Abstract
Herpesvirus virions are highly organized structures built through specific protein-protein interactions. Thus, revelation of the protein interactions among virion proteins will shed light on the processes and the mechanisms of virion formation. Recently, we identified 24 virion proteins of Kaposi's sarcoma-associated herpesvirus (KSHV), using a proteomic approach (F. X. Zhu et al., J. Virol. 79:800-811, 2005). In the current study, a comprehensive analysis of protein-protein interaction between KSHV virion proteins was carried out using yeast two-hybrid (Y2H) and coimmunoprecipitation (co-IP) approaches. Every pairwise combination between KSHV tegument and capsid proteins, between tegument and envelope proteins, and among tegument proteins was tested for possible binary interaction. Thirty-seven protein-protein interactions were identified by both Y2H and co-IP analyses. The results revealed interactions between tegument and capsid proteins such as that of open reading frame 64 (ORF64) with ORF25 (major capsid protein [MCP]), ORF62 (triplex-1 [TRI-1]), and ORF26 (TRI-2). Many interactions were detected among the tegument proteins. ORF64 was found to interact with several tegument proteins including ORF11, ORF21, ORF33, ORF45, ORF63, ORF75, and ORF64 itself, suggesting that ORF64 may serve as a hub protein and play a role in recruiting tegument proteins during tegumentation and virion assembly. Our investigation also revealed redundant interactions between tegument proteins and envelope glycoproteins. These interactions are believed to contribute to final envelopment in virion assembly. Overall, this study allows us to establish a virion-wide protein interaction map, which provides insight into the architecture of the KSHV virion and sets up a foundation for exploring the functions of these proteins in viral particle assembly.
Kaposi's sarcoma (KS)-associated herpesvirus (KSHV), also known as human herpesvirus 8 (HHV-8), is a DNA tumor virus. It is associated with the endothelial neoplasm KS and certain B-cell lymphoproliferative disorders like primary effusion lymphoma and multicentric Castleman's disease (6, 19). Like all herpesviruses, KSHV displays two alternative life cycles, latent and lytic. During latent infection, the viral genome is maintained as an episome, and only a few viral genes are expressed. Under appropriate conditions, latent genomes can be activated to express a full panel of viral genes, which leads to the release of progeny virus particles (18, 21). In KS lesions, most spindle cells of endothelial origin are latently infected with KSHV, but in a small percentage of the cells, the viruses spontaneously undergo lytic replication (11, 20). Several observations have suggested the importance of the lytic life cycle of KSHV in the development of KS. Antiviral drugs that specifically block herpesvirus lytic replication dramatically reduce the incidence of KS development in high-risk individuals (13). Lytic infection of KSHV helps the formation of KS lesions by facilitating virus spread to the target sites and through the expression of paracrine factors (encoded by viral lytic genes) that support the growth of KS tumor cells (1, 4, 10). A recent study has also shown that KSHV episomes in latently infected cells are unstable and, hence, could be rapidly lost as infected cells proliferate. KSHV lytic replication and constant infection of fresh cells are, hence, essential to maintain the population of infected cells and are critical for viral pathogenesis (10).
A lytic life cycle of a herpesvirus consists of several essential steps: viral lytic gene expression, DNA replication, virion assembly, and egress. Among these steps, viral gene expression and DNA replication have been intensively studied. In contrast, little research has been done and little is known about KSHV virion assembly and egress. A herpesvirus virion contains more than 30 virus-encoded proteins assembled into four morphologically distinct components of the virion: the inner nucleoprotein core, which contains the double-stranded viral DNA genome; the icosadeltahedral capsid shell, which encloses the viral DNA core; the outer lipid envelope that bears various membrane glycoproteins on the surface; and the electron-dense material between the capsid and the envelope, defined as the tegument (reviewed in references 22 and 23). Motivated to learn more about the KSHV virion structure, assembly, and egress, we recently purified extracellular KSHV virions from tetradecanoylphorbol acetate-induced BCBL-1 cell culture through double-gradient ultracentrifugation. Virion component proteins were determined by mass spectrometry analysis. This study led to the identification of 24 virion-associated proteins, which included five capsid proteins, eight envelope glycoproteins, and eleven tegument or putative tegument proteins (28). Tegument proteins encoded by open reading frame 21 (ORF21), ORF33, and ORF45 were characterized and found to be resistant to protease digestion when purified virions were treated with trypsin, confirming that they are located within the virion particles. The ORF64-encoded large tegument protein was found to be associated with capsid but was also sensitive to protease treatment, suggesting its unique structure and array in KSHV virions (28).
The structure of KSHV capsid has been well defined by cryoelectron microscopy (26). However, the structure of the KSHV tegument and that of other herpesviruses are largely unknown. Earlier, the tegument of herpesviruses was considered to be an amorphous layer of proteins, but recent studies have indicated the presence of ordered tegument structures in both herpes simplex virus type 1 (HSV-1) (27) and human cytomegalovirus (HCMV) (7). Growing evidence now suggests that the herpesvirus tegument is an organized structure built through specific protein-protein interaction. Furthermore, during virion assembly, the processes of tegumentation and envelopment are driven by specific protein-protein interactions (reviewed in references 16 and 17). Thus, the revelation of protein interactions among the different KSHV virion proteins would not only provide information about the tegument structure of the virus but should also shed light on the processes and the mechanisms of virion formation.
Systematic protein interaction maps for the viral proteins have been reported for T7 bacteriophage (2), vaccinia virus (14), varicella-zoster virus, KSHV (24), and Epstein-Barr virus (EBV) (5). These protein interaction maps based on the genome-wide screens, however, were not focused on the virion proteins. For example, Uetz et al. performed a genome-wide yeast two-hybrid (Y2H) screen for KSHV and identified 123 nonredundant protein-protein interactions. Fifty percent of these interactions were confirmed by coimmunoprecipitation (co-IP). However, very few of the interactions among KSHV virion proteins were revealed in this study (24). Hence, the need arises for studies that could provide more insights into specific interactions among the virion proteins.
All the viral proteins can be roughly divided into two categories: virion proteins that are present in extracellular virions and infected cell proteins that accumulate in infected cells but are absent from uninfected cells. These infected cell proteins in general are not incorporated into virion particles during lytic infection. Given that virions are built through specific protein-protein interactions, we believe that there exists a protein-protein interaction network among the virion proteins and that this network could possibly direct the correct assembly of virion particles. To reveal this network, we systematically studied interactions among the virion proteins of KSHV, aiming to establish a map of the protein interaction network within KSHV virions. Through analyzing 330 possible binary interactions by Y2H as well as co-IP, many potential interactions between tegument and capsid proteins, tegument and glycoproteins, and among tegument proteins were identified. This study thus allowed the establishment of a virion-wide protein interaction map. It also helped to provide more insights into the hitherto less-well-known KSHV tegument architecture, providing a foundation with which to further explore the functions of the KSHV tegument proteins.
MATERIALS AND METHODS
Plasmids for the Y2H screen.
The cDNAs for the different KSHV tegument proteins (the bait) were synthesized by two approaches. The first approach involved amplification of the tegument protein cDNA by PCR, using appropriate cosmid-cloned KSHV fragments as DNA templates (29). Specific primers were designed to amplify the different ORFs based on the published KSHV genomic sequence (GenBank accession number U75698). The second approach involved excision of the appropriate tegument protein cDNA (previously cloned into the pCR.3.1 plasmid) by restriction enzyme digestion. The cDNAs generated by either of these approaches were subsequently cloned into the bait vector pAS2-1 (Clontech) in frame with the GAL4 DNA-binding domain.
Similarly, cDNAs for the virion proteins listed in Table 1 were prepared by either PCR or restriction enzyme digestion of existing expression vectors and were cloned into the prey plasmid pACT-2 (Clontech) in frame with the GAL4 activation domain. All of the bait and prey plasmids used in the Y2H assay are listed in the Table 1.
TABLE 1.
Characteristics of the plasmids of KSHV virion proteins used for Y2H and co-IP assays
| Vector or plasmid | Construct | Characteristics |
|---|---|---|
| Virion protein expression vectors | pCR3.1-ORF11 | Full-length cDNA of ORF11 (1,224 bp) cloned in pCR3.1 |
| pCR3.1-ORF33 | Full-length cDNA of ORF33 (1,005 bp) cloned in pCR3.1 | |
| pCR3.1-ORF45 | Full-length cDNA of ORF45 (1,224 bp) cloned in pCR3.1 | |
| pCR3.1-ORF64 | Full-length cDNA of ORF64 (8,245 bp) cloned in pCR3.1 | |
| Bait plasmids | pAS2-ORF11 | The full-length ORF11 cDNA excised from pCR3.1-ORF11 and subcloned into pAS2-1 |
| pAS2-ORF21 | Full-length cDNA of ORF21 (1,743 bp) cloned in pAS2-1 | |
| pAS2-ORF27 | Full-length cDNA of ORF27 (873 bp) cloned in pAS2-1 | |
| pAS2-ORF33 | Full-length ORF11 cDNA excised from pCR3.1-ORF11 and subcloned into pAS2-1 | |
| pAS2-ORF45 | Full-length ORF11 cDNA excised from pCR3.1-ORF11 and subcloned into pAS2-1 | |
| pAS2-ORF52 | Full-length cDNA of ORF52 (396 bp) cloned in pAS2-1 | |
| pAS2-ORF63 | Full-length cDNA of ORF63 (2,784 bp) cloned in pAS2-1 | |
| pAS2-ORF64N | 2,548-bp fragment (nucleotides 1-2,548) of ORF64 cDNA subcloned into pAS2-1 | |
| pAS2-ORF64M | 3,993-bp fragment (nucleotides 1,800-5,793) of ORF64 cDNA subcloned into pAS2-1 | |
| pAS2-ORF64C | 3,093-bp fragment (nucleotides 4,815-7,908) of ORF64 cDNA subcloned into pAS2-1 | |
| pAS2-ORF75 | Full-length cDNA of ORF75 (3,891 bp) cloned in pAS2-1 | |
| Prey plasmids | pACT2-ORF8 | Full-length cDNA of ORF8 (gB) (2,538 bp) cloned in pACT2 |
| pACT2-ORF8i1 | 2,130-bp fragment (nucleotides 1-2,130) of ORF8 cDNA cloned in pACT2 | |
| pACT2-ORF8i2 | 285-bp fragment (nucleotides 2,253-2,538) of ORF8 cDNA cloned in pACT2 | |
| pACT2-ORF11 | Full-length cDNA of ORF11 (1,224 bp) cloned in pACT2 | |
| pACT2-ORF21 | Full-length cDNA of ORF21 (1,743 bp) cloned in pACT2 | |
| pACT2-ORF22 | Full-length cDNA of ORF22 (gH) (2,193 bp) cloned in pACT2 | |
| pACT2-ORF22i1 | 1,683-bp fragment (nucleotides 1-1,683) of ORF22 cDNA cloned in pACT2 | |
| pACT2-ORF22i2 | 26-bp fragment (nucleotides 2,167-2,193 of ORF22 cDNA cloned in pACT2 | |
| pACT2-ORF25N | 2,040-bp fragment (nucleotides 1-2,040) of ORF25 cDNA cloned in pACT2 | |
| pACT2-ORF25C | 2,091-bp (nucleotide32041-4,131) of ORF25 cDNA cloned in pACT2 | |
| pACT2-ORF26 | Full-length cDNA of ORF26 (918 bp) cloned in pACT2 | |
| pACT2-ORF27 | Full-length cDNA of ORF27 (873 bp) cloned in pACT2 | |
| pACT2-ORF33 | Full-length cDNA of ORF33 (1,005 bp) cloned in pACT2 | |
| pACT2-ORF39 | Full-length cDNA of ORF39 (gM) (1,200 bp) cloned in pACT2 | |
| pACT2-ORF39i2 | 68-bp fragment (nucleotides 304-372) of ORF39 cDNA cloned in pACT2 | |
| pACT2-ORF39i3 | 236-bp fragment (nucleotides 517-615) of ORF39 cDNA cloned in pACT2 | |
| pACT2-ORF39i5 | 207-bp fragment (nucleotides 966-1,176) of ORF39 cDNA cloned in pACT2 | |
| pACT2-ORF45 | Full-length cDNA of ORF45 (1,224 bp) excised from pCR3.1-ORF45 and subcloned in pACT2 | |
| pACT2-ORF47 | Full-length cDNA of ORF47 (gL) (504 bp) cloned in pACT2 | |
| pACT2-ORF47i1 | 83-bp fragment (nucleotides 286-371) of ORF47 cDNA cloned in pACT2 | |
| pACT2-ORF47i2 | 86-bp fragment (nucleotides 418-504) of ORF47 cDNA cloned in pACT2 | |
| pACT2-ORF52 | Full-length cDNA of ORF52 (396 bp) cloned in pACT2 | |
| pACT2-ORF53 | Full-length cDNA of ORF53 (gN) (333 bp) cloned in pACT2 | |
| pACT2-ORF62 | Full-length cDNA of ORF62 (996 bp) cloned in pACT2 | |
| pACT2-ORF63 | Full-length cDNA of ORF63 (2,784 bp) cloned in pACT2 | |
| pACT2-ORF64N | 2,548-bp fragment (nucleotide 1-2,548) of ORF64 cDNA cloned in pACT2 | |
| pACT2-ORF64M | 3,993-bp fragment (nucleotides 1,800-5,793) of ORF64 cDNA cloned in pACT2 | |
| pACT2-ORF64C | 3,093-bp fragment (nucleotide 4,815-7,908) of ORF64 cDNA cloned in pACT2 | |
| pACT2-ORF65 | Full-length cDNA of ORF65 (513 bp) cloned in pACT2 | |
| pACT2-ORF75 | Full-length cDNA of ORF75 (3,891 bp) cloned in pACT2 | |
| Gateway plasmids (V5 tagged) | pcDNA3.1/nV5-DEST-ORF11 | Full-length cDNA of ORF11 (1,224 bp) cloned in pcDNA3.1/nV5-DEST plasmid |
| pcDNA3.1/nV5-DEST-ORF21 | Full-length cDNA of ORF21 (1,743 bp) cloned in pcDNA3.1/nV5-DEST plasmid | |
| pcDNA3.1/nV5-DEST-ORF25 | Full-length cDNA of ORF25 (4,131 bp) cloned in pcDNA3.1/nV5-DEST plasmid | |
| pcDNA3.1/nV5-DEST-ORF26 | Full-length cDNA of ORF26 (918 bp) cloned in pcDNA3.1/nV5-DEST plasmid | |
| pcDNA3.1/nV5-DEST-ORF27 | Full-length cDNA of ORF27 (873 bp) cloned in pcDNA3.1/nV5-DEST plasmid | |
| pcDNA3.1/nV5-DEST-ORF33 | Full-length cDNA of ORF33 (939 bp) cloned in pcDNA3.1/nV5-DEST plasmid | |
| pcDNA3.1/nV5-DEST-ORF45 | Full-length cDNA of ORF45 (1,224 bp) cloned in pcDNA3.1/nV5-DEST plasmid | |
| pcDNA3.1/nV5-DEST-ORF52 | Full-length cDNA of ORF52 (396 bp) cloned in pcDNA3.1/nV5-DEST plasmid | |
| pcDNA3.1/nV5-DEST-ORF62 | Full-length cDNA of ORF62 (996 bp) cloned in pcDNA3.1/nV5-DEST plasmid | |
| pcDNA3.1/nV5-DEST-ORF63 | Full-length cDNA of ORF63 (2,784 bp) cloned in pcDNA3.1/nV5-DEST plasmid | |
| pcDNA3.1/nV5-DEST-ORF65 | Full-length cDNA of ORF65 (513 bp) cloned in pcDNA3.1/nV5-DEST plasmid | |
| pcDNA3.1/nV5-DEST-ORF75 | Full-length cDNA of ORF75 (3,891 bp) cloned in pcDNA3.1/nV5-DEST plasmid | |
| pCMV-3Tag (Myc-tagged) plasmids | pCMV-3Tag2C-ORF11 | Full-length cDNA of ORF11 (1,224-pb) cloned in pCMV-3Tag-2 |
| pCMV-3Tag2C-ORF21 | Full-length cDNA of ORF21 (1,743 bp) cloned in pCMV-3Tag-2 | |
| pCMV-3Tag2B-ORF27 | Full-length cDNA of ORF27 (873 bp) cloned in pCMV-3Tag-2 | |
| pCMV-3Tag2A-ORF33 | Full-length cDNA of ORF33 (939 bp) cloned in pCMV-3Tag-2 | |
| pCMV-3Tag2A-ORF45 | Full-length cDNA of ORF45 (1,224 bp) cloned in pCMV-3Tag-2 | |
| pCMV-3Tag2A-ORF52 | Full-length cDNA of ORF52 (396 bp) cloned in pCMV-3Tag-2 | |
| pCMV-3Tag2C-ORF63 | Full-length cDNA of ORF63 (2,784 bp) cloned in pCMV-3Tag-2 | |
| pCMV-3Tag2C-ORF64N | 2,548-bp fragment (nucleotides 1-2,548) of ORF64 cDNA cloned into pCMV-3Tag-2 | |
| pCMV-3Tag2C ORF64M | 3,993-bp fragment (nucleotides 1,800-5,793) of ORF64 cDNA cloned in pCMV-3Tag-2 | |
| pCMV-3Tag2C-ORF64C | 3,093-bp fragment (nucleotides 4,815-7,908) of ORF64 cDNA cloned in pCMV-3Tag-2 | |
| pCMV-3Tag2C-ORF75 | Full-length cDNA of ORF75 (3,891 bp) cloned in pCMV-3Tag-2 | |
| pCMV-3Tag (Flag-tagged) plasmids | pCMV-3Tag1B-ORF8 | Full-length cDNA of ORF8 (2,538 bp) cloned in pCMV-3Tag-1 |
| pCMV-3Tag1B-ORF22 | Full-length cDNA of ORF22 (2,193 bp) cloned in pCMV-3Tag-1 | |
| pCMV-3Tag1C-ORF39 | Full-length cDNA of ORF 39 (1,239 bp) cloned in pCMV-3Tag-1 | |
| pCMV-3Tag1B-ORF47 | Full-length cDNA of ORF47 (504 bp) cloned in pCMV-3Tag-1 | |
| pCMV-3Tag1B-ORF53 | Full-length cDNA of ORF53 (333 bp) cloned in pCMV-3Tag-1 | |
| pCMV-3Tag1C-ORF64N | 2,548 bp fragment (nucleotides 1-2,548) of ORF64 cDNA cloned into pCMV-3Tag-1 | |
| pCMV-3Tag1C-ORF64M | 3,993-bp fragment (nucleotides 1,800-5,793) of ORF64 cDNA cloned in pCMV-3Tag-1 | |
| pCMV-3Tag1C-ORF64C | 3,093-bp fragment (nucleotides 4,815-7,908) of ORF64 cDNA cloned in pCMV-3Tag-1 |
Y2H screening.
Y2H screening was performed by using a mating assay for potential interactions of each of the KHSV tegument proteins (the bait) with the different prey proteins (tegument, capsid, and envelope glycoproteins) (Table 2). The haploid Saccharomyces cerevisiae yeast strains MaV103 (MATa) and MaV203 (MATα) (gifts from Marc Vidal at Massachusetts General Hospital) were transformed individually with the bait and the prey plasmids, respectively. Transformation of the yeast strains with the respective plasmids was performed using the small-scale lithium acetate transformation procedure (as per the Clontech Yeast Protocols handbook). Subsequently, a clone from each bait transformant was mated with a clone from each prey transformant and grown overnight in 0.5 ml of yeast extract-peptone-dextrose medium (Clontech). One hundred-microliter volumes from each of the mating cultures were plated on minimal synthetic dropout agar medium with the dropout supplement lacking the amino acids leucine, tryptophan, and histidine (His−-Trp−-Leu−; BD Biosciences Clontech). In addition, the medium was also incorporated with 20 to 50 mM of 3-amino-1,2,4-triazole (3-AT) (Sigma). The diploid colonies, which grew on the triple selection plates after 4 days of incubation at 30°C, were also screened for β-galactosidase activity, using the standard colony filter assay. Briefly, yeast colonies from the triple selection plates were carefully transferred onto a dry, sterile filter paper. Yeast cells were lysed by the freeze-thaw method. The filter paper with the lysed yeast cells was placed onto another filter presoaked with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal) substrate solution. β-Galactosidase activities were tested following a few hours of incubation at 30°C. Enzymatic positivity was scored if the X-gal substrate was hydrolyzed to a blue-colored product by β-galactosidase enzyme liberated from the lysed yeast cells. Thus, the interaction between a bait protein and a prey protein was defined as positive only if there was activation of both the HIS3 and the lacZ genes (Table 2).
TABLE 2.
Protein-protein interaction of KSHV virion proteins determined by Y2H and co-IP analyses
| Prey proteina | Bait protein (ORF or domain) assayed in the presence of 3-AT (concn [mM])
|
|||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ORF11 (20)
|
ORF21 (20)
|
ORF27 (20)
|
ORF33 (30)
|
ORF45 (30)
|
ORF52 (20)
|
ORF63 (30)
|
ORF64N (30)b
|
ORF64M (50)b
|
ORF64C (20)b
|
ORF64b
|
ORF75 (20)
|
|||||||||||||
| His+ LacZ+ | Co-IP | His+ LacZ+ | Co-IP | His+ LacZ+ | Co-IP | His+ LacZ+ | Co-IP | His+ LacZ+ | Co-IP | His+ LacZ+ | Co-IP | His+ LacZ+ | Co-IP | His+ LacZ+ | Co-IP | His+ LacZ+ | Co-IP | His+ LacZ+ | Co-IP | His+ LacZ+ | Co-IP | His+ LacZ+ | Co-IP | |
| Capsid proteins | ||||||||||||||||||||||||
| MCP (full length) | − | + | − | − | − | − | − | + | − | − | + | − | ||||||||||||
| MCP (ORF25N) | − | +− | − | − | − | − | − | + | + | − | − | |||||||||||||
| MCP (ORF25C) | − | − | − | − | − | − | − | + | − | − | − | |||||||||||||
| TRI-2 (ORF26) | − | − | − | − | − | − | − | + | − | + | + | − | − | − | − | − | − | − | − | + | − | − | ||
| TRI-1 (ORF62) | − | − | + | + | − | − | − | − | + | + | − | − | − | − | + | + | − | − | − | − | + | + | − | |
| SCIP (ORF65) | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | − | ||
| Tegument proteins | ||||||||||||||||||||||||
| ORF11 | − | − | − | − | − | − | − | − | + | + | − | − | − | − | − | − | + | − | − | − | + | − | − | |
| ORF21 | + | − | − | + | − | − | − | − | + | + | − | − | − | + | + | + | + | + | + | + | + | − | + | |
| ORF27 | − | − | − | − | − | − | − | − | + | − | − | − | − | − | − | − | − | − | − | − | − | − | ||
| ORF33 | − | − | − | − | − | − | − | − | + | + | − | − | − | − | − | − | − | − | − | − | + | − | − | |
| ORF45 | − | − | − | − | − | − | − | − | − | − | + | + | + | + | + | + | + | − | − | − | + | + | − | |
| ORF52 | − | − | − | − | − | − | + | − | − | − | − | − | − | + | − | − | − | − | − | − | + | − | ||
| ORF63 | − | − | − | − | − | − | − | − | + | + | − | − | − | − | + | + | + | − | − | − | + | − | − | |
| ORF64N | − | − | + | + | − | − | − | − | − | − | +− | − | + | + | + | + | + | + | − | − | + | − | − | |
| ORF64M | − | − | − | − | − | − | − | − | + | + | − | − | + | + | + | + | − | + | − | − | + | + | + | |
| ORF64C | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | |
| ORF75 | − | − | − | + | − | − | − | − | + | + | + | + | − | − | − | − | + | + | − | − | + | − | − | |
| Envelope proteins | ||||||||||||||||||||||||
| gB (ORF8) | − | − | − | − | − | − | + | + | − | − | − | − | − | − | + | + | − | + | − | − | ||||
| gB (i2)a | − | − | − | − | − | − | − | − | − | − | − | |||||||||||||
| gM (ORF39) | − | − | − | − | − | − | − | − | − | − | + | + | − | − | + | + | − | + | − | + | ||||
| gM (i2) | − | − | + | − | − | + | − | + | + | − | − | |||||||||||||
| gM (i3) | − | − | − | +− | + | + | − | − | + | − | + | |||||||||||||
| gM (i5) | − | − | − | − | − | − | − | − | − | − | + | |||||||||||||
| gH (ORF22) | − | − | − | − | − | − | − | − | − | − | − | − | − | − | − | + | − | + | − | − | ||||
| gH (i1) | − | + | +− | + | + | − | − | − | + | − | − | |||||||||||||
| gH (i2) | − | − | − | − | − | − | − | − | + | − | − | |||||||||||||
| gL (ORF47) | − | − | − | − | + | + | − | − | + | + | − | − | − | + | − | + | + | − | − | − | ||||
| gL (i1) | − | − | − | − | + | − | − | − | − | − | − | |||||||||||||
| gL (i2) | + | +− | − | − | − | + | + | − | + | − | − | |||||||||||||
| gN (ORF53) | + | + | + | + | + | + | + | + | + | + | + | + | + | + | − | − | − | − | + | + | ||||
Prefix i stands for intracellular or intravirion domain and is numbered (e.g., 1, 2, 3, 5) according to the order of the domain in the molecule.
Fragment length for ORF64N was aa 1 to 849, aa 600 to 1931 for ORF64M, aa 1605 to 2636 for ORF64C, and full length for ORF64.
Plasmids for co-IP.
cDNAs of the KSHV tegument, capsid, and envelope proteins were cloned into one of the following vectors: (i) the pcDNA3.1/nV5-DEST vector with a V5 tag near the N terminus (Gateway system; Invitrogen); (ii) the pCMV-3Tag mammalian protein expression vector with either a three-Flag (pCMV-3Tag-1) or a three-Myc tag (pCMV-3Tag-2) (Stratagene); or (iii) the pCR3.1 vector (Table 1). All of these vectors allow specific protein expression under the control of the CMV promoter in human 293T cells.
Cloning into the pcDNA3.1/nV5-DEST gateway vector was carried out by a two-step procedure. The first step involved amplification of the respective KSHV cDNAs by PCR, followed by cloning into the entry vector pENTR/synthetic dropout/D/TOPO (Invitrogen). To enable directional cloning, all forward primers were designed with a CACC overhang at the 5′ end of the primer to allow the cDNAs to base pair with a GTGG overhang sequence in the pENTR TOPO vector. Subsequently, cDNAs from the entry clone were transferred into the destination vector (pcDNA3.1/nV5-DEST) backbone by mixing both of the plasmids with a Gateway LR Clonase enzyme mixture. The resulting recombination reaction was then transformed into Escherichia coli, and the destination plasmid was selected.
For cloning the cDNAs of the different KSHV virion proteins into the pCMV-3Tag vectors, the cDNAs were obtained through either PCR or restriction enzyme digestion of existing clones (precloned into either the pACT2 or the pAS2-1 vectors) and cloned into either the pCMV-3Tag1 (three-Flag tag) or the pCMV-3Tag2 (three-Myc tag) plasmid or both (Table 1).
Co-IP assay.
Human embryonic kidney 293T cells were cultured and maintained in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and antibiotics.
293T cells grown in 100-mm dishes were cotransfected with 10 μg of the respective bait (tegument) and prey (tegument, capsid, or glycoproteins) plasmids by the calcium phosphate transfection method. Caution was exercised at this point to make sure that the bait (tegument) protein and the prey (tegument, capsid, and envelope glycoproteins) were on different tagged mammalian expression vectors. At 48 h posttransfection, cells were washed twice with 1× phosphate-buffered saline and lysed with ice-cold lysis buffer (50 mM Tris-HCl [pH 7.4], 150 mM NaCl, 30 mM NaF, 5 mM EDTA, 10% glycerol, 40 mM α-glycerophosphate, 1 mM phenylmethylsulfonyl fluoride [PMSF], 1% Nonidet P-40, 1 mM sodium orthovanadate) supplemented with protease inhibitor cocktail (Roche). The cells transfected with envelope glycoproteins were lysed with ice-cold lysis buffer with a slightly different composition compared to the former (10 mM Tris [pH 8], 150 mM NaCl, 10 mM EDTA, 1% Nonidet P-40, 0.5% deoxycholic acid, 1 mM PMSF, supplemented with the protease inhibitor cocktail). This buffer allows for more efficient solubilization of membrane-associated proteins.
The cell lysates were homogenized and clarified by high-speed centrifugation at 4°C. Subsequently, they were tested for protein expression by Western blotting, using the appropriate antibodies. Immunoprecipitation was then performed by incubating the cell lysates with an antibody against the tag of the prey protein (such as either monoclonal anti-V5 or anti-Myc antibodies) for 2 h at 4°C. Subsequently, proteins were immunoprecipitated with protein G agarose beads (Invitrogen) for 2 h at 4°C. Immunoprecipitation of proteins cloned into a Flag-tagged vector was done by incubating the cell lysates with anti-Flag M2 Affinity gel (Sigma) for 2 h at 4°C.
Immunoprecipitated complexes were then thoroughly washed with the appropriate lysis buffer at least five times. A reverse immunoprecipitation was also performed as described above by incubating cell lysates with the antibody directed against the tag of the bait proteins. Precipitates were resuspended in 100 μl of sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer and boiled for 10 min. Complexes were then separated on SDS-PAGE 8 to 12% bis-Tris gels (Invitrogen) and transferred to nitrocellulose membranes. Membranes were blocked in 5% dried milk in 1× phosphate-buffered saline plus 0.2% Tween 20. Western blots were incubated with diluted mouse monoclonal anti-V5 or anti-Myc or anti-Flag antibodies. Mouse monoclonal anti-ORF45 and rabbit polyclonal anti-ORF64 antibodies were also used to specifically detect the respective proteins. Anti-mouse or anti-rabbit immunoglobulin G antibodies conjugated to horseradish peroxidase (Pierce) were used as secondary antibodies. An enhanced chemiluminescence-based system (SuperSignal West Dura extended duration substrate; Pierce) was used for the detection of the interacting protein pairs.
RESULTS AND DISCUSSION
Screens for potential binary protein interactions among individual virion proteins by Y2H analysis.
Growing evidence suggests that herpesvirus virions are highly organized structures built through specific protein-protein interactions. During virion assembly, the processes of capsid formation, tegumentation, and envelopment are driven by such interactions (16). Thus, insights into the protein interactions among the different KSHV virion proteins will shed light on the processes and the mechanisms of KSHV virion formation. To this end, we decided to systematically study interactions among virion proteins of KSHV, aiming to establish a map of the protein interaction network within KSHV virions.
Potential interactions between virion proteins were first determined using a Y2H system. The cDNAs for 11 tegument proteins or fragments were cloned into a Clontech Matchmaker system two-bait vector pAS2-1, which allows expression of a fusion protein of the DNA binding domain of yeast GAL4 protein and a tegument protein. cDNAs of all KSHV virion proteins (capsid, tegument, and envelope glycoproteins) were cloned into Matchmaker prey vector pACT2, generating the prey array listed in Table 1. For glycoproteins, both the full-length proteins as well as their cytoplasmic domains (especially their cytoplasmic tails) were cloned separately into the pACT2 vector. Each tegument bait vector was tested for possible binary interaction with each virion protein prey, as follows. The yeast strain MaV103 (a mating type) transformed with the tegument bait plasmids was mated with the MaV203 yeast strain (α mating type) transformed with the prey plasmids. The positive diploid yeast cells were selected on His−-Trp−-Leu− plates with 3-AT for the Y2H interaction and subsequently assayed for β-galactosidase activities. Through analyzing 330 possible binary interactions by Y2H screening, many potential interactions among the KSHV proteins were identified and are listed in Table 2.
Analysis of binary protein interactions among the individual virion proteins by co-IP assay.
Y2H screens are known to generate significant numbers of false positive and sometimes false negatives as well. Therefore, potential interactions between virion proteins were also tested by co-IP assays. The cDNAs of the different virion proteins or fragments (as listed in Table 1) were cloned into the pCMV-3Tag-1 or -2 mammalian expression vector. This allowed for the expression of each of the proteins or the fragments with a three-Flag tag or a three-Myc tag, respectively, in cells. In addition, some proteins or fragments were also cloned into the pcDNA3.1/nV5-DEST vector (Gateway system; Invitrogen), which allowed for the expression of V5-tagged polypeptides in cells. 293T cells were cotransfected with vectors for an epitope-tagged tegument protein (bait) and a prey protein (either a capsid or tegument or envelope protein) with a different epitope tag. At 48 h posttransfection, whole-cell extracts were prepared and subjected to immunoprecipitation with an antibody against the tag epitope attached to the prey protein. The protein precipitates were subsequently analyzed by Western blotting with antibodies against the tags in both bait and prey proteins, respectively. Co-IP studies revealed many interactions of the KSHV tegument proteins with capsid, other tegument, and envelope proteins. A total of 43 protein-protein interactions were detected by both Y2H and co-IP analyses, which included 31 interactions seen only in one bait-prey orientation and an additional six pairs of interaction detected in both of the orientations. The results of the Y2H screen and co-IP analyses are summarized in Table 2.
Reliability assessment of the interactions among the KSHV proteins.
Each pairwise combination of KSHV virion proteins was tested by both Y2H and co-IP methodologies, allowing us to assess the reliability of the data for virion protein interactions obtained in this study. Among 60 pairs of interactions that were positive in the Y2H screen, 51 pairs were also examined by co-IP assay. Forty-three of the 51 interactions tested showed positivity by the co-IP assay, which accounts for 84% of the Y2H positives. This percentage of correlation between both of the assays was higher than those obtained earlier by others with KSHV (48%) and EBV (47%) interactome maps (5, 24). One possible explanation for such a higher correlation between the two assays could be attributed to the fact that we had optimized the Y2H system for each bait plasmid. To control for the autoactivation that occurred with some baits, the optimal concentrations of 3-AT were determined for each bait on plates containing 3-AT of 0, 10, 20, 30, 50, and 100 mM. The lowest concentration of 3-AT that completely inhibited yeast growth with each bait in the histidine-free medium was used for the assay. The concentrations of 3-AT used for different baits are shown in Table 2. We believe that carefully determining the optimal concentration of 3-AT is critical for controlling false positives in Y2H assays.
Y2H is known to be associated with high rates of false positivity. However, less attention has been paid to the issues concerning false negativity. Six pairs of interactions positive in the co-IP assays were negative in the Y2H analyses. These could have been due to either a false negativity by the Y2H or a false positivity by co-IP. Furthermore, because of the availability of a specific antibody against ORF64, we have also performed co-IP assays wherein we used both the untagged full-length ORF64 (cloned into pCR3.1 vector), as well as tagged ORF64 fragments (cloned into pCMV-3Tag vectors) as bait. The interactions of ORF64 with ORF21, ORF45, ORF63, and ORF75, as well as ORF64 self-association, were detected with both untagged and tagged ORF64 as baits. However, three additional interactions, consisting of ORF64 with ORF26, ORF11, and ORF33, were detected only with the full-length untagged ORF64 bait but not with the tagged and truncated fragments of ORF64 (Table 2). Hence, it is tempting to speculate here that either the truncation or the tag attachment to the protein could have contributed to the false-negative results with the tagged ORF64 fragments.
Interactions between tegument and capsid proteins.
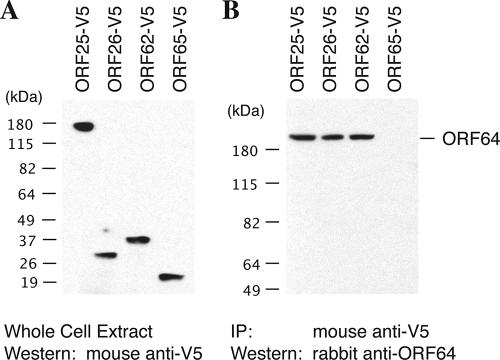
Seven interactions between tegument and capsid proteins were identified by both the Y2H and the co-IP assays, as follows: interactions between ORF64 and ORF25 (the major capsid protein [MCP]), ORF64 and ORF62 (triplex-1 [TRI-1]), ORF64 and ORF26 (TRI-2), ORF21 and ORF25, ORF21 and ORF62, ORF52 and ORF26, and ORF45 and ORF62. Findings of interactions of full-length ORF64 with the capsid proteins are shown in Fig. 1. Among these interactions, that between ORF64 and MCP has been reported in other herpesviruses, such as the interaction between the tegument proteins VP1/2 and the MCP VP5 in HSV-1 (15). Furthermore, an electron cryomicroscopy study of HSV-1 has demonstrated a filamentous and convoluted material of approximately 200 Å long and 40 Å thick, extending from the surface of the pentons of the HSV-1 capsid. This density extends from the interface between the upper domains of the adjacent VP5 subunits in the penton. The visualized filamentous material is likely to be VP1/2. The reconstructed three-dimensional image showed interactions of the tegument protein(s) (VP1/2) with MCP (VP5), TRI-2, and TRI-1 (27). These findings suggest that the interactions of ORF64 with the three capsid proteins (MCP, TRI-1, and TRI-2) are conserved among all herpesviruses.
FIG. 1.
Interaction of tegument protein ORF64 with capsid proteins, by a co-IP assay. 293T cells were cotransfected with pCR3.1-ORF64 and each of the expression vectors of V5-tagged capsid proteins. Forty-eight hours posttransfection, whole-cell extracts were prepared from the transfected cells, and the expression of tagged capsid proteins (A) and ORF64 (data not shown) was examined by Western blotting. The cell extracts were subjected to immunoprecipitation with an anti-V5 antibody. Precipitated samples were separated on an SDS-polyacrylamide gel, followed by Western analysis using a specific antibody against ORF64 (B).
Interactions between tegument proteins.
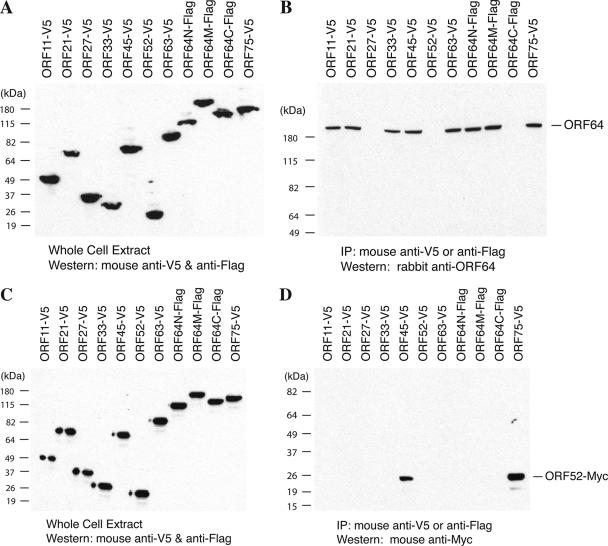
Fifteen pairs of binary interactions between tegument proteins were identified by both the Y2H and co-IP assays. Among them, six pairs of interactions could be confirmed in both of the bait-prey orientations, which are interactions of ORF64N-ORF21, ORF64N-ORF45, ORF64N-ORF64M, ORF64N-ORF63, ORF64M-ORF75, and ORF63-ORF45 (Table 2). Nine other interactions however were detected in only one direction. For example, the interactions of ORF45-ORF11, ORF45-21, and ORF45-ORF33 could be seen only when ORF45 was used as the bait and targeted by the specific antibody with ORF11, ORF21, and ORF33 as the prey on tagged vectors. However, the interactions were not seen the other way round. These discrepancies could be caused by improper folding of the proteins related to the attachment of certain tag peptides or by other unknown reasons. The interactions of ORF64 and ORF52 with other tegument proteins revealed by co-IP are shown in Fig. 2.
FIG. 2.
Interaction of the tegument proteins ORF64 and ORF52 with other tegument proteins by co-IP assays. 293T cells were cotransfected with the bait expression vector (pCR3.1-ORF64 or pCMV-3Tag2A-ORF52) and each of the expression vectors of V5-tagged or Flag-tagged tegument proteins or fragments, as indicated. Forty-eight hours posttransfection, whole-cell extracts were prepared from the transfected cells, and the expression levels of prey tegument proteins (A and C) and that of bait proteins (ORF64 and Myc-tagged ORF52, data not shown) were examined by Western blotting. The cell extracts were subjected to immunoprecipitation with anti-V5 or anti-Flag antibodies. Precipitated samples were separated on an SDS-polyacrylamide gel and then subjected to Western analysis using specific antibodies against ORF64 (B) and Myc tag (D).
ORF64 was found to interact with most of the tegument proteins (Fig. 2). This observation leads to a speculation that ORF64 may function as a hub protein and may play a critical role in tegumentation by recruiting these tegument proteins during virion assembly. ORF64 was also seen to interact with itself (Table 2 and Fig. 2). This self-association suggests dimerization or multimerization of the ORF64 protein in virions or in infected cells. Since the self-association appears to involve different regions in ORF64N (amino acids 1 to 849) and ORF64M (amino acids 600 to 1931) fragments, the possibility exists that the self-association may contribute to intramolecular folding of the protein. Such a self-association has also been reported in the HSV-1 analogue UL36 and the EBV analogue BPLF1 (5, 25).
In addition to ORF64, ORF45 was also found to interact with a wide range of other tegument proteins including ORF11, ORF21, ORF27, ORF33, ORF63, ORF64, and ORF75 (Table 2). This suggests that ORF45 possibly plays a critical role in the virion tegumentation process along with ORF64.
Among the interactions that we identified between the tegument proteins, the ORF64-ORF63 interaction has been reported between their analogues in alphaherpesviruses HSV-1 and pseudorabies virus (12, 25). ORF52-ORF75 interaction was also shown between the corresponding homologues (BLRF2 and BNRF1) in EBV (5). The rest of the interactions found between the tegument proteins of KSHV in our study have not been reported for any other herpesviruses.
Interactions between tegument proteins and envelope glycoproteins.
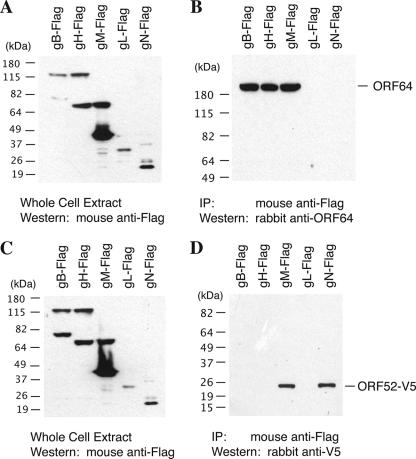
Proteins containing transmembrane regions are theoretically likely to give false-negative results in Y2H screens, since both bait and prey proteins have to be transported into the nucleus in order to transactivate the promoters of the reporter genes in yeast. Therefore, for Y2H analyses of tegument-envelope glycoprotein interaction, cDNAs for each of the full-length glycoproteins as well as their cytoplasmic domains (especially their cytoplasmic tails) were cloned separately into the prey vectors (see Table 1). Some full-length glycoproteins and their cytoplasmic domain fragments showed positive signals in their interactions with certain tegument proteins in Y2H assays (Table 2). Interactions of the full-length glycoproteins with the different tegument proteins were also tested by co-IP. To our surprise, the data obtained with both the Y2H screen and co-IP correlated quite well. Of the 45 pairwise analyses tested, 42 correlated, by both the methods, accounting for 93% of the pairwise interactions (Table 2). The interactions of ORF63 with glycoprotein L (gL) and ORF75 with gM, though negative by Y2H analysis, were positive by co-IP, suggesting the existence of false negatives in Y2H testing, as predicted. However, the Y2H analysis did detect interactions of ORF63 with an intracellular domain (i2) of gL and ORF75 with two intracellular domains (i3 and i5) of gM.
Seventeen interactions between the tegument and envelope glycoproteins were identified in this study, including interactions of ORF64 with gB, gM, and gH and ORF52 with gM and gN (Fig. 3). It was both surprising and exciting to detect numerous interactions between the tegument and envelope proteins. Thus, it could be suggested that the interactions between the tegument and the envelope proteins could contribute to the final envelopment. The redundancy of the interactions allows us to predict that interactions of the tegument-capsid complex and the viral envelope proteins are very stable and the envelopment process is hardly affected if one interaction is interrupted or if one tegument protein is missing. An illustration of this thinking could be seen in alphaherpesviruses, where the tegument protein VP22 interacts with glycoproteins (9). However a VP22-null virus showed no defect in virion assembly (8). This may be due to the redundancy of interactions between the remaining tegument proteins and glycoproteins of HSV-1, thereby compensating for the VP22 deficiency.
FIG. 3.
Interaction of tegument proteins ORF64 and ORF52 with envelope glycoproteins by co-IP assays. 293T cells were cotransfected with the bait expression vector (pCR3.1-ORF64 or pcDNA3.1/nV5-DEST-ORF52) and each of the prey expression vectors (Flag-tagged glycoproteins). Forty-eight hours posttransfection, whole-cell extracts were prepared from the transfected cells, and the expression levels of tagged glycoproteins (A and C) and bait tegument proteins (ORF64 and V5-tagged ORF52, data not shown) were examined by Western blotting. The cell extracts were subjected to immunoprecipitation with anti-Flag antibody. Precipitated samples were separated on SDS-polyacrylamide gels and subjected to Western analyses using specific antibodies against ORF64 (B) and V5 tag (D), respectively.
Besides virion proteins, some cellular proteins have also been shown to be present in KSHV virion particles (3, 28). Although we believe that some virus-host protein interaction may be very important for virion assembly, in the current study, we did not involve any cellular proteins but focused only on virion proteins. We wanted to initiate the study with a simplified system and in the future move to a more complex system which takes both virion and cellular proteins into consideration.
In summary, this report described our efforts to systematically investigate the protein-protein interactions within the KSHV virion. The salient features of this study are as follows.
(i) Thirty-seven interactions between the virion proteins were identified by both Y2H and co-IP analyses. Eighty-four percent of interactions found to be positive by the Y2H test were validated by co-IP, while 83% of interactions found positive by co-IP were also positive by Y2H. The high validation rates in both directions suggest a high reliability of the interaction data obtained through these systematic studies. The vast majority of interactions reported here are novel and have not been reported previously in any herpesviruses.
(ii) The tegument structure of KSHV, as well as other herpesviruses, is largely unknown. The data generated in this study provide insights into the architecture of the tegument and virion of KSHV. In the past, the tegument of herpesviruses used to be considered an amorphous layer of proteins, but recent studies suggest ordered tegument structures in both HSV-1 (27) and HCMV (7). The protein-protein interactions within KSHV virions identified in the current study provide further evidence for an ordered structure of the herpesvirus tegument.
(iii) The study led to the establishment of a protein interaction map of the KSHV virion, setting up a foundation on which to further explore the functions of these proteins that could ultimately shed light on the mechanisms of viral particle assembly. For example, in an earlier study from our laboratory, following the identification of KSHV virion proteins, we noticed and reported a peculiar behavior of ORF64. This tegument protein was found to be tightly associated with capsid; however, it was degraded when intact virion particles were treated with trypsin (28), unlike other tegument proteins that were protected from digestion by the virion envelope. In the current study, identifying the interactions of ORF64 with a number of envelope glycoproteins led to a hypothesis that the filamentous ORF64 protein binds to the capsid proteins (ORF25, ORF26, and ORF62) with one end and attaches to the envelope (interacting with gB, gM, and gH) with the other. Thus, this finding predicts a function of ORF64 in virion assembly in positioning the DNA-filled capsid at the cell membrane or trans-Golgi apparatus network by interacting with the membrane proteins where virion maturation and egress occur.
Acknowledgments
We thank Marc Vidal at Massachusetts General Hospital for yeast strains MaV103 and MaV203. We thank all members of the Yuan laboratory for critical reading of the manuscript and helpful discussion.
This work was supported by National Institutes of Health grant R01CA86839.
Footnotes
Published ahead of print on 5 March 2008.
REFERENCES
- 1.Bais, C., A. van Geelen, P. Eroles, A. Mutlu, C. Chiozzini, S. Dias, R. L. Silverstein, S. Rafii, and E. A. Mesri. 2003. Kaposi's sarcoma associated herpesvirus G protein-coupled receptor immortalizes human endothelial cells by activation of the VEGF receptor-2/ KDR. Cancer Cell 3131-143. [DOI] [PubMed] [Google Scholar]
- 2.Bartel, P. L., J. A. Roecklein, D. SenGupta, and S. Fields. 1996. A protein linkage map of Escherichia coli bacteriophage T7. Nat. Genet. 1272-77. [DOI] [PubMed] [Google Scholar]
- 3.Bechtel, J. T., R. C. Winant, and D. Ganem. 2005. Host and viral proteins in the virion of Kaposi's sarcoma-associated herpesvirus. J. Virol. 794952-4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boshoff, C., Y. Endo, P. D. Collins, Y. Takeuchi, J. D. Reeves, V. L. Schweickart, M. A. Siani, T. Sasaki, T. J. Williams, P. W. Gray, P. S. Moore, Y. Chang, and R. A. Weiss. 1997. Angiogenic and HIV-inhibitory functions of KSHV-encoded chemokines. Science 278290-294. [DOI] [PubMed] [Google Scholar]
- 5.Calderwood, M. A., K. Venkatesan, L. Xing, M. R. Chase, A. Vazquez, A. M. Holthaus, A. E. Ewence, N. Li, T. Hirozane-Kishikawa, D. E. Hill, M. Vidal, E. Kieff, and E. Johannsen. 2007. Epstein-Barr virus and virus human protein interaction maps. Proc. Natl. Acad. Sci. USA 1047606-7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 2661865-1869. [DOI] [PubMed] [Google Scholar]
- 7.Chen, D., H. H. Jiang, M. Lee, F. Liu, and Z. H. Zhou. 1999. Three-dimensional visualization of tegument/capsid interactions in the intact human cytomegalovirus. Virology 26010-16. [DOI] [PubMed] [Google Scholar]
- 8.del Rio, T., H. C. Werner, and L. W. Enquist. 2002. The pseudorabies virus VP22 homologue (UL49) is dispensable for virus growth in vitro and has no effect on virulence and neuronal spread in rodents. J. Virol. 76774-782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuchs, W., B. G. Klupp, H. Granzow, C. Hengartner, A. Brack, A. Mundt, L. W. Enquist, and T. C. Mettenleiter. 2002. Physical interaction between envelope glycoproteins E and M of pseudorabies virus and the major tegument protein UL49. J. Virol. 768208-8217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grundhoff, A., and D. Ganem. 2004. Inefficient establishment of KSHV latency suggests an additional role for continued lytic replication in Kaposi sarcoma pathogenesis. J. Clin. Investig. 113124-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katano, H., T. Sata, T. Suda, T. Nakamura, N. Tachikawa, H. Nishizumi, S. Sakurada, Y. Hayashi, M. Koike, A. Iwamoto, T. Kurata, and S. Mori. 1999. Expression and antigenicity of human herpesvirus 8 encoded ORF59 protein in AIDS-associated Kaposi's sarcoma. J. Med. Virol. 59346-355. [DOI] [PubMed] [Google Scholar]
- 12.Klupp, B. G., W. Fuchs, H. Granzow, R. Nixdorf, and T. C. Mettenleiter. 2002. Pseudorabies virus UL36 tegument protein physically interacts with the UL37 protein. J. Virol. 763065-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin, D. F., B. D. Kuppermann, R. A. Wolitz, A. G. Palestine, H. Li, and C. A. Robinson. 1999. Oral ganciclovir for patients with cytomegalovirus retinitis treated with a ganciclovir implant. N. Engl. J. Med. 3401063-1070. [DOI] [PubMed] [Google Scholar]
- 14.McCraith, S., T. Holtzman, B. Moss, and S. Fields. 2000. Genome-wide analysis of vaccinia virus protein-protein interactions Proc. Natl. Acad. Sci. USA 974879-4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McNabb, D., and R. J. Courtney. 1992. Characterization of the large tegument protein (ICP1/2) of herpes simplex virus type 1. Virology 190221-232. [DOI] [PubMed] [Google Scholar]
- 16.Mettenleiter, T. C. 2002. Herpesvirus assembly and egress. J. Virol. 761537-1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mettenleiter, T. C. 2004. Budding events in herpesvirus morphogenesis. Virus Res. 106167-180. [DOI] [PubMed] [Google Scholar]
- 18.Miller, G., L. Heston, E. Grogan, L. Gradoville, M. Rigsby, R. Sun, D. Shedd, Y. M. Kushnaryov, S. Grossberg, and Y. Chang. 1997. Selective switch between latency and lytic replication of Kaposi's sarcoma herpesvirus and Epstein-Barr virus in dually infected body cavity lymphoma cells. J. Virol. 71314-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moore, P. S., and Y. Chang. 2001. Kaposi's sarcoma-associated herpesvirus, p. 2803-2833. In D. Knipe, P. Howley, D. Griffin, R. Lamb, M. Martin, and S. Straus (ed.), Fields virology, 4th ed., vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 20.Parravicini, C., B. Chandran, M. Corbellino, E. Berti, M. Paulli, P. S. Moore, and Y. Chang. 2000. Differential viral protein expression in Kaposi's sarcoma-associated herpesvirus-infected diseases: Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. Am. J. Pathol. 156743-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Renne, R., W. Zhong, B. Herndier, M. Mcgrath, N. Abbey, D. Kedes, and D. Ganem. 1996. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 2342-346. [DOI] [PubMed] [Google Scholar]
- 22.Roizman, B., and D. M. Knipe. 2001. Herpes simplex viruses and their replication, p. 2399-2459. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology. Lippincott Williams & Wilkins, Philadelphia, PA.
- 23.Roizman, B., and P. E. Pellett. 2001. The family Herpesviridae: a brief introduction, p. 2381-2398. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology. Lippincott Williams & Wilkins, Philadelphia, PA.
- 24.Uetz, P., Y. A. Dong, C. Zeretzke, C. Atzler, A. Baiker, B. Berger, S. V. Rajagopala, M. Roupelieva, D. Rose, E. Fossum, and J. Haas. 2006. Herpesviral protein networks and their interaction with the human proteome. Science 311239-242. [DOI] [PubMed] [Google Scholar]
- 25.Vittone, V., E. Diefenbach, D. Triffett, M. W. Douglas, A. L. Cunningham, and R. J. Diefenbach. 2005. Determination of interactions between tegument proteins of herpes simplex virus type 1. J. Virol. 799566-9571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu, L., P. Lo, X. Yu, J. K. Stoops, B. Forghani, and Z. H. Zhou. 2000. Three-dimensional structure of the human herpesvirus 8 capsid. J. Virol. 749646-9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou, Z., D. Chen, J. Jakana, F. J. Rixon, and W. Chiu. 1999. Visualization of tegument-capsid interactions and DNA in intact herpes simplex virus type 1 virions. J. Virol. 733210-3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhu, F. X., J. M. Chong, L. Wu, and Y. Yuan. 2005. Virion proteins of Kaposi's sarcoma-associated herpesvirus. J. Virol. 79800-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu, F. X., T. Cusano, and Y. Yuan. 1999. Identification of the immediate-early transcripts of Kaposi's sarcoma-associated herpesvirus. J. Virol. 735556-5567. [DOI] [PMC free article] [PubMed] [Google Scholar]