IL-12 (rmIL-12) or PBS in a 50-μl volume by IN instillation every 3 days beginning 24 h after inoculation of P. carinii.
CD4+ T-lymphocyte depletion.
Mice were depleted of CD4+ T lymphocytes by intraperitoneal injection of 0.3 mg of anti-CD4+ monoclonal antibody (hybridoma GK 1.5; ATCC) in 0.1 ml PBS each week. This treatment produces a sustained and profound depletion of CD4+ lymphocytes from the blood and spleen, allowing progressive P. carinii pneumonia (49).
Treatment with AdIL-12.
Gene transfer of IL-12 was designed as a treatment protocol in animals with established P. carinii infection. Control mice or CD4-depleted mice were inoculated with P. carinii as described above. Twelve days after inoculation, half the mice from each group were administered 2 × 108 PFU of an adenoviral vector encoding IL-12 (AdIL-12), and the other half were administered 2 × 108 of an adenoviral vector encoding luciferase (AdLuc). Mice were then sacrificed 4 weeks after inoculation, and lung tissue was assayed for the burden of P. carinii.
Statistical analysis.
Data are reported as mean ± standard deviation (SD) or standard error of the mean (SEM), as noted. Scalar comparisons were made by the t test. Data collected at multiple times were corrected for multiple comparisons using analysis of variance and Newman-Keuls follow-up testing (56). Significance was accepted when the P value was less than 0.05.
RESULTS
Endogenous IL-12 during infection with P. carinii.
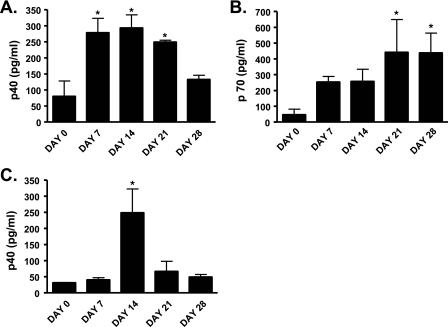
We first sought to determine whether IL-12 release was part of the normal host response to infection with P. carinii. Normal mice were inoculated with P. carinii, and IL-12 was assayed in lung homogenate and in BAL fluid. Using this protocol, infection with P. carinii is self limited with pathogen clearance from lung tissue by 1 month after inoculation (3). The p40 subunit of IL-12 was detectable in lung homogenate and lavage fluid by as early as 7 days after inoculation, peaking at 14 days in both lung homogenate (Fig. 1A) and BAL fluid (Fig. 1C). When lung tissue was assayed for the entire p70 complex, IL-12 was detectable in lung homogenate by day 7 and then persisted to day 28 (Fig. 1B). IL-12 p70 was not detectable in lavage fluid. These results indicate that the release of IL-12 is part of the normal host response to P. carinii.
FIG. 1.
IL-12 protein concentration in lung homogenate and BAL fluid of wild-type mice infected with Pneumocystis carinii. Mice were infected with 2 × 105 Pneumocystis carinii organisms IT on day 0 and sacrificed immediately or on days 7, 14, 21, or 28 postinfection. IL-12 p40 and p70 cytokine levels in lung homogenate (A and C) or BAL fluid (B) were determined by multiplex cytokine assay (Bio-Plex; Bio-Rad, Hercules, CA). IL-12 p70 was not detectable in the BAL fluid. Data are reported as mean ± SEM, n = 3 to 10. * indicates P < 0.01 compared to day 0.
Clearance of P. carinii in IL-12-deficient mice.
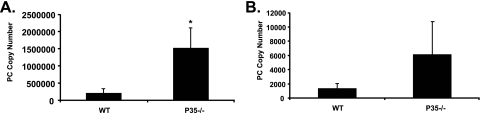
To determine whether endogenous IL-12 is critical to host defense against P. carinii, we inoculated P. carinii into wild-type mice and mice deficient in the P35 and P40 subunits of IL-12. Mice were sacrificed 2 and 4 weeks after inoculation, and P. carinii was assayed in lung tissue by real-time PCR. In comparison to wild-type mice, infection burden in mice deficient in P35 was significantly increased at 2 weeks (Fig. 2A) and was also increased at 4 weeks, although not statistically significant (Fig. 2B). The P35-deficient mice were ultimately able to clear the infection (data not shown). Additional experiments showed that clearance of infection was also impaired in P40-deficient mice (data not shown). Thus, the observation that clearance of P. carinii infection is impaired in P35-deficient mice indicates that IL-12 is required but not essential for optimal host defense against this pathogen.
FIG. 2.
Lung tissue burden of P. carinii in P35 KO and control mice. Data are for mice sacrificed 2 (A) and 4 (B) weeks after inoculation with P. carinii (PC). P. carinii infection burden was measured as real-time PCR for P. carinii rRNA, expressed as copy number per lung. Each data point represents mean ± SD for at least six animals. * indicates P < 0.05 compared to control. WT, wild type.
Local administration of rmIL-12.
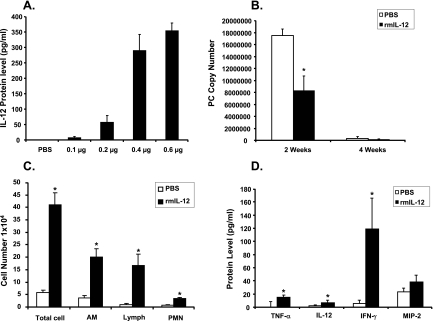
In a dose-ranging study, mice were administered 25 μl of rmIL-12 at various doses by IN instillation. Twenty-four hours later the mice were sacrificed, and the lungs lavaged. IL-12 was assayed by ELISA in the first milliliter of recovered lavage fluid. The results are shown in Fig. 3A. There was a dose-dependent increase in lavage fluid IL-12. There were no animal deaths, even at the highest dose of IL-12.
FIG. 3.
Treatment of mice with rmIL-12. (A) Mice were administered increasing doses of rmIL-12 by IN instillation. IL-12 in the first ml of recovered BAL fluid was assayed by ELISA 24 h later. (B to D) Mice were challenged with 2 × 105 P. carinii organisms IT and then by IN inoculation of 0.4 μg rmIL-12 (or PBS) every 3 days. Mice then were sacrificed 2 or 4 weeks after infection. Each data point represents mean ± SD for at least six animals. * indicates P < 0.05 compared to PBS. (B) Infection burden assayed as rRNA copy number/lung. (C) Total and differential cell counts in lavage fluid at 2 weeks. (D) Concentrations of TNF-α, IL-12, IFN-γ, and MIP-2 in lavage fluid by ELISA at 2 weeks.
Treatment of P. carinii-infected mice with recombinant IL-12.
Based on the results shown in Fig. 3A, we established a treatment regimen in which mice were treated with 0.4 μg rmIL-12 by IN instillation every 3 days. Normal mice were inoculated with P. carinii and then treated 24 h later with IN rmIL-12 or an equal volume of PBS. These treatments continued every 3 days. Groups of mice were then sacrificed at 2 and 4 weeks after P. carinii inoculation, and lung tissue burden was assayed by real-time PCR. The results are shown in Fig. 3B. As expected, mice treated with PBS were able to clear their lungs of P. carinii with nearly complete eradication of infection by 4 weeks. However, the mice treated with IL-12 showed an enhanced clearance of infection with a significantly lower fungal burden at 2 weeks in comparison to mice treated only with PBS. These results indicate that exogenous IL-12 can accelerate clearance of P. carinii in immunocompetent mice.
Treatment with rmIL-12 enhances inflammatory cell recruitment into lung tissue.
To investigate the effect of IN IL-12 on inflammatory cell recruitment, mice were inoculated with P. carinii and then treated with either IN rmIL-12 or PBS as described above. Two weeks after P. carinii challenge, the mice were sacrificed for BAL. Recovered lavage fluid was centrifuged, and the cell supernatant reserved for cytokine analysis (see below). In comparison to mice treated with IN PBS, the mice treated with IL-12 showed an increased total lavage cell count and significantly increased numbers of alveolar macrophages, lymphocytes, and polymorphonuclear leukocytes (Fig. 3C).
Treatment with rmIL-12 enhances cytokine release into lung tissue.
Cell-free supernatants of lavage fluid from IL-12- and PBS-treated mice were analyzed 2 weeks after inoculation of P. carinii for concentrations of proinflammatory cytokines (TNF-α, IL-12 [P70], IFN-γ, and MIP-2). The results are shown in Fig. 3D. Intranasal treatment with rmIL-12 resulted in significantly increased lavage fluid concentrations of TNF-α, IL-12, and IFN-γ in comparison to PBS-treated mice. The concentrations of MIP-2 were not significantly different in the IL-12 mice compared to the PBS mice.
Effect of IT administration of AdIL-12 on lung tissue IL-12.
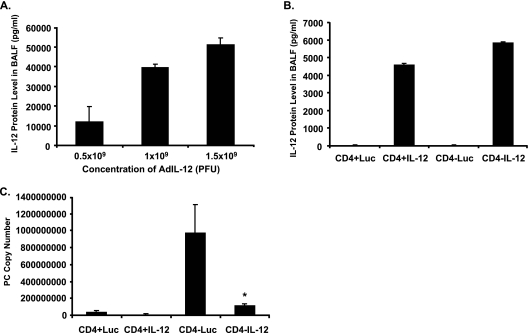
Normal mice were inoculated with AdIL-12 at various doses by IT administration. Three days later, mice were sacrificed for BAL, and IL-12 was assayed by ELISA in lavage fluid. As shown in Fig. 4A, there was a dose-dependent increase in IL-12 three days following gene transfer, indicating that IL-12 gene expression could be achieved in vivo. Unfortunately, the mice did not tolerate these doses of AdIL-12 well, and many of them died after the 3-day time interval. Accordingly, we scaled our dose of AdIL-12 back to 2 × 108 PFU. This dose was better tolerated and resulted in detectable IL-12 in lavage fluid 1 week following gene transfer in both control mice and mice depleted of CD4+ lymphocytes (Fig. 4B). At this dose of AdIL-12, there was minimal stimulation of endogenous IL-12 due to the vector alone (designated Luc). As we have shown previously for a TNF-α adenoviral vector (29), gene expression was enhanced in mice depleted of CD4+ T lymphocytes in comparison to control mice.
FIG. 4.
Gene transfer of rmIL-12 using an adenoviral vector. (A) Wild-type control mice received various doses of IL-12 in the adenoviral vector by IT inoculation, and IL-12 was assayed in lavage fluid after 3 days. (B) IL-12 concentrations in lavage fluid in control (CD4+) and CD4-depleted (CD4−) mice 1 week after administration of 2 × 108 PFU of AdIL-12 or AdLuc (Luc). (C) Control and CD4-depleted BALB/c mice were challenged with 2 × 105 P. carinii (PC) organisms. Twelve days after challenge, mice were administered either 2 × 108 PFU AdIL-12 or AdLuc by IT inoculation. Mice were sacrificed at 4 weeks, and lung homogenate was assayed via real-time PCR for P. carinii rRNA. Data are reported as mean ± SD, n = 5 or 6. * indicates P < 0.05 compared to AdLuc.
Effect of IT administration of AdIL-12 on clearance of P. carinii.
Control mice and CD4-depleted mice were inoculated with P. carinii followed by a single dose of 2 × 108 PFU of AdIL-12 or AdLuc given at 12 days after inoculation. CD4-depleted mice received 0.3 mg of GK 1.5 by intraperitoneal injection 3 days prior to P. carinii inoculation and at weekly intervals until the sacrifice. All mice were then sacrificed at 4 weeks, and lung tissue was assayed for fungal burden by RT-PCR. The results are shown in Fig. 4C. The treatment of the normal (CD4+) mice with AdIL-12 did not significantly change the P. carinii fungal burden at 4 weeks, but clearance was virtually complete in normal mice at this time point (CD4+Luc). In contrast, mice depleted of CD4+ T lymphocytes showed an increased fungal burden at 4 weeks, consistent with previous studies (49). In these mice, treatment with AdIL-12 significantly decreased lung P. carinii copy number in comparison to CD4-depleted mice given the control adenoviral vector (CD4−Luc). Similar results were observed at 2 weeks after P. carinii challenge (data not shown). These results support the idea that gene transfer of IL-12 can restore host defenses against P. carinii in immunosuppressed mice.
Effect of IT administration of AdIL-12 on lymphocyte recruitment.
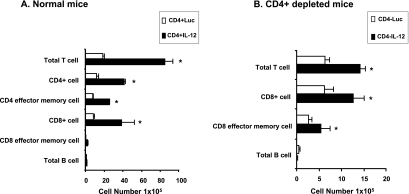
Control mice and CD4-depleted mice were inoculated with P. carinii and then given a single dose of IT AdIL-12 or AdLuc, as described above. The mice were then sacrificed at 4 weeks for flow cytometry of lavaged cells. For control (CD4+) mice, treatment with AdIL-12 significantly increased lavaged total T lymphocytes, CD4+ T lymphocytes, effector memory CD4+ T lymphocytes, and CD8+ T lymphocytes above comparison levels seen in mice treated with the control vector (CD4+Luc) (Fig. 5A). The numbers of effector memory CD8+ T lymphocytes and B lymphocytes did not change in response to AdIL-12. In CD4-depleted mice, the numbers of lavage lymphocytes were lower than those in CD4+ mice. Treatment with AdIL-12 stimulated significantly increased numbers of lavaged total T lymphocytes, CD8+ T lymphocytes, and effector memory CD8+ T lymphocytes in comparison to mice treated with AdLuc (Fig. 5B). B-lymphocyte numbers were unchanged. As expected, negligible numbers of CD4+ T lymphocytes were recovered in both AdIL-12 and AdLuc-treated mice depleted of CD4+ T cells. These results indicate that gene transfer of IL-12 stimulates the recruitment of a broad distribution of lymphocyte subsets, both in control mice and in mice depleted of CD4+ T lymphocytes.
FIG. 5.
Lung lymphocyte phenotypes after gene transfer of rmIL-12 using an adenoviral vector. Control and CD4-depleted BALB/c mice were challenged with 2 × 105 P. carinii organisms. Twelve days after challenge, mice were administered either 2 × 108 PFU AdIL-12 or AdLuc by IT inoculation. Mice were sacrificed at 4 weeks, and lavaged lymphocytes were assayed by flow cytometry. Numbers of lavaged total T cells, CD4+ cells, CD4+ effector/memory cells, CD8+ cells, CD8+ effector/memory cells, and B cells are shown for CD4+ mice (A) and CD4-depleted mice (B). Negligible numbers of CD4+ T cells were observed in the CD4-depleted mice. Data are reported as mean ± SD, n = 5 or 6. * indicates P < 0.05 compared to AdLuc.
Effect of IT administration of AdIL-12 on clearance of P. carinii in scid mice.
Mice bearing the scid mutation have no functional T or B lymphocytes (5) and develop progressive and ultimately fatal pneumonia after the inoculation of P. carinii (8). We investigated the effect of AdIL-12 on P. carinii infection in these immunocompromised mice. scid mice were inoculated with P. carinii, followed by AdIL-12 or AdLuc 12 days later. The mice were then sacrificed at 3 and 4 weeks after P. carinii challenge, and the fungal burden was assayed in lung tissue by RT-PCR. There was no effect of AdIL-12 on the severity of infection in comparison to scid mice treated with the control vector (AdLuc) at either 3 or 4 weeks. The P. carinii copy number in scid mice at 3 weeks was (0.82 + 0.02) × 106 for AdLuc versus (0.93 + 0.07) × 106 for AdIL-12 (P was not significant). At 4 weeks, copy numbers were (1.1 + 0.03) × 106 for AdLuc versus (2.4 + 0.06) × 106 for AdIL-12 (P was not significant). These results suggest that the therapeutic effect of IL-12 against P. carinii is mediated through T or B lymphocytes other than CD4+ lymphocytes.
DISCUSSION
IL-12 is a proinflammatory cytokine that has been postulated to serve as a bridge between innate and adaptive immune responses. IL-12 was first recognized because of its ability to stimulate the release of IFN-γ by NK cells (54). Subsequent investigations showed that IL-12 is critical for Th1 lymphocyte responses and for the production of cytotoxic CD8+ T lymphocytes (53). Infection with a number of bacterial and fungal pathogens results in the release of IL-12 from macrophages, dendritic cells, and neutrophils (52). In contrast, IL-12 is not essential for host defense against viruses and Chlamydia pneumoniae infection (16, 39). Little is known about the role of IL-12 in host defense against the fungal pathogen P. carinii. Blood mononuclear cells from HIV-positive subjects release less IL-12 in response to P. carinii in vitro than cells from HIV-negative subjects (19). Alveolar macrophages from asymptomatic HIV-positive subjects spontaneously release IL-12 but show a suboptimal release of IL-12 in response to in vitro stimulation with Staphylococcus aureus strain Cowan 1 (11). Our data indicate that the challenge of normal mice with this pathogen results in an early release of IL-12 into lung tissue. The p40 subunit of IL-12 could be detected in both lung homogenate and lavage fluid, while the p70 complex was detectable only in tissue homogenate. Discordance at different times between lung homogenate and lavage fluid p40 concentrations may reflect different cellular sources of p40 in the respective compartments (dendritic cells, for example [47]) and/or various expression levels of p40 as a monomer versus a homodimer (9). In general, p40 is secreted in excess over IL-12 p70 (9). The fact that we observed IL-12 in BAL fluid supports a cellular source of IL-12 within the alveolar space, most likely alveolar macrophages. We cannot rule out other cellular sources of this cytokine, however.
IL-12 is not absolutely required for the host to resolve this infection but is necessary for optimal host defense. This conclusion can be drawn from our results showing delayed clearance of infection in P35 KO mice infected with P. carinii. IL-12 is a heterodimer composed of a 34-kDa light chain (P35) and a 40-kDa heavy chain (P40) (27). The P35 subunit is unique to IL-12, while the P40 subunit is shared with another dimeric cytokine, IL-23 (42). Our demonstration that clearance of P. carinii is impaired in P40 KO mice is also consistent with a role for IL-23 in host defense against this pathogen, as reported in a previous publication (48). The fact that IL-12 is complementary to but not critical for protection against P. carinii is consistent with the redundant role of cytokines in pulmonary host defense (51). For example, IL-12−/− mice show an early impaired host response to infection with Listeria monocytogenes but ultimately are able to clear the infection, probably due to IL-12-independent production of IFN-γ by CD8+ T cells (43).
Administration of IL-12 might be useful to enhance host defense against P. carinii, particularly in immunocompromised hosts. Although IL-12 has been investigated most extensively in the treatment of cancer (37), a considerable body of literature supports IL-12 therapy for infectious disease as well. For example, systemic IL-12 has been shown to enhance host defense in animal models of infection with Listeria monocytogenes (55), Mycobacterium tuberculosis (13), Toxoplasma gondii (14), coxsackievirus (46), herpes simplex virus (28), bacteria (17), mycobacteria (26), cryptococcus (24), and Leishmania major (20). Local delivery of IL-12 has been used to treat experimental infections with Klebsiella pneumoniae (18) and Franciscella tularensis (12, 44). In human subjects, IL-12 therapy has been investigated as adjunctive therapy for hepatitis C infection (45) and HIV infection (23). There have been no studies of IL-12 therapy of P. carinii infection, although recombinant IL-12 has been shown not to enhance in vitro proliferation of blood mononuclear cells in response to P. carinii (19). To test the potential of exogenous IL-12 therapy in pulmonary infection with P. carinii, we utilized both IN administration of IL-12 protein and in vivo transfer of the IL-12 gene by IT instillation. Intranasal IL-12 has been shown to be less toxic than systemic IL-12 in murine models (22), while the gene transfer of cytokines such as IL-12 provides more sustained gene expression and avoids the need for repetitive administration. Our results clearly demonstrate that exogenous administration of IL-12 either as recombinant protein or expressed through an adenoviral vector can augment host defenses against P. carinii, accelerating clearance of infection in normal mice and restoring clearance in mice deficient in CD4+ T lymphocytes. This latter observation is particularly relevant to human P. carinii pneumonia, which is most often observed in the setting of HIV infection where CD4+ T lymphocytes are dysfunctional or severely depleted.
The mechanisms through which IL-12 therapy enhances host defense against P. carinii are likely to be multiple. IL-12 is a key initiator of cell-mediated immunity promoting the development of Th1 T-lymphocyte responses to antigen (21, 34), and local delivery of IL-12 has been shown to amplify antigen-specific immune responses in the lungs of mice (50). Thus, in our experiments with P. carinii-infected normal mice, IL-12 therapy may augment Th1 lymphocyte responses to P. carinii. IL-12 has also been shown to enhance the numbers of CD8+ cytotoxic T lymphocytes at mucosal surfaces (4). This mechanism of action is supported in our studies by the observed increase in CD8+ T lymphocytes and CD8+ cells bearing effector memory markers in P. carinii-infected animals depleted of CD4+ T cells treated with AdIL-12. The absence of a therapeutic effect of IL-12 in scid mice with no functional B or T cells strongly suggests that IL-12's effect is mediated through CD8+ T lymphocytes. Preliminary studies from our laboratory also show that IL-12 therapy of CD4-depleted, P. carinii-infected mice increases the numbers of lung CD8+ T lymphocytes containing intracellular IFN-γ (data not shown). Thus, IL-12 administration may facilitate host clearance of P. carinii by augmenting antigen-specific lymphocyte responses involving both CD4+ and CD8+ T cells.
In addition to stimulating antigen-specific immune responses, IL-12 therapy may also enhance innate immunity. For example, IL-12 has been shown to enhance resistance to Toxoplasma gondii in a T-cell-independent fashion through the stimulation of IFN-γ production by NK cells and the activation of macrophages (15). IL-12 therapy may also increase the recruitment of lymphocytes through enhanced production of IFN-γ-inducible lymphocyte chemokines, particularly those of the CXCR3 class (36). This is particularly the case, given the high induction of IFN-γ following IL-12 administration in our model. A nonspecific effect of IL-12 in our model of P. carinii infection is supported by the broad increase in the recruitment of macrophages, lymphocytes, and neutrophils into lung tissue and the increase in proinflammatory cytokines associated with IL-12 administration. Research is ongoing to characterize the specific therapeutic mechanism(s) of IL-12 therapy in P. carinii pneumonia in normal and immunosuppressed mice.
In conclusion, the present study shows that IL-12 is part of the normal host response to infection with P. carinii and is required but not essential for optimal clearance of pulmonary infection. IL-12 therapy can enhance host resistance to infection in both normal mice and mice depleted of CD4+ T lymphocytes. A treatment effect of IL-12 is mediated through enhanced inflammatory cell recruitment into lung tissue and increased tissue concentrations of proinflammatory cytokines. These results support a potential role for IL-12 as an adjunctive therapy for pneumonia due to P. carinii.
Acknowledgments
We thank Ping Zhang and the LSUHSC Immunology Core for assistance with flow cytometry and Robert Kutner of the LSUHSC Vector Core for assistance with AdIL-12.
This work was supported by NIH grants HL076100 (NHLBI) and AI51677 (NIAID) and by the LSUHSC Alcohol Research Center.
We do not have a commercial or other association (pharmaceutical stock ownership, consultancy, advisory board membership, related patents, or research funding) that might pose a conflict of interest.
Editor: A. Casadevall
Footnotes
Published ahead of print on 10 March 2008.
REFERENCES
- 1.Arulanandam, B. P., M. O'Toole, and D. W. Metzger. 1999. Intranasal interleukin-12 is a powerful adjuvant for protective mucosal immunity. J. Infect. Dis. 180940-949. [DOI] [PubMed] [Google Scholar]
- 2.Beck, J., M. Warnock, J. Curtis, M. Sniezek, S. Arrag-Peffer, H. Kaltreider, and J. Shellito. 1991. Inflammatory responses to Pneumocystis carinii in mice selectively depleted of helper T lymphocytes. Am. J. Respir. Cell Mol. Biol. 5186-197. [DOI] [PubMed] [Google Scholar]
- 3.Beck, J., M. Warnock, H. Kaltreider, and J. Shellito. 1993. Host defenses against Pneumocystis carinii in mice selectively depleted of CD4+ lymphocytes. Chest 103116S-118S. [DOI] [PubMed] [Google Scholar]
- 4.Belyakov, I. M., J. D. Ahlers, B. Y. Brandwein, P. Earl, B. L. Kelsall, B. Moss, W. Strober, and J. A. Berzofsky. 1998. The importance of local mucosal HIV-specific CD8(+) cytotoxic T lymphocytes for resistance to mucosal viral transmission in mice and enhancement of resistance by local administration of IL-12. J. Clin. Investig. 1022072-2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosma, G. C., R. P. Custer, and M. J. Bosma. 1983. A severe combined immunodeficiency mutation in the mouse. Nature 301527-530. [DOI] [PubMed] [Google Scholar]
- 6.Bramson, J., M. Hitt, W. S. Gallichan, K. L. Rosenthal, J. Gauldie, and F. L. Graham. 1996. Construction of a double recombinant adenovirus vector expressing a heterodimeric cytokine: in vitro and in vivo production of biologically active interleukin-12. Hum. Gene Ther. 7333-342. [DOI] [PubMed] [Google Scholar]
- 7.Carreno, V., and J. A. Quiroga. 1997. Biological properties of interleukin-12 and its therapeutic use in persistent hepatitis B virus and hepatitis C virus infection. J. Viral Hepat. 4(Suppl. 2)83-86. [DOI] [PubMed] [Google Scholar]
- 8.Comley, J. C., and A. M. Sterling. 1994. Artificial infections of Pneumocystis carinii in the SCID mouse and their use in the in vivo evaluation of antipneumocystis drugs. J. Eukaryot. Microbiol. 41540-546. [DOI] [PubMed] [Google Scholar]
- 9.Cooper, A. M., and S. A. Khader. 2007. IL-12p40: an inherently agonistic cytokine. Trends Immunol. 2833-38. [DOI] [PubMed] [Google Scholar]
- 10.Crothers, K., C. B. Beard, J. Turner, G. Groner, M. Fox, A. Morris, S. Eiser, and L. Huang. 2005. Severity and outcome of HIV-associated Pneumocystis pneumonia containing Pneumocystis jirovecii dihydropteroate synthase gene mutations. AIDS 19801-805. [DOI] [PubMed] [Google Scholar]
- 11.Denis, M., and E. Ghadirian. 1994. Dysregulation of interleukin 8, interleukin 10, and interleukin 12 release by alveolar macrophages from HIV type 1-infected subjects. AIDS Res. Hum. Retrovir. 101619-1627. [DOI] [PubMed] [Google Scholar]
- 12.Duckett, N. S., S. Olmos, D. M. Durrant, and D. W. Metzger. 2005. Intranasal interleukin-12 treatment for protection against respiratory infection with the Francisella tularensis live vaccine strain. Infect. Immun. 732306-2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flynn, J. L., M. M. Goldstein, K. J. Triebold, J. Sypek, S. Wolf, and B. R. Bloom. 1995. IL-12 increases resistance of BALB/c mice to Mycobacterium tuberculosis infection. J. Immunol. 1552515-2524. [PubMed] [Google Scholar]
- 14.Gazzinelli, R. T., S. Hieny, T. A. Wynn, S. Wolf, and A. Sher. 1993. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon gamma by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc. Natl. Acad. Sci. USA 906115-6119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gazzinelli, R. T., M. Wysocka, S. Hayashi, E. Y. Denkers, S. Hieny, P. Caspar, G. Trinchieri, and A. Sher. 1994. Parasite-induced IL-12 stimulates early IFN-gamma synthesis and resistance during acute infection with Toxoplasma gondii. J. Immunol. 1532533-2543. [PubMed] [Google Scholar]
- 16.Geng, Y., K. Berencsi, Z. Gyulai, T. Valyi-Nagy, E. Gonczol, and G. Trinchieri. 2000. Roles of interleukin-12 and gamma interferon in murine Chlamydia pneumoniae infection. Infect. Immun. 682245-2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goebel, A., E. Kavanagh, A. Lyons, I. B. Saporoschetz, C. Soberg, J. A. Lederer, J. A. Mannick, and M. L. Rodrick. 2000. Injury induces deficient interleukin-12 production, but interleukin-12 therapy after injury restores resistance to infection. Ann. Surg. 231253-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenberger, M., S. Kunkel, R. Strieter, N. Lukacs, J. Bramson, J. Gauldie, F. Graham, M. Hitt, J. Danforth, and T. Standiford. 1996. IL-12 gene therapy protects mice in lethal Klebsiella pneumonia. J. Immunol. 1573006-3012. [PubMed] [Google Scholar]
- 19.Harrison, T. S., and S. M. Levitz. 1996. Role of IL-12 in peripheral blood mononuclear cell responses to fungi in persons with and without HIV infection. J. Immunol. 1564492-4497. [PubMed] [Google Scholar]
- 20.Heinzel, F. P., F. Ahmed, A. M. Hujer, and R. M. Rerko. 1995. Immunoregulation of murine leishmaniasis by interleukin-12. Res. Immunol. 146575-581. [DOI] [PubMed] [Google Scholar]
- 21.Hsieh, C. S., S. E. Macatonia, C. S. Tripp, S. F. Wolf, A. O'Garra, and K. M. Murphy. 1993. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science 260547-549. [DOI] [PubMed] [Google Scholar]
- 22.Huber, V. C., B. P. Arulanandam, P. M. Arnaboldi, M. K. Elmore, C. E. Sheehan, B. V. Kallakury, and D. W. Metzger. 2003. Delivery of IL-12 intranasally leads to reduced IL-12-mediated toxicity. Int. Immunopharmacol. 3801-809. [DOI] [PubMed] [Google Scholar]
- 23.Jacobson, M. A., J. Spritzler, A. Landay, E. Chan, D. Katzenstein, B. Schock, L. Fox, J. Roe, S. Kundu, and R. Pollard. 2002. A phase I, placebo-controlled trial of multi-dose recombinant human interleukin-12 in patients with HIV infection. AIDS 161147-1154. [DOI] [PubMed] [Google Scholar]
- 24.Kawakami, K., M. Tohyama, Q. Xie, and A. Saito. 1996. IL-12 protects mice against pulmonary and disseminated infection caused by Cryptococcus neoformans. Clin. Exp. Immunol. 104208-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kazanjian, P. H., D. Fisk, W. Armstrong, Q. Shulin, H. Liwei, Z. Ke, and S. Meshnick. 2004. Increase in prevalence of Pneumocystis carinii mutations in patients with AIDS and P. carinii pneumonia, in the United States and China. J. Infect. Dis. 1891684-1687. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi, K., T. Kasama, J. Yamazaki, M. Hosaka, T. Katsura, T. Mochizuki, K. Soejima, and R. M. Nakamura. 1995. Protection of mice from Mycobacterium avium infection by recombinant interleukin-12. Antimicrob. Agents Chemother. 391369-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi, M., L. Fitz, M. Ryan, R. M. Hewick, S. C. Clark, S. Chan, R. Loudon, F. Sherman, B. Perussia, and G. Trinchieri. 1989. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J. Exp. Med. 170827-845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kobayashi, M., H. Takahashi, D. N. Herndon, R. B. Pollard, and F. Suzuki. 2003. Therapeutic effects of IL-12 combined with benzoylmesaconine, a non-toxic aconitine-hydrolysate, against herpes simplex virus type 1 infection in mice following thermal injury. Burns 2937-42. [DOI] [PubMed] [Google Scholar]
- 29.Kolls, J., D. Lei, G. Odom, S. Nelson, W. Summer, M. Gerber, and J. Shellito. 1996. Transient CD4-lymphocyte depletion prolongs transgene expression of E1-deleted adenoviral vectors. Hum. Gene Ther. 7489-497. [DOI] [PubMed] [Google Scholar]
- 30.Kolls, J., C. Vazquez, D. Lei, P. Schwarzenberger, P. Ye, S. Nelson, W. Summer, and J. Shellito. 1999. Interferon-gamma and CD8+ T-cells restore host defense against P. carinii in mice lacking CD4+ T-cells. J. Immunol. 1622890-2894. [PubMed] [Google Scholar]
- 31.Lieberman, L. A., and C. A. Hunter. 2002. Regulatory pathways involved in the infection-induced production of IFN-gamma by NK cells. Microbes Infect. 41531-1538. [DOI] [PubMed] [Google Scholar]
- 32.Lundberg, B. E., A. J. Davidson, and W. J. Burman. 2000. Epidemiology of Pneumocystis carinii pneumonia in an era of effective prophylaxis: the relative contribution of non-adherence and drug failure. AIDS 142559-2566. [DOI] [PubMed] [Google Scholar]
- 33.MacDonald, H. R., R. C. Budd, and J. C. Cerottini. 1990. Pgp-1 (Ly 24) as a marker of murine memory T lymphocytes. Curr. Top. Microbiol. Immunol. 15997-109. [DOI] [PubMed] [Google Scholar]
- 34.Manetti, R., P. Parronchi, M. G. Giudizi, M. P. Piccinni, E. Maggi, G. Trinchieri, and S. Romagnani. 1993. Natural killer cell stimulatory factor (interleukin 12 [IL-12]) induces T helper type 1 (Th1)-specific immune responses and inhibits the development of IL-4-producing Th cells. J. Exp. Med. 1771199-1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masur, H., M. Michelis, J. Greene, I. Onorato, R. Vande Stouwe, R. Holzman, G. Wormser, L. Brettman, M. Lange, H. Murray, and S. Cunningham-Rundles. 1981. An outbreak of community-acquired Pneumocystis carinii pneumonia: initial manifestation of cellular immune dysfunction. N. Engl. J. Med. 3051431-1438. [DOI] [PubMed] [Google Scholar]
- 36.McAllister, F., S. Ruan, C. Steele, M. Zheng, L. McKinley, L. Ulrich, L. Marrero, J. E. Shellito, and J. K. Kolls. 2006. CXCR3 and IFN protein-10 in Pneumocystis pneumonia. J. Immunol. 1771846-1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melero, I., G. Mazzolini, I. Narvaiza, C. Qian, L. Chen, and J. Prieto. 2001. IL-12 gene therapy for cancer: in synergy with other immunotherapies. Trends Immunol. 22113-115. [DOI] [PubMed] [Google Scholar]
- 38.Meshnick, S. R., P. A. Hossler, K. S. Enger, P. Kazanjian, J. S. Rest, D. Mindell, B. Li, C. H. Lee, L. F. Nimri, J. L. Carter, C. B. Beard, and L. Huang. 2001. Distribution of DHPS mutations among ITS subtypes of P. carinii f. sp. hominis. J. Eukaryot. Microbiol. 48(Suppl.)126S-128S. [DOI] [PubMed] [Google Scholar]
- 39.Monteiro, J. M., C. Harvey, and G. Trinchieri. 1998. Role of interleukin-12 in primary influenza virus infection. J. Virol. 724825-4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morris, A., J. D. Lundgren, H. Masur, P. D. Walzer, D. L. Hanson, T. Frederick, L. Huang, C. B. Beard, and J. E. Kaplan. 2004. Current epidemiology of Pneumocystis pneumonia. Emerg. Infect. Dis. 101713-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okamura, H., S. Kashiwamura, H. Tsutsui, T. Yoshimoto, and K. Nakanishi. 1998. Regulation of interferon-gamma production by IL-12 and IL-18. Curr. Opin. Immunol. 10259-264. [DOI] [PubMed] [Google Scholar]
- 42.Oppmann, B., R. Lesley, B. Bloom, J. Timans, Y. Xu, B. Hunte, F. Vega, N. Yu, J. Wang, K. Singh, F. Zonin, E. Vaisberg, T. Churakova, M. Liu, D. Gorman, J. Wagner, S. Zurawski, Y. Liu, J. Abrams, K. Moore, D. Rennick, R. Waal-Malefyt, C. Hannum, J. Bazan, and R. Kastelein. 2000. Novel p19 protein engages IL-12 p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13715-725. [DOI] [PubMed] [Google Scholar]
- 43.Oxenius, A., U. Karrer, R. M. Zinkernagel, and H. Hengartner. 1999. IL-12 is not required for induction of type 1 cytokine responses in viral infections. J. Immunol. 162965-973. [PubMed] [Google Scholar]
- 44.Pammit, M. A., V. N. Budhavarapu, E. K. Raulie, K. E. Klose, J. M. Teale, and B. P. Arulanandam. 2004. Intranasal interleukin-12 treatment promotes antimicrobial clearance and survival in pulmonary Francisella tularensis subsp. novicida infection. Antimicrob. Agents Chemother. 484513-4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pockros, P. J., K. Patel, C. O'Brien, M. Tong, C. Smith, V. Rustgi, R. L. Carithers, J. G. McHutchison, E. Olek, and M. F. Debruin. 2003. A multicenter study of recombinant human interleukin 12 for the treatment of chronic hepatitis C virus infection in patients nonresponsive to previous therapy. Hepatology 371368-1374. [DOI] [PubMed] [Google Scholar]
- 46.Potvin, D. M., D. W. Metzger, W. T. Lee, D. N. Collins, and A. I. Ramsingh. 2003. Exogenous interleukin-12 protects against lethal infection with coxsackievirus B4. J. Virol. 778272-8279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rothfuchs, A. G., A. Bafica, C. G. Feng, J. G. Egen, D. L. Williams, G. D. Brown, and A. Sher. 2007. Dectin-1 interaction with Mycobacterium tuberculosis leads to enhanced IL-12p40 production by splenic dendritic cells. J. Immunol. 1793463-3471. [DOI] [PubMed] [Google Scholar]
- 48.Rudner, X. L., K. I. Happel, E. A. Young, and J. E. Shellito. 2007. Interleukin-23 (IL-23)-IL-17 cytokine axis in murine Pneumocystis carinii infection. Infect. Immun. 753055-3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shellito, J., V. Suzara, W. Blumenfeld, J. Beck, H. Steger, and T. Ermak. 1990. A new model of Pneumocystis carinii infection in mice selctively depleted of helper T lymphocytes. J. Clin. Investig. 851686-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stampfli, M. R., G. Scott Neigh, R. E. Wiley, M. Cwiartka, S. A. Ritz, M. M. Hitt, Z. Xing, and M. Jordana. 1999. Regulation of allergic mucosal sensitization by interleukin-12 gene transfer to the airway. Am. J. Respir. Cell Mol. Biol. 21317-326. [DOI] [PubMed] [Google Scholar]
- 51.Strieter, R. M., J. A. Belperio, and M. P. Keane. 2003. Host innate defenses in the lung: the role of cytokines. Curr. Opin. Infect. Dis. 16193-198. [DOI] [PubMed] [Google Scholar]
- 52.Trinchieri, G. 1998. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv. Immunol. 7083-243. [DOI] [PubMed] [Google Scholar]
- 53.Trinchieri, G. 1998. Proinflammatory and immunoregulatory functions of interleukin-12. Int. Rev. Immunol. 16365-396. [DOI] [PubMed] [Google Scholar]
- 54.Trinchieri, G., and P. Scott. 1995. Interleukin-12: a proinflammatory cytokine with immunoregulatory functions. Res. Immunol. 146423-431. [DOI] [PubMed] [Google Scholar]
- 55.Wagner, R. D., H. Steinberg, J. F. Brown, and C. J. Czuprynski. 1994. Recombinant interleukin-12 enhances resistance of mice to Listeria monocytogenes infection. Microb. Pathog. 17175-186. [DOI] [PubMed] [Google Scholar]
- 56.Zar, J. 1974. Biostatistical analysis. Prentice-Hall, Inc., Englewood Cliffs, NJ.