Abstract
Yersinia pestis, the highly virulent agent of plague, is a biological weapon. Strategies that prevent plague have been sought for centuries, and immunization with live, attenuated (nonpigmented) strains or subunit vaccines with F1 (Caf1) antigen is considered effective. We show here that immunization with live, attenuated strains generates plague-protective immunity and humoral immune responses against F1 pilus antigen and LcrV. Y. pestis variants lacking caf1 (F1 pili) are not only fully virulent in animal models of bubonic and pneumonic plague but also break through immune responses generated with live, attenuated strains or F1 subunit vaccines. In contrast, immunization with purified LcrV, a protein at the tip of type III needles, generates protective immunity against the wild-type and the fully virulent caf1 mutant strain, in agreement with the notion that LcrV can elicit vaccine protection against both types of virulent plague strains.
Plague epidemics occur infrequently, often separated by centuries with sporadic human disease (26). Nevertheless, the fulminant spread and high mortality of plague probably killed more people worldwide than any other infectious disease (53). Many different species of mammals, including rats, squirrels, mice, prairie dogs, and gerbils, represent animal reservoirs for the plague pathogen, which is transmitted to humans via flea bite, aerosol, or contact (47). Flea-bite transmission limits Yersinia pestis replication initially to local lymph nodes, with characteristic swellings (bubos) and disease symptoms that frequently progress to systemic spread of the pathogen and the lethal outcome of bubonic plague (7). Aerosol transmission of Y. pestis, whether during plague epidemics or deliberate dissemination, causes pneumonic plague, a disease with rapid fatality and few characteristic symptoms (36, 50). The ubiquitous spread of zoonotic reservoir and insect vectors for plague, in addition to the possible illegitimate use of Y. pestis as a weapon, demands the urgent development of plague vaccines that protect humans against bubonic and pneumonic plague (30).
Although bubonic plague infection is associated with high mortality, individuals that survive the disease are considered immune to subsequent plague infection (27). This discovery prompted a search for vaccines, derived from either live, attenuated strains or purified bacterial subunits, to generate protection and countermeasures against future plague pandemics (10, 11, 13, 27). The ultimate goal of plague vaccine research is the development of safe products that generate protective immunity in humans but cannot be defeated by naturally occurring Y. pestis strains or their mutant variants (1, 17, 28, 42, 55, 56). Two subunit antigens, purified F1 pilin (2), i.e., recombinant capsular fraction 1 (Caf1) (5, 23), and LcrV (10, 48), a protein residing at the tip of type III needle complexes (43), are currently thought of as the only protective antigens for plague vaccines (41, 60) (see Fig. 1A). Nevertheless, the utility of these two antigens, either alone or as combined vaccine preparations, has been questioned. Y. pestis variants lacking Caf1 capsule have been proposed to cause lethal plague infections (15, 22, 58, 61), and LcrV-mediated immune responses have not yet been demonstrated to generate protective immunity against pneumonic plague in nonhuman primates (55). In contrast to purified subunit vaccines, live, attenuated vaccines are comprised of large arrays of naturally occurring antigens, working either alone or in synergy to generate protective immunity (51). Concerns regarding the stability of attenuated Y. pestis strains and unpredictable and serious side effects (including death) following human immunization, as well as the undefined antigenicity of vaccines, diminished the interest in whole-cell immunization of humans (37, 38), even though early pioneering work demonstrated its efficacy for plague protection in clinical studies that involved thousands of subjects (24, 27).
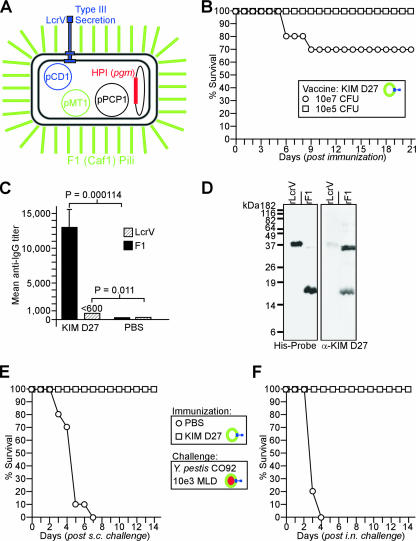
FIG. 1.
Immune responses to live, attenuated (Δpgm) plague vaccine strains. (A) Protective antigens of Y. pestis include LcrV (pCD1-encoded protein [blue] at the tip of type III secretion needles) and F1 (caf1 pilus assembly operon on pMT1) pili (green) that resemble a capsular coat. The HPI and pigmentation segment (pgm) locus (red) is flanked by IS100 insertional elements and can be lost spontaneously, giving rise to attenuated strains that express both protective antigens. (B) Groups of BALB/c mice (n = 10) were immunized by intramuscular injection (1 × 105 or 1 × 107 CFU) of the live, attenuated vaccine strain KIM D27 and monitored over 21 days. Data are representative of the results of two independent experiments. (C) Twenty-one days following immunization, sera (n = 5) were analyzed for antibody titers specific for either purified rF1 or rLcrV by ELISA. Error bars show standard deviations. (D) Purified antigens, 200 ng of rLcrV or rF1 protein, were electrotransferred onto PVDF membranes and detected using alkaline-phosphate-conjugated His probe. Antibodies in diluted serum (1:1,000) of immunized animals were revealed with secondary antibody conjugates to mouse IgG. Molecular size markers are shown on the left. α, anti. (E) Twenty-one days following immunization, BALB/c mice (n = 10) were challenged by subcutaneous (s.c.) injection with 1,000 MLD of Y. pestis CO92 and monitored over 14 days. Data are representative of the results of two independent experiments. (F) Twenty-one days following immunization, anesthetized BALB/c mice (n = 10) were challenged by intranasal (i.n.) inoculation with 1,000 MLD (400,000 CFU) Y. pestis CO92 and monitored over 14 days. Data are representative of the results of two independent experiments. Colored-ring symbols in keys are as described for panel A.
The majority of Y. pestis strains employed in past live vaccine studies were attenuated due to mutations in the pigmentation locus (pgm) (8, 25), a genotype that has been attributed by Fetherston and colleagues to spontaneous deletion of a 102-kb chromosomal fragment comprising the high-pathogenicity island (HPI) involved in bacterial iron uptake (46) and the pigmentation segment (pgm) associated with Congo red staining of colonies from virulent Y. pestis isolates (20, 34). Immunization experiments with live, attenuated Y. pestis pgm strains focused largely on determining the levels of protection afforded by vaccination via intravenous, subcutaneous, intradermal, intraocular, or aerosolized routes in terms of morbidity and mortality rather than actual characterization of the host's immune response to strains postinoculation (37). Thus, even though immunization with live, attenuated strains is considered the definitive standard for the generation of plague-protective immunity, the molecular basis for immunity against bubonic or pneumonic disease has not yet been determined (12).
MATERIALS AND METHODS
Bacterial strains and plasmids.
Y. pestis KIM D27 (KIM 5) (8) was used as the parental strain for allelic replacement. The KIM D27 ΔV variant was created via allelic exchange, by inserting a stop codon followed by a plus-1 frameshift mutation immediately after the start codon (16, 49). The mutation was generated by amplifying 1-kb regions upstream and downstream of the second codon with the following primers: YpV5Sac (5′-GAGCTCCCCTCATACTTTGTCTGGCA-3′), ECOYpV5 (5′-AAGAATTCATTAAATCATATTAAATAATTTGCCCTC-3′), ECOYpV3 (5′-AAGAATTCAGCCTACGAACAAAACCC-3′), and PSTYpV3 5′-AACTGCAGCTGTCGTCTCTTGTTGCATAA-3′). The amplified products, a 5′ SacI/EcoRI fragment and a 3′ EcoRI/PstI fragment, were ligated by three-way ligation into the SacI/PstI sites of pLC28 (14).
Y. pestis ΔF1 was created via allelic exchange by deleting the caf1 open reading frame (35). The mutation was generated by amplifying 1-kb DNA segments upstream of the start codon and downstream of the stop codon with the primers 5′Caf1XbaI (5′-ATTCTAGAATACTAGAAACGATTGCCG-3′), 5′Caf1BamHI (5′-TAGGATCCCATATATTACCTCTATCG-3′), 3′Caf1Bam (5′-TAGGATCCTAATATCTAACCAATAATCC-3′), and 3′Caf1SacI (5′-TAGAGCTCTACTGGCTTTGCGCCACCG-3′). The amplified products, a 5′ XbaI/BamHI fragment and a 3′ BamHI/SacI fragment were ligated by three-way ligation into the XbaI/SacI sites of pLC28. The recombinant plasmids were electroporated into KIM D27, and single-crossover events were selected by plating on heart infusion agar (HIA) supplemented with 20 μg/ml chloramphenicol. The resolution of replication-defective plasmid cointegrates within pCD1 for lcrV and pMT1 for caf1 was achieved by plating on HIA supplemented with 5% sucrose as counter-selection for sacB, which is located on the pLC28 vector (14). Chloramphenicol-sensitive and sucrose-resistant colonies were examined by PCR to reveal mutant genotypes.
Using the same methods, the double-mutant strain ΔVF1 was generated by deleting the caf1 open reading frame in the ΔV variant. Mutations in CO92 were generated with a similar strategy, utilizing pCVD422 (Ampr) for allelic replacement, according to the select agent rules for antibiotic use in virulent plague strains. For complementation, the caf1 open reading frame was PCR amplified with primers 5′CAF1NdeI (5′-TACATATGAAAAAAATCAGTTCCGTTATCGCC-3′) and 3′CAF1BamHI (5′-TAGGATCCTTATTGGTTAGATACGGTTACGG-3′). The amplified product, an NdeI/BamHI fragment, was cloned into plasmid pDA41, downstream from a constitutive promoter, to yield the plasmid pF1.
Immunofluorescence microscopy.
Y. pestis was grown in 4 ml heart infusion broth (HIB) overnight at 37°C, and bacteria in the culture were sedimented by centrifugation (5 min at 6,000 × g). The bacteria in the sediment were washed with 1 ml of phosphate-buffered saline (PBS; 10 mM sodium phosphate), fixed with 2.5% paraformaldehyde and 0.006% glutaraldehyde in 30 mM PBS (pH 7.4) for 20 min at room temperature, and washed three times with 1 ml PBS. Bacterial suspensions (30 μl) were applied to l-polylysine-coated coverslips for 5 min, washed three times with 60 μl PBS to remove nonadherent cells, and allowed to dry. Plague bacteria were rehydrated in 60 μl of PBS for 5 min and blocked with 3% bovine serum albumin in PBS for 45 min, followed by 1 h of incubation with purified anti-F1 polyclonal rabbit serum in 3% bovine serum albumin in PBS. The anti-F1 polyclonal rabbit serum was purified by incubating the serum for 2 h with acetone-precipitated antigen derived from whole-cell preparations of strain Y. pestis ΔF1. The purified serum was used at a final concentration of 1:1,000. Bacteria were washed 10 times with 100 μl PBS and incubated for 1 h in the dark with Alexa Fluor 647 goat anti-rabbit immunoglobulin G (IgG) 1:200 (Invitrogen). Cells were washed 15 times with 60 μl PBS, and slides prepared for microscopy and viewed with a Leica SP5 AOBS spectral 2-photon confocal microscope or a Leica DMI6000 inverted microscope with conventional fluorescence (100 W Hg) and differential interference contrast optics under a 63× oil objective (numerical aperture, 1.4) with automatically optimized confocal pinhole apertures. Images were captured by using a chilled photomultiplier tube fluorescence detector (digital spectral definition in 1-nm increments) plus one transmitted light detector with 12-bit output and 6.5× and 13.5× digital zoom. Fluorescence z-scans were captured sequentially with a 633-nm-line (10 mW) HeNe laser. Resonant scanning galvanometer mirrors (8,000 Hz scan rate) were used to collect frame-averaged (n = 64) z-series scans sampled in 125-nm steps. The captured images were analyzed with Image J software.
Antibody detection.
Serum IgG levels with specific antigen binding activity were determined with a custom enzyme-linked immunosorbent assay (ELISA) at the GLRCE Immunology Core at The University of Chicago (17).
Immunization.
Attenuated Y. pestis strains were grown overnight in HIB at 26°C, diluted 1:100 into fresh media, and grown for 3 h at 26°C. The bacteria in each culture were sedimented by centrifugation, washed, and diluted in PBS to the required concentration. Groups of 6- to 8-week-old female BALB/c mice (Charles River Labs, MA) were immunized by intramuscular injection into the hind leg with 0.1-ml aliquots of 1 × 105 CFU of KIM D27 or its isogenic variants suspended in PBS. Following injection, mice were monitored for 21 days. Blood sampling and challenge occurred at day 21. For subunit vaccines, groups of 6- to 8-week-old female BALB/c mice (Charles River Labs, MA) were immunized by intramuscular injection into the hind leg with 0.1-ml aliquots of 50 μg of recombinant LcrV (rLcrV) or recombinant F1 (rF1) in 25% Alhydrogel on days 0 and 21. Blood sampling or plague challenge occurred on day 42.
Plague challenge experiments.
For the bubonic plague model, mice were challenged by subcutaneous injection with 0.1-ml aliquots of 1,000 mean lethal dose (MLD) Y. pestis CO92 (1 × 103 CFU) (17). For this experiment, Y. pestis CO92 was grown in HIB at 26°C overnight. The plague bacilli were washed and diluted in sterile PBS to the required concentration. Mice were infected by subcutaneous injection with bacterial suspensions and observed for morbidity, mortality, and recovery over a course of 14 days. For the pneumonic plague model, 21 days following immunization, the mice were anesthetized with a cocktail of 17 mg/ml ketamine (Ketsed:Vedco) and 0.7 mg/ml xylazine (Sigma) (administered intraperitoneally) and challenged by intranasal inoculation with 20 μl of 1,000 MLD Y. pestis CO92 (4 × 105 CFU) (17). For this experiment, Y. pestis CO92 was grown in HIB supplemented with 2.5 mM calcium at 37°C overnight. The plague bacteria were washed and diluted in sterile PBS at the required concentration. The mice were observed for morbidity, mortality, and recovery over a course of 14 days. Fisher's exact test was used to compare mortality between groups. The two-tailed Student's t test was used to compare bacterial recovery data. All animal and plague experiments were performed in accordance with institutional guidelines following experimental protocol review and approval by the institutional biosafety committee, select agent committee, and the institutional animal care and use committee at The University of Chicago.
RESULTS
Plague immunity generated with live, attenuated Y. pestis (Δpgm) strains.
We examined the virulence and vaccine attributes of Y. pestis KIM D27 (biovar Medievalis), a Δ(pgm) strain harboring all three virulence plasmids (pCD1, pMT1, and pPCP1) (18) (Fig. 1A). Groups of 6- to 8-week-old BALB/c mice (n = 10) were immunized by intramuscular injection with 1 × 105 CFU and 1 × 107 CFU of Y. pestis KIM D27 suspended in PBS or with PBS alone. Animals were monitored for morbidity and mortality over the course of 21 days. In contrast to animals injected with PBS (all of which remained healthy), mice immunized with 1 × 107 CFU of Y. pestis KIM D27 presented clinical symptoms (ruffled fur and lethargy) and 30% mortality (Fig. 1B). Animals immunized with the lower dose (1 × 105 CFU) presented similar symptoms; however, all mice recovered 6 to 8 days postimmunization (Fig. 1B). To avoid vaccine mortality, the sublethal immunization dose (1 × 105 CFU) was chosen for future experiments.
Twenty-one days following immunization with live, attenuated Y. pestis KIM D27, mice (n = 10) were challenged by subcutaneous injection with 1,000 MLD of Y. pestis CO92 (1,000 CFU), a fully virulent strain (17). As expected (39), mock-immunized animals inoculated with PBS died between days 4 and 7, whereas animals immunized with KIM D27 were completely protected against bubonic plague challenge (KIM D27 versus PBS, P < 0.001) (Fig. 1E). Anesthetized BALB/c mice were inoculated intranasally with 1,000 MLD (4 × 105 CFU) of Y. pestis CO92, which precipitates pneumonic plague (17). Mock-immunized animals inoculated with PBS died within 4 days, whereas animals immunized by inoculation with KIM D27 were completely protected against pneumonic plague challenge (KIM D27 versus PBS, P < 0.001) (Fig. 1F). Serum samples from groups of five immunized mice each were analyzed on day 21 for antibodies against protective antigens, using ELISA and purified rLcrV or rF1 antigen (Fig. 1C). Mice immunized with KIM D27 harbored antibody titers against rF1 at a dilution of 1:13,000, and animals inoculated with PBS did not generate F1 antibodies. When tested for immune reactivity against purified rLcrV, mice immunized with KIM D27 harbored low levels of antibodies at dilutions at or below 1:600, whereas mice inoculated with PBS did not (Fig. 1C). As an additional test for the generation of specific antibodies, 200 ng of purified (His-tagged) rLcrV and rF1 was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), electrotransferred, and immobilized on polyvinylidene difluoride (PVDF) membrane. The mobilities of the purified proteins were detected with His probes, revealing rLcrV at the expected molecular size (38 kDa); rF1 migrated as both a monomer (17 kDa) and a dimer (35 kDa) (62) (Fig. 1D). Diluted serum was used to probe PVDF membranes, which revealed also that animals immunized with KIM D27 harbored serum antibodies against F1 and LcrV. Of note, F1 antibodies preferentially bound rF1 pilin species that migrated as dimers (35 kDa) rather than the more-abundant monomer species (17 kDa) (62) (Fig. 1D). This observation is in agreement with the hypothesis that F1 antibodies preferentially recognize folded, assembled pilin protein rather than the denatured, monomeric polypeptide (40).
Nonpigmented plague vaccine strains lacking lcrV and caf1.
Allelic replacement was used to generate mutants of Y. pestis KIM D27 that lacked pCD1-encoded LcrV (V) or pMT1 (pFra)-encoded Caf1 (F1) (Fig. 2A). Depending on the deletion, these strains were named ΔV [Δ(lcrV)], ΔF1 [Δ(caf1)], and ΔVF1 [Δ(lcrV caf1)], respectively. To monitor the expression of specific genes in each mutant strain, yersiniae were grown at 37°C in HIB. The cultures were centrifuged, and the bacterial proteins in the sediment solubilized in sample buffer. Following the separation of proteins by SDS-PAGE and immunoblotting with specific rabbit antibodies (anti-F1, anti-LcrV, and anti-RpoA), the expression of LcrV, F1, and RNA polymerase subunit A (loading control) could be detected in samples of the wild-type parent strain Y. pestis KIM D27 (Fig. 2A). As expected, the ΔV strain did not express LcrV, whereas the ΔF1 and ΔVF1 strains did not express F1 or LcrV and F1, respectively. The transformation of the ΔF1 mutant strain with a plasmid encoding wild-type caf1 (pF1) restored F1 pilin expression (Fig. 2A). Immunofluorescent microscopy with anti-F1 antibodies was used to determine whether Y. pestis strain KIM D27 assembled F1 pili on the bacterial surface as previously reported for strain EV76 (19). Both conventional (Fig. 2B) and confocal z-stacked fluorescent images (Fig. 2C) obtained for anti-F1-stained Y. pestis KIM D27 revealed bacterial deposition of F1 pilin into a thick, capsular surface coat. F1 deposition was not detected for the ΔF1 mutant strain (Fig. 2B).
FIG. 2.
Genetic analysis of (Δpgm) plague vaccine strains. (A) Bacterial extracts derived from the wild-type (WT) attenuated vaccine strain (Y. pestis KIM D27) [Δ(pgm)] and its isogenic variants lacking lcrV (ΔV), caf1 (ΔF1, without and with complementing plasmid pF1), and lcrV and caf1 (ΔVF1), were examined by immunoblotting with antisera raised against purified proteins, including plague pilus antigen capsular fraction 1 (Caf1 [α-F1]), low-calcium-response V antigen (LcrV [α-LcrV]), or RNA polymerase subunit A (α-RpoA) as a loading control. −, absent. (B) Fluorescence microscopy of F1 capsule (α-F1) on the surface of plague bacteria. Y. pestis strains KIM D27 and ΔF1 were grown at 37°C, fixed, and immobilized on coverslips. Capsule was detected with anti-F1 rabbit IgG and secondary antibodies labeled with Alexa Fluor 647 (red). Fluorescence microscopy and differential interference contrast (DIC) images were captured with a charge-coupled-device camera. (C) Confocal microscopy images were captured by laser scanning confocal microscopy (z-series; 366-nm increments labeled 0 to 2,562 nm). (D) Groups of 6- to 8-week-old BALB/c mice (n = 5) were immunized by intramuscular inoculation with 1 × 105 CFU of wild-type Y. pestis KIM D27 (WT) or its isogenic variants. After 21 days, antibody titers specific for either purified Caf1 pilin protein (F1) or LcrV were detected by ELISA in diluted serum. Error bars show standard deviations. (E) BALB/c mice (n = 10) were immunized by intramuscular inoculation with 1 × 105 CFU of Y. pestis KIM D27 or isogenic variants ΔV, ΔF1, and ΔF1 complemented with pF1, and the results were compared to those for mock-immunized control animals (PBS). Twenty-one days following immunization, experimental animals were challenged by subcutaneous (s.c.) inoculation with 1,000 MLD of Y. pestis CO92. Data are representative of the results of two independent experiments. (F) BALB/c mice (n = 10) were immunized as described for panel E. Twenty-one days following immunization, experimental animals were challenged by intranasal (i.n.) inoculation with 1,000 MLD of fully virulent Y. pestis CO92, and survival monitored over 14 days. Data are representative of the results of two independent experiments. Colored-ring symbols are as described for Fig. 1A. α, anti.
Plague immunity generated with lcrV or caf1 mutant strains.
Mice (n = 10) were immunized by intramuscular injection with 1 × 105 CFU of Y. pestis KIM D27 or its variants lacking lcrV, caf1, or both genes and monitored over 21 days. As before, mice immunized with Y. pestis KIM D27 presented clinical symptoms and recovered 6 to 8 days postimmunization. Mice immunized with the ΔF1 variant displayed similar signs of morbidity for the first 4 days but recovered earlier, on day 5. Finally, animals immunized with the ΔV or ΔVF1 variant did not show clinical signs of morbidity postinoculation (data not shown). Twenty-one days following immunization, serum samples of five mice in each immunization group were analyzed by ELISA for antibodies against purified rLcrV and rF1. Mice immunized with KIM D27 and ΔV harbored antibody titers against rF1 at a dilution of 1:13,000, whereas animals immunized with the ΔF1 or ΔVF1 variant did not generate F1 antibodies (Fig. 2D). Animals immunized with the ΔF1 variant that had been complemented with plasmid [ΔF1(pF1)] harbored serum antibodies against F1 at a dilution of 1:2,500; reduced antibody titers are likely due to the loss of the pF1 plasmid from variants following inoculation into mice (Fig. 2D). When tested by ELISA for immune reactivity to purified rLcrV, mice immunized with the KIM D27, ΔF1, or ΔF1(pF1) variant generated low levels of antibodies (dilutions at or below 1:600), whereas mice immunized with the ΔV or ΔVF1 variant did not harbor antibodies against LcrV (Fig. 2D).
Twenty-one days following immunization, mice (n = 10) were challenged by subcutaneous injection with 1,000 MLD Y. pestis CO92. Mock-immunized animals, injected with PBS, died within 4 days, whereas animals immunized by inoculation with KIM D27 were completely protected against bubonic plague challenge (KIM D27 versus PBS, P < 0.001) (Fig. 2E). A similar result was observed for animals that had been inoculated with the ΔV variant (ΔV versus PBS, P < 0.001), indicating that immune responses against LcrV are not required to generate protective immunity against bubonic plague following immunization with live, attenuated strains. In contrast, animals inoculated with the ΔF1 or ΔVF1 variant succumbed to bubonic plague challenge (ΔF1 versus PBS, P = 0.23, and ΔVF1 versus PBS, P = 1.0). All ΔVF1 variant-immunized animals died, albeit with a delay of 2 days compared to the times of death of mock-inoculated animals, whereas 20% of ΔF1 variant-immunized animals survived the challenge. The phenotypic defect of ΔF1 variants in generating protective immunity could be rescued by transformation with the pF1 plasmid, which was associated with only 10% mortality following bubonic plague challenge. The partial-restoration phenotype is likely due to the reduced level of F1 antibody observed in the serum of animals inoculated with the ΔF1(pF1) variant compared to the level in KIM D27-immunized mice (Fig. 2E). It appears, therefore, that F1 antibodies, generated during immunization with live, attenuated strains (KIM D27), are required for protective immunity against bubonic plague challenge. Antibodies against LcrV also contribute to protective immunity; however, in the absence of F1-specific IgG (ΔF1-immunized animals), low-level humoral immune responses to V antigen cannot protect against bubonic plague. Antibodies against other plague antigens may play a role in generating protective immunity against bubonic plague challenge, as animals immunized with the ΔVF1 variant gain a small increase in time to death over mock-immunized mice (Fig. 2E).
For pneumonic plague challenge, immunized mice (n = 10) were inoculated intranasally with 1,000 MLD (4 × 105 CFU) of Y. pestis CO92 (Fig. 2F). Mock-immunized animals (PBS) died within 3 days, whereas animals immunized by inoculation with KIM D27 were completely protected against pneumonic plague challenge (KIM D27 versus PBS, P < 0.001). Animals immunized by intramuscular injection with the ΔV, ΔF1, or ΔVF1 variant all lacked protection against pneumonic plague challenge and succumbed to Y. pestis infection within 3 to 4 days (Fig. 2F). Thus, in contrast to bubonic plague, immune responses against both F1 pilin and LcrV appear to be absolutely required for the protection of experimental animals immunized with live, attenuated vaccine strains against pneumonic plague challenge.
Plague variants lacking lcrV or caf1.
Y. pestis CO92 variants that lacked lcrV or caf1 were also generated by allelic replacement and, depending on the deletion, these strains were named CO92 ΔV [Δ(lcrV)] and CO92 ΔF1 [Δ(caf1)], respectively. To account for the presence of the pgm locus, colonies of the wild type and CO92 variants were formed on Congo red agar. In contrast to a nonpigmented control [Y. pestis CO92 Δ(pgm)], colonies of wild-type Y. pestis CO92, CO92 ΔV, and CO92 ΔF1 retained Congo red pigment (Fig. 3A). PCR amplification with specific primers confirmed the absence of Δ(lcrV) and Δ(caf1) in deletion strains and the presence of pgm (data not shown). Yersinia strains were grown at 26°C or 37°C in HIB. The cultures were centrifuged, and the proteins in the bacterial sediment solubilized in sample buffer. Following separation of proteins on SDS-PAGE and immunoblotting with specific rabbit antibodies (anti-F1, anti-LcrV, and anti-RpoA [RNA polymerase subunit A; loading control]), the expression of LcrV and F1 was detected in samples obtained from the wild-type parent, but only at 37°C (Fig. 3B). As expected, the CO92 ΔV strain failed to express LcrV, whereas CO92 ΔF1 did not express F1 (Fig. 3B).
FIG. 3.
Plague virulence attributes of the caf1 (F1) mutant Y. pestis CO92. (A) Pigmentation phenotypes of wild-type Y. pestis CO92 (WT) and its variants lacking caf1 (ΔF1), lcrV (ΔV), or the pgm locus (Δpgm). Bacteria were spread on HIA-Congo red agar and incubated at 26°C for 48 h. (B) Bacterial extracts derived from wild-type Y. pestis CO92 (WT) and its isogenic variants ΔV and ΔF1 were examined by immunoblotting with antisera raised against purified F1 (α-F1) and LcrV (α-LcrV) or RpoA (α-RpoA) as a loading control. α, anti. (C) BALB/c mice (n = 10) were challenged by subcutaneous (s.c.) inoculation with 20 CFU of the fully virulent Y. pestis CO92 strain or with increasing doses of its isogenic variant lacking caf1 (ΔF1), ranging from 7 to 50 CFU. Mice were monitored over 14 days. (D) Bacterial dissemination during pneumonic plague. BALB/c mice were challenged by intranasal inoculation with 1 × 104 CFU of fully virulent Y. pestis CO92 (n = 15) or its isogenic variant lacking caf1 (ΔF1) (n = 15). Starting 24 h postinfection and continuing in 24-h intervals, five mice per group were killed and their spleens and lungs removed, homogenized, and plated on Congo red agar for colony formation and enumeration. On day 3 following infection, only three mice infected with CO92 were still alive and could be used for bacterial load analysis. (E) BALB/c mice were challenged by intranasal inoculation with 1 × 104 CFU of fully virulent Y. pestis CO92 or its isogenic variant lacking caf1 (ΔF1) (n = 15). Starting 24 h postinfection and continuing at 24-h intervals, two mice per group were killed and their lungs removed and fixed in 25% formalin. Tissue was embedded in paraffin, thin sectioned, and stained with hematoxylin and eosin, and images captured by video microscopy. (F) BALB/c mice (n = 10) were challenged by intranasal (i.n.) inoculation with 10 MLD (4 × 103 CFU) of Y. pestis CO92 or with increasing doses of its isogenic variant lacking caf1 (ΔF1), ranging from 2.5 × 102 to 6.5 × 104 CFU. Colored-ring symbols are as described for Fig. 1A.
Virulence attributes of a plague variant lacking caf1.
To determine the 50% lethal dose of the plague variants, groups of 10 animals were inoculated by subcutaneous injection with increasing numbers of bacteria, from 1 × 101 to 1 × 106 CFU. As a control, 20 CFU (20 MLD) of the parent strain, Y. pestis CO92, were used for inoculation. The CO92 ΔV strain was completely avirulent, and all inoculated mice survived challenge (data not shown). In contrast, the CO92 ΔF1 variant appeared to be fully virulent: an infectious dose of 15 CFU killed all infected mice, while 7 CFU caused a lethal infection in 80% of the experimental animals. We conclude that there is no significant difference in the MLD of the parent Y. pestis CO92 (1 to 2 CFU) and the CO92 ΔF1 variant (Fig. 3C). Nevertheless, CO92 ΔF1 variants displayed an increased time to death in the bubonic plague model compared to that for the wild-type parent (9.5 [±2.9] [mean ± standard deviation] days versus 6.2 [±1.54] days; P = 0.006). To examine the ability of the caf1 mutant strain to cause pneumonic plague, anesthetized animals were infected by intranasal inoculation with 1 × 104 CFU of wild-type and mutant strains. At timed intervals, animals were killed, lungs and spleens removed, and organ tissues subjected to measurements of bacterial load, as well as histopathology. On the first day following infection, 1 × 104 CFU Y. pestis CO92 was detected in lung tissue; however, the spleens of infected animals were sterile, indicating that bacterial dissemination had not yet occurred during the first 24 h (Fig. 3D). On the second day, the load of Y. pestis in lung tissue was dramatically increased and bacterial dissemination to the spleen had commenced. On the third day of illness, shortly before animals succumbed to pneumonic plague, a uniformly elevated load of Y. pestis was detectable in lung and spleen tissue (108 to 109 CFU). The caf1 mutant strain displayed growth and dissemination properties similar to those of the wild-type parent strain (Fig. 3D). The bacterial loads of wild-type and ΔF1 strains in lung tissues on each day were similar (P value of 0.89 for day 1, P value of 0.9 for day 2, and P value of 0.78 for day 3). Histopathology of hematoxylin and eosin-stained lung sections revealed evidence of pneumonia, manifested by immune cell infiltration, large sections of hemorrhage and necrosis, loss of alveolar architecture with consolidation of lung parenchyma, and bacterial infiltrates (Fig. 3E). These features were indistinguishable in animals infected with Y. pestis CO92 or its caf1 mutant. To assess the virulence of caf1 mutants for pneumonic plague, animals were inoculated with increasing doses of bacteria and mortality from lung infection monitored over 14 days (Fig. 3F). Y. pestis CO92 causes lethal infections with a 50% lethal dose of 390 CFU (17), whereas the dose of CO92 ΔF1 required to kill 50% of experimental animals via pneumonic plague was calculated to be two- or threefold higher (1,090 CFU). Considering the extraordinary properties of Y. pestis during lung infection, the caf1 mutant must be considered as fully virulent.
Y. pestis CO92 caf1 variants and live, attenuated plague vaccines.
Animals immunized with KIM D27 were challenged by subcutaneous injection with wild-type Y. pestis CO92 or CO92 ΔF1, which revealed that the nonpigmented vaccine strain protected mice from bubonic plague with either challenge strain (Fig. 4A and B). We sought to determine whether immunity was based on antibodies against F1 and LcrV. Serum from animals that had been immunized with Y. pestis KIM D27 was transferred by intraperitoneal injection into naïve mice (Table 1). Passively immunized animals were subsequently challenged by subcutaneous injection with 20 CFU (MLD) of Y. pestis CO92 (wild type) or the ΔF1 mutant strain (Table 1). Compared to the results with control serum, the passive transfer of serum from KIM D27-immunized animals protected BALB/c mice against lethal plague challenge with the wild-type strain Y. pestis CO92. The dilution of serum from 1:1 or 1:2 to 1:4 was associated with loss of protective immunity (Table 1). Serum from KIM D27-immunized animals afforded very little or no protection following passive transfer when mice were challenged with the ΔF1 variant strain (one of five animals survived the challenge) (Table 1); further dilution of the serum abolished all protection. These data further corroborate the hypothesis that protective immunity following immunization with the live, attenuated, nonpigmented strain KIM D27 is largely based on immune responses against F1 pilin. Further, this plague immunity cannot be expanded to ΔF1 mutant strains.
FIG. 4.
Escape of Y. pestis F1 mutants from plague-protective immunity. (A and B) BALB/c mice (n = 10) were immunized by intramuscular inoculation with 1 × 105 CFU of Y. pestis KIM D27 or the lcrV (ΔV) mutant of Y. pestis CO92, and the results were compared to those for mock-immunized control animals (PBS). Twenty-one days following immunization, experimental animals were challenged by subcutaneous (s.c.) inoculation with 1,000 MLD of the wild-type strain Y. pestis CO92 (A) and its isogenic F1 variant (B). (C and D) BALB/c mice (n = 10) were immunized by intramuscular inoculation with 1 × 105 CFU of Y. pestis KIM D27 or the lcrV (ΔV) mutant of Y. pestis CO92, and the results were compared to those for mock-immunized control animals (PBS). Twenty-one days following immunization, experimental animals were challenged by intranasal (i.n.) inoculation with 1,000 MLD of the wild-type strain Y. pestis CO92 (C) and its isogenic F1 variant (D). (E and F) BALB/c mice (n = 10) were immunized by intramuscular inoculation with 1 × 105 or 1 × 107 CFU of Y. pestis KIM 10, an lcrV (ΔV) mutant of Y. pestis KIM lacking the pCD1 and pPCP1 plasmids but harboring pMT1. Twenty-one days following immunization, experimental animals were challenged by subcutaneous (s.c.) inoculation with 1,000 MLD of the wild-type strain Y. pestis CO92 (E) and its isogenic F1 variant (F). (G) Groups of 6- to 8-week-old BALB/c mice (n = 5) were immunized by intramuscular inoculation with 1 × 105 CFU of Y. pestis KIM D27, 1 × 105 CFU of Y. pestis CO92 ΔV, or 1 × 105 CFU or 1 × 107 CFU of Y. pestis KIM 10. Twenty-one days following immunization, antibody titers specific for either purified rF1 or rLcrV were detected by ELISA in diluted serum. Error bars show standard deviations. Colored-ring symbols are as described for Fig. 1A.
TABLE 1.
Passive immunization of mice with serum from animals that had been immunized with Y. pestis KIM D27
| Challenge straina | No. of animals with 14-day survival/no. of animals challengedb with:
|
|||
|---|---|---|---|---|
| Naïve mouse serum at dilution of 1:1 | Anti-KIM D27 serum at dilution of:
|
|||
| 1:1 | 1:2 | 1:4 | ||
| Y. pestis CO92, wild type | 0/5 | 4/5 | 5/5 | 1/5 |
| Y. pestis CO92 ΔF1 | 0/5 | 1/5 | 0/5 | 0/5 |
Passively immunized BALB/c mice were challenged by subcutaneous inoculation with 20 CFU of Y. pestis strains.
Passive immunization occurred 1 h prior to challenge by intraperitoneal injection of 250 μl of serum. Serum was diluted in PBS.
As a test of whether pgm and HPI encode additional vaccine antigens, mice were immunized by inoculation with Y. pestis CO92 ΔV; upon bubonic plague challenge with wild-type Y. pestis CO92, all animals were protected (ΔV versus PBS, P < 0.001) (Fig. 4A). In contrast, bubonic plague challenge with CO92 ΔF1 revealed that CO92 ΔV immunization afforded only partial protection (ΔV versus PBS, P = 0.016) (Fig. 4B). This result documents that, in the absence of LcrV and F1, immune responses derived from infection with attenuated Y. pestis strains cannot generate full plague protection.
Upon pneumonic plague challenge with the wild-type strain CO92, mice immunized with KIM D27 displayed the expected protection (Fig. 4C). One animal died during the experiment; this isolated lethal event is unrelated to plague infection and could not be reproduced in subsequent experiments (data not shown). Immunization with CO92 ΔV afforded only partial protection against wild-type CO92 challenge, in agreement with the hypothesis that live, attenuated strains must generate immune responses against both LcrV and F1 to achieve pneumonic plague protection (Fig. 4C). If so, challenge with CO92 ΔF1 should break through pneumonic plague protection generated via KIM D27 immunization. This was indeed observed, as both KIM D27- and CO92 ΔV-immunized animals remained sensitive to intranasal challenge with 1,000 MLD of Y. pestis CO92 ΔF1 (P = 0.49) (Fig. 4D).
As an additional test for the protective value of antigens encoded by HPI and pgm, we used Y. pestis KIM 10, a pigmented strain harboring pMT1 (F1) but lacking pCD1 (lcrV) and pPCP1 (18). Immunization of mice with 105 or 107 CFU KIM 10 generated humoral immune responses against F1, but not against LcrV (Fig. 4G). As expected, these immune responses protected mice against bubonic plague challenge with wild-type Y. pestis CO92 (1,000 MLD) similarly to the protection afforded by immunization with ΔV variants of the nonpigmented vaccine strain KIM D27 (Fig. 4E). KIM 10 immunization-derived immune responses also achieved significant protection (105 CFU, P = 0.043, and 107 CFU, P < 0.001) against bubonic plague challenge with 1,000 MLD of CO92 ΔF1. Thus, unlike the ΔVF1 variant of KIM D27 but similarly to CO92 ΔV, the HPI (pgm) locus of KIM 10 enables the live, attenuated vaccine strain to develop immune responses that generate protection against bubonic plague challenge.
LcrV subunit vaccines protect against Y. pestis CO92 caf1 variants.
Purified subunit vaccines, rLcrV or rF1, when offered to the immune system together with adjuvant, generate elevated immune responses that protect against bubonic and pneumonic plague (1, 28, 33, 42). We wondered whether these vaccines protect also against the caf1 mutant strain of CO92. To test this, we purified rLcrV and rF1 from Escherichia coli (Fig. 5A). Purified protein emulsified in aluminum hydroxide was injected twice intramuscularly into mice, with an intervening interval of 21 days. With this protocol, average specific antibody titers to rLcrV and rF1 were detected at dilutions of 1:100,000 and 1:80,000, respectively. Each immune response alone, anti-rLcrV and anti-rF1, was sufficient to generate protection against lethal bubonic or pneumonic plague challenge with 1,000 MLD Y. pestis CO92 (Fig. 5C and E). Immune responses against rLcrV also protected against bubonic and pneumonic plague challenge with 1,000 MLD of the caf1 mutant strain (CO92 ΔF1). Anti-rF1-specific immune responses afforded no protection against CO92 ΔF1 challenge, thereby documenting that anti-F1 antibodies generate protective immunity only by targeting F1 pili, but not any of the other nine fimbrial operons encoded within the genome of Y. pestis CO92 (45).
FIG. 5.
Plague subunit vaccines and protection against the Y. pestis F1 mutant. (A) Histidine affinity-tagged LcrV (rLcrV) and F1 (rF1) were purified from lysates of recombinant E. coli bacteria, separated by SDS-PAGE, and stained with Coomassie brilliant blue. M, molecular size markers. (B) Purified rLcrV and rF1 (50 μg each) were emulsified with Alhydrogel and injected intramuscularly into BALB/c mice. Following two immunizations with intervening intervals of 21 days, animals were examined for humoral immune responses against rLcrV and rF1 by ELISA (n = 5). Error bars show standard deviations. (C and D) BALB/c mice (n = 10) were immunized by intramuscular injection with rLcrV and rF1, and the results were compared to those for mock-immunized control animals (PBS). Twenty-one days following a second booster immunization, experimental animals were challenged by subcutaneous (s.c.) inoculation with 1,000 MLD of the wild-type strain Y. pestis CO92 (C) and its isogenic F1 variant (D). (E and F) BALB/c mice (n = 10) were immunized by intramuscular injection with rLcrV and rF1, and the results were compared to those for mock-immunized control animals (PBS). Twenty-one days following a second booster immunization, experimental animals were challenged by intranasal (i.n.) inoculation with 1,000 MLD of the wild-type strain Y. pestis CO92 (E) and its isogenic F1 variant (F). Colored-ring symbols are as described for Fig. 1A.
DISCUSSION
Pneumonic plague is a disease dreaded by mankind (36). Historical records of the 14th and 17th centuries documented that large segments of European and Asian populations were decimated by plague epidemics (44) where aerosol transmission of Y. pestis caused fulminant disease, rapidly killing infected individuals unless they had acquired immunity via immunization or prior plague disease (25). Few characteristic symptoms herald the onset of pneumonic plague, and infected individuals disseminate the disease via aerosol transmission, precipitating its epidemic spread, particularly in crowded settings (31, 50). Early efforts to conquer plague derived whole-cell vaccines from the pathogen and demonstrated vaccine protection (27). These efforts were expanded, firmly demonstrating that live, attenuated vaccine strains, but not whole-cell killed preparations, generate immunity in animals or humans against bubonic and pneumonic plague (37). Live, attenuated vaccine strains therefore serve as the definitive standard for the development of new plague vaccines from purified subunits, designed to lack the serious side effects of whole-cell vaccines (38).
Here we report that Y. pestis mutants lacking the F1 pilin subunit, and with it capsular fraction 1, remain fully virulent in bubonic and pneumonic plague models and are able to break through the protection generated by immunization with live, attenuated vaccine strains. In agreement with the hypothesis that plague immunity derived from live, attenuated strains is largely based on immune responses against F1 (Caf1) (13), vaccine strain mutants lacking the F1 or LcrV antigen are unable to protect against pneumonic plague challenge, even when challenged with the wild-type strain. Naturally occurring F1 mutant strains have been isolated and presumably cause human disease similarly to wild-type strains (15, 22). We wonder whether F1 mutant strains may also break through human immunity derived via bubonic plague infection with a wild-type strain. An answer to this question cannot currently be obtained; however, the experiment whose results are shown in Fig. 4D addresses a similar problem. The immunization of mice with Y. pestis CO92 ΔV, an lcrV mutant strain that harbors both pgm and pMT1 (F1), cannot generate protective immunity against pneumonic plague challenge with F1 mutant strains. We conclude that immunization with live, attenuated strains (with or without pgm and lcrV) is likely not able to provide for adequate vaccine protection against F1 mutant strains.
Earlier work reported that some plague strains require pFra (pMT1) for full virulence and described plasmid mutations in CO92 that not only abolished F1 pilin expression but also abrogated the ability of mutant strains to cause plague (57, 58). However, the requirement for pFra (pMT1) and F1 pili does not appear to be universal (3). For example, Y. pestis Java9, a strain isolated from Indonesian rats, lacks the caf1 operon, as well as the ability to generate F1 pili. Moreover, Java9 was determined to be fully virulent in several different animal models of bubonic and pneumonic plague (21). Our studies here contribute to the appreciation of F1 by demonstrating that the deletion of the caf1 coding region in Y. pestis CO92 has no effect on bacterial virulence in bubonic and pneumonic plague models.
Considering the biological threat of F1 mutant strains of Y. pestis, what plague vaccines are capable of providing protection? Previous work showed that LcrV and its variants, when used as a subunit vaccine, generate high titers of specific antibodies that confer protective immunity against bubonic and pneumonic plague in mice (17, 41). We report here that this immunity extends also to the fully virulent F1 mutant strain (Fig. 5F). Importantly, while our observations have been obtained with mice, appropriate studies with LcrV vaccines and the F1 mutant strain have not yet been performed with nonhuman primates or other animal models. Current efforts to generate subunit vaccines for human use have focused on F1 plus LcrV and F1-LcrV fusion protein vaccines, as the combination of two protective antigens generates higher levels of vaccine protection than individual components (28, 59). In view of the data presented here, however, the inclusion of F1 into subunit vaccines leaves open the possibility that protection does not extend toward fully virulent F1 mutant plague strains; these strains should therefore be included in future efficacy testing for plague vaccines.
Taylor et al. reported that mice inoculated by oral gavage with a deoxy-adenosine methylase (dam) mutant of Yersinia pseudotuberculosis IP32953, which also lacked the pYV virulence plasmid (encoding lcrV), were protected against bubonic plague challenge with fully virulent Y. pestis strain GB (54). As Y. pseudotuberculosis IP32953 lacks both F1 (caf1) and lcrV, the observed immunity is thought to be provided by antigens that are shared between Y. pseudotuberculosis and Y. pestis (54). Earlier work, using intravenous inoculation of pYV virulence plasmid-cured Y. pseudotuberculosis into mice, also generated immune protection against subsequent challenge with Y. pestis 6/69 M, which was inoculated subcutaneously as an otherwise lethal bubonic plague challenge (52). We asked a similar question, namely whether Y. pestis antigens exist with similar ability to incite a protective response against plague infection without relying on antibodies against either F1 or LcrV. The results shown in Fig. 2E and F suggest that the F1 antigen in the nonpigmented vaccine strain is absolutely essential for the generation of protective immunity against bubonic plague. Thus, within the genetic context of the nonpigmented Y. pestis strain KIM D27 and its isogenic variants, Y. pestis appears to harbor only two protective antigens (F1 and LcrV).
Y. pestis nonpigmented [Δ(pgm)] variants lack 83 open reading frames that are distributed between the 68-kb pigmentation segment and the 35-kb Yersinia HPI (9). HPI encompasses 11 ybt genes required for biosynthesis and iron-scavenging via the siderophore yersiniabactin (Ybt), including psn, the structural gene for pesticin receptor (an outer-membrane protein also involved in bacteriocin sensitivity) and two additional outer-membrane proteins (6, 9). HPI genes are conserved in Y. pestis, Y. pseudotuberculosis, and Yersinia enterocolitica biotype 1B strains, all of which are not only pathogenic for humans but able to disseminate in other mammalian hosts. In contrast, Y. enterocolitica biotypes 2 to 5 lack HPI genes; although pathogenic for humans, these strains are seemingly unable to disseminate in other mammalian hosts (4). The pigmentation segment encompasses the hmsSFRH cluster, associated with the pigmentation phenotype of Y. pestis on Congo red agar (34). Initially attributed to hemin storage, the hms operon is, however, required for the transmission of Y. pestis by its flea vector (32). In accordance with the proposed function of hmsSFRH in poly-N-acetylglucosamine (PNAG) biosynthesis (29), hms mutants form neither PNAG exopolysaccharide nor biofilm at temperatures below 30°C, phenotypes involved in bacterial blockade of the insect digestive tract and in transmission to new mammalian hosts (32). The pigmentation segment encodes further a fimbrial operon with similarity to the hifABCDE cluster of Haemophilus influenzae, a two-component regulatory system homologous to Bordetella pertussis bvgAS, genes that contribute to histidine and arginine utilization, as well as genes specifying unknown transport functions (9, 18).
We considered that Y. pseudotuberculosis carrying both HPI and the pigmentation segment but lacking pYV and, therefore, lcrV, can generate protective immunity against bubonic plague challenge, whereas the Y. pestis pgm strain KIM D27 with a deletion in caf1 cannot. Indeed, the immunization of mice with the pigmented strain KIM 10 (lacking lcrV) can generate protective immunity against a bubonic plague challenge with the Y. pestis F1 variant. One interpretation of this experimental result is that protective antigen properties may be encoded by HPI or the pigmentation segment, and its candidates would therefore be the pigmentation segment-encoded fimbrial gene cluster, PNAG, as well as HPI (ybt)-encoded outer-membrane proteins, including psn. Future work must consider such possibilities and further pursue envelope antigens from live vaccine strains by genetic subtraction in an effort to identify plague-protective vaccine antigens.
Acknowledgments
We thank Andrea DeDent for help with immunofluorescence microscopy and confocal microscopy and members of our laboratory for critical comments on the manuscript. The following reagent was obtained though the NIH Biodefense and Emerging Infections Research Resources Repository, NIAID, NIH: Yersinia pestis, strain KIM 10+.
The authors acknowledge membership in and support from the Region V “Great Lakes” Regional Center of Excellence in Biodefense and Emerging Infectious Diseases Consortium (NIH award 1-U54-AI-057153).
Editor: J. B. Bliska
Footnotes
Published ahead of print on 17 March 2008.
REFERENCES
- 1.Anderson, G. W., Jr., S. E. C. Leary, E. D. Williamson, R. C. Titball, S. C. Welkos, P. L. Worsham, and A. M. Friedlander. 1996. Recombinant V antigen protects mice against pneumonic and bubonic plague caused by F1-capsule-positive and -negative strains of Yersinia pestis. Infect. Immun. 644580-4585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andrews, G. P., D. G. Heath, G. W. Anderson, Jr., S. L. Welkos, and A. M. Friedlander. 1996. Fraction 1 capsular antigen (F1) purification from Yersinia pestis CO92 and from an Escherichia coli recombinant strain and efficacy against lethal plague challenge. Infect. Immun. 642180-2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anisimov, A., L. Lindler, and G. Pier. 2004. Intraspecific diversity of Yersinia pestis. Clin. Microbiol. Rev. 17434-464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bach, S., C. Buchrieser, M. Prentice, A. Guiyoule, T. Msadek, and E. Carniel. 1999. The high-pathogenicity island of Yersinia enterocolitica Ye8081 undergoes low-frequency deletion but not precise excision, suggesting recent stabilization in the genome. Infect. Immun. 675091-5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baker, E. E., H. Sommer, L. W. Foster, E. Meyer, and K. F. Meyer. 1952. Studies on immunization against plague. I. The isolation and characterization of the soluble antigen of Pasteurella pestis. J. Immunol. 68131-145. [PubMed] [Google Scholar]
- 6.Bearden, S., J. Fetherston, and R. Perry. 1997. Genetic organization of the yersiniabactin biosynthetic region and construction of avirulent mutants in Yersinia pestis. Infect. Immun. 651659-1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boisier, P., L. Rahalison, M. Rasolomaharo, M. Ratsitorahina, M. Mahafaly, M. Razafimahefa, J.-M. Duplantier, L. Ratsifasomanana, and S. Chanteau. 2002. Epidemiologic features of four successive annual outbreaks of bubonic plague in Mahajanga, Madagascar. Emerg. Infect. Dis. 8311-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brubaker, R. R. 1969. Mutation rate to nonpigmentation in Pasteurella pestis. J. Bacteriol. 981404-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchrieser, C., C. Rusniok, L. Frangeul, E. Couve, A. Billault, F. Kunst, E. Carniel, and P. Glaser. 1999. The 102-kilobase pgm locus of Yersinia pestis: sequence analysis and comparison of selected regions among different Yersinia pestis and Yersinia pseudotuberculosis strains. Infect. Immun. 674851-4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burrows, T. W. 1956. An antigen determining virulence in Pasteurella pestis. Nature 177426-427. [DOI] [PubMed] [Google Scholar]
- 11.Burrows, T. W. 1963. Virulence of Pasteurella pestis and immunity to plague. Ergebn. Mikrobiol. 3759-113. [DOI] [PubMed] [Google Scholar]
- 12.Burrows, T. W., and G. A. Bacon. 1956. The basis of virulence in Pasteurella pestis: antigen determining virulence. Br. J. Exp. Pathol. 37481-493. [PMC free article] [PubMed] [Google Scholar]
- 13.Burrows, T. W., and G. A. Bacon. 1958. The effect of loss of different virulence determinants on the virulence and immunogenicity of strains of Pasteurella pestis. Br. J. Exp. Pathol. 39278-291. [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng, L. W., D. M. Anderson, and O. Schneewind. 1997. Two independent type III secretion mechanisms for YopE in Yersinia enterocolitica. Mol. Microbiol. 24757-765. [DOI] [PubMed] [Google Scholar]
- 15.Davis, K. J., D. L. Fritz, M. L. M. Pitt, S. L. Welkos, P. L. Worsham, and A. M. Friedlander. 1996. Pathology of experimental pneumonic plague produced by fraction 1-positive and fraction 1-negative Yersinia pestis in African green monkeys (Cercopitheus aethiops). Arch. Pathol. Lab. Med. 120156-163. [PubMed] [Google Scholar]
- 16.DeBord, K., V. T. Lee, and O. Schneewind. 2001. On the role of LcrG and LcrV during the type III targeting of effector Yops by Yersinia enterocolitica. J. Bacteriol. 1834588-4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DeBord, K. L., D. M. Anderson, M. M. Marketon, K. A. Overheim, R. W. DePaolo, N. A. Ciletti, B. Jabri, and O. Schneewind. 2006. Immunogenicity and protective immunity against bubonic and pneumonic plague by immunization of mice with the recombinant V10 antigen, a variant of LcrV. Infect. Immun. 744910-4914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng, W., V. Burland, G. R. Plunkett, A. Boutin, G. F. Mayhew, P. Liss, N. T. Perna, D. J. Rose, B. Mau, S. Zhou, D. C. Schwartz, J. D. Fetherston, L. E. Lindler, R. R. Brubaker, G. V. Plano, S. C. Straley, K. A. McDonough, M. L. Nilles, J. S. Matson, F. R. Blattner, and R. D. Perry. 2002. Genome sequence of Yersinia pestis KIM. J. Bacteriol. 1844601-4611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Du, Y., R. Rosqvist, and A. Forsberg. 2002. Role of fraction 1 antigen of Yersinia pestis in inhibition of phagocytosis. Infect. Immun. 701453-1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fetherston, J. D., P. Schuetze, and R. D. Perry. 1992. Loss of the pigmentation phenotype in Yersinia pestis is due to the spontaneous deletion of 102 kb of chromosomal DNA which is flanked by a repetitive element. Mol. Microbiol. 62693-2704. [DOI] [PubMed] [Google Scholar]
- 21.Filippov, A. A., N. S. Solodovnikov, L. M. Kookleva, and O. A. Protsenko. 1990. Plasmid content in Yersinia pestis strains of different origin. FEMS Microbiol. Lett. 6745-48. [DOI] [PubMed] [Google Scholar]
- 22.Friedlander, A. M., S. L. Welkos, P. L. Worsham, G. P. Andrews, D. G. Heath, G. W. Anderson, Jr., L. M. Pitt, J. Estep, and K. Davis. 1995. Relationship between virulence and immunity as revealed in recent studies of the F1 capsule of Yersinia pestis. Clin. Infect. Dis. 21S178-S181. [DOI] [PubMed] [Google Scholar]
- 23.Galyov, E. E., O. Y. Smirnov, A. V. Karlishev, K. I. Volkovoy, A. I. Denesyuk, I. V. Nazimov, K. S. Rubtsov, V. M. Abramov, S. M. Dalvadyanz, and V. P. Zav'yalov. 1990. Nucleotide sequence of the Yersinia pestis gene encoding F1 antigen and the primary structure of the protein. Putative T and B cell epitopes. FEBS Lett. 17230-232. [DOI] [PubMed] [Google Scholar]
- 24.Girard, G. 1955. Plague. Annu. Rev. Microbiol. 9253-277. [DOI] [PubMed] [Google Scholar]
- 25.Girard, G., and J. Robic. 1942. L'état actuel de la peste à Madagascar et la prophylaxie vaccinale par le virus-vaccin E. V. Bull. Soc. Pathol. Exot. 3542-49. [Google Scholar]
- 26.Gottfried, R. S. 1983. The black death. Natural and human disaster in medieval Europe. The Free Press, New York, NY.
- 27.Haffkine, W. M. 1897. Remarks on the plague prophylactic fluid. Br. Med. J. 11461-1462. [Google Scholar]
- 28.Heath, D. G., G. W. Anderson, Jr., J. M. Mauro, S. L. Welkos, G. P. Andrews, J. J. Adamovicz, and A. M. Friedlander. 1998. Protection against experimental bubonic and pneumonic plague by a recombinant capsular F1-V antigen fusion protein vaccine. Vaccine 161131-1137. [DOI] [PubMed] [Google Scholar]
- 29.Heilmann, C., O. Schweitzer, C. Gerke, N. Vanittanakom, D. Mack, and F. Gotz. 1996. Molecular basis of intercellular adhesion in the biofilm-forming Staphylococcus epidermidis. Mol. Microbiol. 201083-1091. [DOI] [PubMed] [Google Scholar]
- 30.Inglesby, T. V., D. T. Dennis, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. D. Fine, A. M. Friedlander, J. Hauer, J. F. Koerner, M. Layton, J. McDade, M. T. Osterholm, T. O'Toole, G. Parker, T. M. Perl, P. K. Russell, M. Schoch-Spana, and K. Tonat. 2000. Plague as a biological weapon: medical and public health management. JAMA 2832281-2290. [DOI] [PubMed] [Google Scholar]
- 31.Kellogg, W. H. 1920. An epidemic of pneumonic plague. Am. J. Public Health 10599-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirillina, O., J. D. Fetherston, A. G. Bobrov, J. Abney, and R. D. Perry. 2004. HmsP, a putative phosphodiesterase, and HmsT, a putative diguanylate cyclase, control Hms-dependent biofilm formation in Yersinia pestis. Mol. Microbiol. 5475-88. [DOI] [PubMed] [Google Scholar]
- 33.Leary, S. E. C., E. D. Williamson, K. F. Griffin, P. Russell, S. M. Eley, and R. W. Titball. 1995. Active immunization with recombinant V antigen from Yersinia pestis protects mice against plague. Infect. Immun. 632854-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lillard, J. W., Jr., S. W. Bearden, J. D. Fetherston, and R. D. Perry. 1999. The haemin storage (Hms+) phenotype of Yersinia pestis is not essential for the pathogenesis of bubonic plague in mammals. Microbiology 145197-209. [DOI] [PubMed] [Google Scholar]
- 35.Lindler, L. E., G. V. Plano, V. Burland, G. F. Mayhew, and F. R. Blattner. 1998. Complete DNA sequence and detailed analysis of the Yersinia pestis KIM 5 plasmid encoding murine toxin and capsular antigen. Infect. Immun. 665731-5742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meyer, K. 1961. Pneumonic plague. Bacteriol. Rev. 25249-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Meyer, K. F. 1970. Effectiveness of live or killed plague vaccines in man. Bull. W. H. O. 42653-666. [PMC free article] [PubMed] [Google Scholar]
- 38.Meyer, K. F., D. C. Cavanaugh, P. J. Bartonelli, and J. Marshall, J. D. 1974. Plague immunization. Part I. Past and present trends. J. Infect. Dis. 129S13-S18. [DOI] [PubMed] [Google Scholar]
- 39.Meyer, K. F., G. Smith, L. E. Foster, J. D. Marshall, and D. C. Cavanaugh. 1974. Plague immunization. IV. Clinical reactions and serological responses to inoculations of Haffkine and freeze-dried plague vaccine. J. Infect. Dis. 129S30-S36. [DOI] [PubMed] [Google Scholar]
- 40.Miller, J., E. D. Williamson, J. H. Lakey, M. J. Pearce, S. M. Jones, and R. W. Titball. 1998. Macromolecular organisation of recombinant Yersinia pestis F1 antigen and the effect of structure on immunogenicity. FEMS Immunol. Med. Microbiol. 21213-221. [DOI] [PubMed] [Google Scholar]
- 41.Motin, V. L., R. Nakajima, G. B. Smirnov, and R. R. Brubaker. 1994. Passive immunity to yersiniae mediated by anti-recombinant V antigen and protein A-V antigen fusion peptide. Infect. Immun. 624192-4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Motin, V. L., Y. A. Nedialkov, and R. R. Brubaker. 1996. V antigen-polyhistidine fusion peptide: binding to LcrH and active immunity against plague. Infect. Immun. 644313-4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mueller, C. A., P. Broz, S. A. Muller, P. Ringler, F. Erne-Brand, I. Sorg, M. Kuhn, A. Engel, and G. R. Cornelis. 2005. The V-antigen of Yersinia forms a distinct structure at the tip of injectisome needles. Science 310674-676. [DOI] [PubMed] [Google Scholar]
- 44.Naphny, W., and A. Spicer. 2004. Plague: black death and pestilence in Europe. Tempus, Stroud, Gloucestershire, United Kingdom.
- 45.Parkhill, J., B. W. Wren, N. R. Thompson, R. W. Titball, M. T. Holden, M. B. Prentice, M. Sebaihia, K. D. James, C. Churcher, K. L. Mungall, S. Baker, D. Dasham, S. D. Bentley, K. Brooks, A. M. Cerdeno-Tarraga, T. Chillingworth, A. Cronin, R. M. Davies, P. Davis, G. Dougan, T. Feltwell, N. Hamlin, S. Holroyd, K. Jagels, A. V. Karlyshev, S. Leather, S. Moule, P. C. Oyston, M. Quail, K. Rutherford, M. Simmonds, J. Skelton, K. Stevens, S. Whitehead, and B. G. Barrell. 2001. Genome sequence of Yersinia pestis, the causative agent of plague. Nature 413523-527. [DOI] [PubMed] [Google Scholar]
- 46.Perry, R. D., P. B. Balbo, H. A. Jones, J. D. Fetherston, and E. DeMoll. 1999. Yersiniabactin from Yersinia pestis: biochemical characterization of the siderophore and its role in iron transport and regulation. Microbiology 1451181-1190. [DOI] [PubMed] [Google Scholar]
- 47.Perry, R. D., and J. D. Fetherston. 1997. Yersinia pestis: etiologic agent of plague. Clin. Microbiol. Rev. 1035-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Perry, R. D., P. A. Harmon, W. S. Bowmer, and S. C. Straley. 1986. A low-Ca2+ response operon encodes the V antigen of Yersinia pestis. Infect. Immun. 54428-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perry, R. D., S. C. Straley, J. D. Fetherston, D. J. Rose, J. Gregor, and F. R. Blattner. 1998. DNA sequencing and analysis of the low-Ca2+-response plasmid pCD1 of Yersinia pestis KIM 5. Infect. Immun. 664611-4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ratsitorahina, M., S. Chanteau, L. Rahalison, L. Ratisofasoamanana, and P. Boisier. 2000. Epidemiological and diagnostic aspects of the outbreak of pneumonic plague in Madagascar. Lancet 355111-113. [DOI] [PubMed] [Google Scholar]
- 51.Russel, P., S. M. Eley, S. E. Hibbs, R. J. Manchee, A. J. Stagg, and R. W. Titball. 1995. A comparison of plague vaccine, USP and EV76 vaccine induced protection against Yersinia pestis in a murine model. Vaccine 131551-1556. [DOI] [PubMed] [Google Scholar]
- 52.Simonet, M., P. Berche, D. Mazigh, and M. Veron. 1985. Protection against Yersinia infection induced by non-virulence-plasmid-encoded antigens. J. Med. Microbiol. 20225-231. [DOI] [PubMed] [Google Scholar]
- 53.Slack, P. 1989. The black death, past and present. Trans. R. Soc. Trop. Med. Hyg. 83461-463. [DOI] [PubMed] [Google Scholar]
- 54.Taylor, V. L., R. W. Titball, and P. C. F. Oyston. 2005. Oral immunization with a dam mutant of Yersinia pseudotuberculosis protects against plague. Microbiology 1511919-1926. [DOI] [PubMed] [Google Scholar]
- 55.Titball, R. W., and E. D. Williamson. 2001. Vaccination against bubonic and pneumonic plague. Vaccine 194175-4184. [DOI] [PubMed] [Google Scholar]
- 56.Titball, R. W., and E. D. Williamson. 2004. Yersinia pestis (plague) vaccines. Expert Opin. Biol. Ther. 4965-973. [DOI] [PubMed] [Google Scholar]
- 57.Welkos, S. L., G. P. Andrews, L. E. Lindler, N. J. Snellings, and S. D. Strachan. 2004. Mu dI1(Ap lac) mutagenesis of Yersinia pestis plasmid pFra and identification of temperature-regulated loci associated with virulence. Plasmid 511-11. [DOI] [PubMed] [Google Scholar]
- 58.Welkos, S. L., K. M. Davis, L. M. Pitt, P. L. Worsham, and A. M. Friedlander. 1995. Studies on the contribution of the F1 capsule-associated plasmid pFra to the virulence of Yersinia pestis. Contrib. Microbiol. Immunol. 13299-305. [PubMed] [Google Scholar]
- 59.Williamson, E. D., S. M. Eley, A. J. Stagg, M. Green, P. Russell, and R. W. Titball. 1997. A sub-unit vaccine elicits IgG in serum, spleen cell cultures and bronchial washings and protects immunized animals against pneumonic plague. Vaccine 151079-1084. [DOI] [PubMed] [Google Scholar]
- 60.Williamson, E. D., H. C. Flick-Smith, C. LeButt, C. A. Rowland, S. M. Jones, E. L. Waters, R. J. Gwyther, J. Miller, P. J. Packer, and M. Irving. 2005. Human immune response to a plague vaccine comprising recombinant F1 and V antigens. Infect. Immun. 733598-3608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Worsham, P. L., M. P. Stein, and S. L. Welkos. 1995. Construction of defined F1 negative mutants of virulent Yersinia pestis. Contrib. Microbiol. Immunol. 13325-328. [PubMed] [Google Scholar]
- 62.Zavialov, A. V., J. Berglund, A. F. Pudney, L. J. Fooks, T. M. Ibrahim, S. MacIntyre, and S. D. Knight. 2003. Structure and biogenesis of the capsular F1 antigen from Yersinia pestis: preserved folding energy drives fiber formation. Cell 113587-596. [DOI] [PubMed] [Google Scholar]