Abstract
CD4+CD25+ regulatory T cells (CD25+ Tregs) play a key role in immune regulation. Since hepatitis C virus (HCV) persists with increased circulating CD4+CD25+ T cells and virus-specific effector T-cell dysfunction, we asked if CD4+CD25+ T cells in HCV-infected individuals are similar to natural Tregs in uninfected individuals and if they include HCV-specific Tregs using the specific Treg marker FoxP3 at the single-cell level. We report that HCV-infected patients display increased circulating FoxP3+ Tregs that are phenotypically and functionally indistinguishable from FoxP3+ Tregs in uninfected subjects. Furthermore, HCV-specific FoxP3+ Tregs were detected in HCV-seropositive persons with antigen-specific expansion, major histocompatibility complex class II/peptide tetramer binding affinity, and preferential suppression of HCV-specific CD8 T cells. Transforming growth factor β contributed to antigen-specific Treg expansion in vitro, suggesting that it may contribute to antigen-specific Treg expansion in vivo. Interestingly, FoxP3 expression was also detected in influenza virus-specific CD4 T cells. In conclusion, functionally active and virus-specific FoxP3+ Tregs are induced in HCV infection, thus providing targeted immune regulation in vivo. Detection of FoxP3 expression in non-HCV-specific CD4 T cells suggests that immune regulation through antigen-specific Treg induction extends beyond HCV.
Hepatitis C virus (HCV) is a highly persistent human pathogen that causes chronic necroinflammatory liver disease with progression to liver failure and cancer (25, 31). While T cells play a critical role in HCV clearance (24, 53), the virus generally persists, along with impaired antiviral effector T-cell responses (13, 59, 63, 69). While the precise underlying mechanisms for HCV persistence and disease pathogenesis are not fully defined, a role for CD4+CD25+ regulatory T cells (CD25+ Tregs) has been proposed based on increased circulating CD4+CD25+ T cells that can suppress HCV-specific T cells in HCV-infected patients (10, 12, 48, 54). CD25+ Tregs are a subset of naturally occurring, thymus-derived CD4+ regulatory T cells that highly express CD25 (the interleukin-2 [IL-2] receptor alpha chain) and play a critical role in immune tolerance to self and nonself antigens (27, 44, 49, 51). The role of CD25+ Tregs in immune regulation has been demonstrated in animal models of organ-specific autoimmune diseases, in which pathology is precipitated by their depletion and ameliorated by their reconstitution (52). The key marker of CD25+ Tregs is the forkhead/winged helix family transcription factor FoxP3 (70). Impaired FoxP3 gene expression results in severe autoimmunity in human immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (8, 67) and in Scurfy mice (11); furthermore, forced FoxP3 expression in FoxP3− T cells confers regulatory properties in vitro (21, 65).
CD25+ Tregs may also contribute to pathogen-specific immune regulation in viral infections, including those with hepatitis B and C, herpes simplex, Friend leukemia, and dengue viruses (14, 22, 23, 34, 36, 45, 47). However, since CD25 is expressed by both activated and regulatory T cells, CD25+ Tregs may be more precisely defined by FoxP3 in inflammatory settings, including viral infections. Furthermore, it is not known if CD25+ Tregs induced during viral infection are distinct from natural Tregs or if they recognize viral antigens. Here, we report that CD25+ FoxP3+ Tregs indistinguishable from natural Tregs are upregulated in HCV-infected persons. We also show that HCV can prime virus-specific FoxP3+ Tregs with antigen-specific expansion and suppression of HCV-specific CD8 T cells. We also show that FoxP3 extends to non-HCV-specific CD4 T cells, suggesting a more global role for antigen-specific Tregs in immune regulation beyond that for HCV infection.
MATERIALS AND METHODS
Study subjects.
HCV-seropositive subjects with chronic viremia (designations beginning with C) or spontaneous resolution of infection (designations beginning with R) and HCV-seronegative uninfected controls were recruited at the Philadelphia Veterans Affairs Medical Center and the Hospital of the University of Pennsylvania with informed consent approved by the institutional review boards. Persons with human immunodeficiency virus (HIV) or hepatitis B virus coinfection, active immunosuppressive or antiviral therapy, autoimmune or alcoholic hepatitis, or conditions precluding phlebotomy as previously described (54, 55) were excluded. HCV viremia was quantified by Roche COBAS Amplicor HCV Monitor or TaqMan HCV (Roche Diagnostics, Indianapolis, IN) and defined as “high” when it was above the upper limit of the HCV Monitor assay (>850,000 IU/ml).
Fluorescent antibodies.
All antibodies were purchased from Becton Dickinson (San Jose, CA) or BD Pharmingen (San Diego, CA) except for anti-glucocorticoid-induced tumor necrosis factor (TNF) receptor (GITR) (clone110416) from R&D Systems (Minneapolis, MN), anti-transforming growth factor β (TGF-β; clone TB21) from IQ Products (Groningen, The Netherlands), and anti-FoxP3 (clone PCH101) from eBioscience (San Diego, CA).
Recombinant HCV proteins and peptides.
Recombinant HCV proteins (known to stimulate CD4 T cells) and control superoxide dismutase (SOD) were kindly provided by Michael Houghton (Chiron Corporation, Emeryville, CA) (15, 56). Antigenic peptides (optimum and overlapping 15-mers) were synthesized as described previously (28, 54).
PBMC.
Peripheral blood mononuclear cells (PBMC) were isolated by Ficoll-Histopaque (Sigma Chemical Co., St. Louis, MO) as described previously (28, 54).
Major histocompatibility complex (MHC)/peptide tetramers.
HLA-A2-restricted class I tetramers were produced at the University of Oxford and used as described previously (26, 55). HLA-DR4-restricted HCV-specific (HCV 1248, GYKVLVLNPSVAATL; HCV1770, SGIQYLAGLSTLPGNPAIASL) and influenza virus-specific hemagglutinin (HA; PRYVKQNTLKLAT) class II tetramers were synthesized at the Benaroya Research Institute and used at 0.5 μg/sample as described previously (17, 42).
Immunophenotyping by flow cytometry (fluorescence-activated cell sorting [FACS]).
Cells were stained with fluorescent antibodies, acquired using FACSCalibur or FACSCanto (Becton Dickinson) and analyzed with Cell Quest (Becton Dickinson) or FlowJo (Tree Star, Inc., San Carlos, CA) software (54, 55), gating on lymphoid cells based on forward and side scatter profile. CD25 positivity was defined by an isotype-defined cutoff (99.9%). High or intermediate CD25 expression (CD25high or CD25int) was defined at a threshold where most CD4-negative cells lost CD25 expression (Fig. 1A). FoxP3 positivity was defined at an isotype-defined threshold where most CD4-negative cells became Foxp3 negative. Since delayed staining or cryopreservation resulted in marked reduction in CD25high T-cell frequency (data not shown), all ex vivo stainings were performed with freshly isolated PBMC.
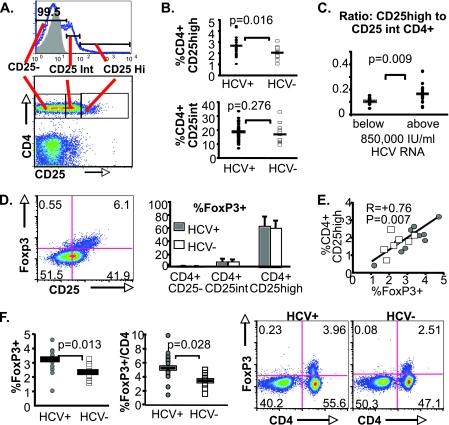
FIG. 1.
Increased CD4+CD25high T-cell frequency in HCV-infected subjects reflects increased FoxP3+ CD4 T-cell frequency. (A) Representative staining characteristics and gating strategies are shown for CD25high, CD25int, and CD25− CD4 T-cell subsets in PBMC (gating on lymphoid cells based on forward and side scatter characteristics). CD25 positivity was defined by a fluorescence cutoff below which 99.9% of isotype-stained cells were negative. CD4+CD25+ T cells were separated into CD25high or intermediate cells at a point where most CD4-negative cells lost CD25 expression. (B, top) CD4+CD25high T-cell frequencies, expressed as percentages, in 35 chronically HCV-infected (HCV+) and 15 uninfected subjects (HCV−) (mean, 2.6% versus 2.0%). (Bottom) Percentages of CD4+CD25int T cells in HCV-infected and uninfected subjects (mean, 18.6% versus 17.1%). Horizontal bars indicate mean values. (C) Ratios between CD4+CD25high and CD4+CD25int T-cell frequencies in HCV-infected patients with HCV RNA titers below or above 850,000 IU/ml (the upper limit of the HCV reverse transcription-PCR assay) (mean, 0.17 versus 0.11). (D) Representative FACS density plots showing intracellular FoxP3 protein expression primarily in CD25+ lymphocytes ex vivo. The bar graph shows mean % FoxP3+ cells among CD4+CD25high, CD4+CD25int, and CD4+CD25− T-cell subsets (58% versus 6% versus 1%; P < 0.001 [Friedman test]). There were no differences in % FoxP3 expression between the chronically HCV-infected (n = 12) and uninfected (n = 8) subjects for any of the T-cell subsets (P not significant). Error bars indicate standard deviations. Similar patterns were shown for FoxP3 mRNA expression in FACS-sorted CD4+CD25high, CD4+CD25int, and CD4+CD25− T cells (data not shown). (E) Correlation between circulating FoxP3+ and CD4+CD25high T-cell frequencies (R = +0.76; P = 0.007 [Spearman rank correlation]). Gray circles represent data points from chronically HCV-infected subjects, and unfilled squares represent data points from uninfected controls. (F) Chronically HCV-infected patients (n = 14) display greater FoxP3+ T-cell frequencies ex vivo than uninfected controls (n = 9) (mean % FoxP3+ per lymphoid cell, 3.1% versus 2.3%; P = 0.013; mean % FoxP3+ per CD4 T cell, 5.5% versus 3.7%; P = 0.028 [Mann-Whitney U]). Multicolor plots show the expression of FoxP3 in CD4 T cells.
Cell sorting.
CD4+CD25high, CD4+CD25int, and CD4+CD25− subsets were sorted by FACSVantage (Becton Dickinson) at the BSL-3 FACS Core Facility at the Children's Hospital of Philadelphia to >90% purity. CD4+CD25+ (>80% CD25high) and CD4+CD25− (>90% CD25−) T-cell subsets were also sorted by autoMACS using the CD4+CD25+ regulatory T-cell isolation system (Miltenyi Biotec Inc., Auburn, CA). Typically, 2 to 5 million CD4+CD25high or CD4+CD25+ T cells were isolated from 200 to 300 million PBMC. Sorting for MHC-II tetramer+ cells was performed using antiphycoerythrin beads (Miltenyi Biotech, Auburn, CA) as described previously (17, 33).
Cytokine analysis.
Intracellular cytokine staining was performed after 5 h of stimulation with or without 10 ng/ml phorbol 12-myristate 13-acetate (PMA; Sigma Chemical Co.), 200 ng/ml ionomycin (Sigma Chemical Co.), and brefeldin A (1 μl/ml) with a Cytofix/Cytoperm kit (BD Pharmingen) (15, 40). Culture supernatants of cells stimulated with PMA-ionomycin or HCV antigens (up to 72 h) were also examined for Th1/Th2 cytokines using the Cytometric Bead Array Th1/Th2 cytokine kit (BD Pharmingen) and for TGF-β using the human TGF-β1 immunoassay kit (R&D Systems).
Ex vivo Treg suppression assay.
FACS-sorted CD4+CD25high or CD4+CD25int “suppressor” T cells were cocultured in triplicate with autologous CD4+CD25− “responders” (50,000 cells/well) in 96-well plates at suppressor/responder ratios of 1:0, 1:1, 0.4:1, 0.2:1, and 0:1 for 3 days with media alone, 2 μg/ml phytohemagglutinin (PHA; Sigma Chemical Co.), or 1 μg/ml plate-bound anti-CD3 (clone UCHT1; BD Pharmingen) with 2 μg/ml anti-CD28 (clone CD28.2; BD Pharmingen) before 16 h of [3H]thymidine (1 μCi/well) uptake (15, 54). Proliferation was expressed as a stimulation index (SI): the mean cpm in stimulated wells divided by the mean cpm in unstimulated wells. T-cell proliferation in each coculture was normalized by proliferation in CD4+CD25− T cells alone and compared to the final CD4+CD25high T-cell content in sorted cells, as verified by FACS.
HCV-specific FoxP3+ Treg cultures.
Treg cultures were established by stimulating PBMC (with or without carboxyfluorescein diacetate succinimidyl ester [CFSE] labeling) for 14 days with HCV (10 μg/ml) or control proteins in the presence of 10 μg/ml anti-CD28 (BD Bioscience) and 1,000 U/ml recombinant IL-2 (rIL-2), followed by twice weekly rIL-2 (with or without weekly TGF-β). In select cultures, 10 ng/ml recombinant TGF-β (R&D Systems) or 1 μM TGF-β type I receptor kinase inhibitor (SD-208) (kindly provided by Linda Higgins, Scios Inc., Fremont, CA) (29, 62) was added. The cutoff for FoxP3 expression was defined by the isotype control (data not shown).
Suppression assay with in vitro-expanded Tregs.
CD4+CD25+ (eTreg) and CD4+CD25− (eCD25−) subsets were sorted from 2-week Treg cultures using the CD4+CD25+ regulatory T-cell isolation system (Miltenyi Biotec Inc.). Sorted cells were added to autologous PBMC and stimulated for 7 days with HCV peptides (2 μM) or 1 μg/ml plate-bound anti-CD3 with 2 μg/ml soluble anti-CD28 before FACS analysis.
FoxP3 mRNA by real-time PCR.
Total RNA was extracted with an RNeasy kit (Qiagen Inc., Valencia, CA), reverse transcribed, and analyzed by real-time PCR for FoxP3 and GAPDH (glyceraldehyde-3-phosphate dehydrogenase) expression with specific primer/probe sets (Applied Biosystems, Foster City, CA), normalizing FoxP3 expression relative to GAPDH.
Microarray analysis.
Total RNA preparations from FACS-sorted CD4+CD25high T cells from four HCV-infected and four uninfected subjects were analyzed at the University of Pennsylvania Microarray Core Facility using the Human Genome U133 Plus 2.0 Array GeneChip (Affymetrix, Santa Clara, CA). The probe set intensities and absent/present/marginal flags were calculated using GC-robust multiarray analysis and Affymetrix algorithms as implemented in Arrayassist Lite (Stratagene, La Jolla, CA). Significance analysis of microarrays (SAM version 2.1; Stanford University, Palo Alto, CA) was performed on 18,207/54,675 probe sets that were flagged as “present” in at least 2/8 samples, resulting in 5 probe sets with at least a twofold difference between HCV-infected and uninfected samples and a false-discovery rate of 36%.
Statistics.
Clinical and immunological parameters were compared with the nonparametric Mann-Whitney U or Kruskal-Wallis test. Differences in phenotype and cytokine profile between T-cell subsets were compared by the nonparametric Wilcoxon signed-rank and Friedman tests. Correlations were tested for statistical significance by Spearman rank correlation.
RESULTS
HCV persists with increased frequencies of CD4+CD25high FoxP3+ Tregs.
Since the regulatory phenotype of CD4+CD25+ T cells correlates with the intensity of CD25 expression (5, 6), we first differentiated between CD4 T cells with high and intermediate levels of CD25 expression (CD25high versus CD25int) (Fig. 1A), as described in Materials and Methods. As shown in Fig. 1B, HCV-infected patients displayed significantly greater CD4+CD25high T-cell frequency but not CD4+CD25int T-cell frequency than uninfected subjects. Notably, the increased CD25high/CD25int CD4 T-cell ratio was associated with high HCV RNA titers above the upper limit of detection (>850,000 IU/ml) (Fig. 1C), suggesting that the balance between CD25high regulatory and CD25int activated effector T cells contributes to viremic control. CD4+CD25high T cells were highly enriched for FoxP3 protein expression regardless of HCV infection, compared to CD4+CD25int or CD4+CD25− T cells (means of 58% versus 6% versus 1%, respectively; P < 0.001) (Fig. 1D), as well as for FoxP3 mRNA (data not shown). Overall, CD4+CD25high and FoxP3+ T-cell frequencies were tightly correlated (R = +0.76; P = 0.007) (Fig. 1E). Importantly, FoxP3+ T-cell frequency was significantly greater among HCV-infected subjects than uninfected subjects in total lymphoid cells (P = 0.013) and in CD4 T cells (P = 0.028) (Fig. 1F). Thus, HCV persistence is associated with increased circulating CD25high FoxP3+ Tregs, rather than CD25+ FoxP3− effector T cells.
FoxP3+ Tregs in HCV-infected patients display a phenotype, cytokine profile, and gene expression pattern similar to those of FoxP3+ Tregs in uninfected subjects.
CD25high FoxP3+ T cells in HCV-infected and uninfected subjects displayed similar phenotypes, expressing higher levels of CD45RO (98%), CD62L (91%), cytotoxic T-lymphocyte antigen 4 (CTLA4; 95%), and HLA-DR (79%) than CD25high FoxP3− and CD25− FoxP3− subsets (Fig. 2A). Despite constitutive expression reported in murine Tregs (43), GITR was expressed only in a minority of FoxP3+ T cells (14%), although at greater levels than in FoxP3− T cells. In addition, FoxP3+ T cells in HCV-infected and uninfected subjects were mostly negative for CD127 (IL-7 receptor α), as reported for natural Tregs (32, 50).
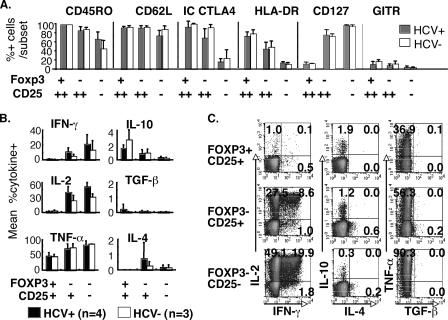
FIG. 2.
FoxP3+ CD4+ T cells from HCV-infected and uninfected subjects display similar phenotype and anergic cytokine profiles. (A) Mean percentages of CD45RO+, CD62L+, intracellular (IC) CTLA4+, HLA-DR+, CD127+, and GITR+ cells in three CD4+ T-cell subsets (FoxP3+ CD25high, FoxP3− CD25high, and FoxP3− CD25−) are shown, with standard deviations indicated by error bars (HCV+, infected subjects; HCV−, uninfected controls) based on data from 14 HCV+ and 7 HCV− subjects. No significant differences between HCV-infected and uninfected subjects for each of these markers are observed. (B) Mean percentages of cytokine+ cells are shown within FoxP3+ CD25+, FoxP3− CD25+, and FoxP3− CD25− CD4 T-cell subsets following PMA-ionomycin stimulation. Error bars indicate standard deviations. Cytokine profiles for FoxP3+ CD25+ and FoxP3− CD25+ CD4 T cells were determined in the autoMACS-sorted CD4+CD25+ T-cell subset; cytokine profiles for FoxP3− C25− T cells were determined in the autoMACS-sorted CD4+CD25− T-cell subset. (C) Representative FACS density plots for intracellular cytokine staining (IFN-γ, IL-2, IL-4, IL-10, TGF-β, and TNF-α) following PMA-ionomycin stimulation in FoxP3+ CD25+, FoxP3− CD25+, and FoxP3− CD25− T-cell subsets from an HCV-infected subject. Longer stimulation with PMA-ionomycin or stimulation with HCV-derived proteins did not enhance IL-10 or TGF-β production by sorted CD4+CD25+ T-cell subsets based on cytokine bead array (IL-10) or enzyme-linked immunosorbent assay (TGF-β) (data not shown).
FoxP3+ T cells displayed an anergic cytokine profile following PMA-ionomycin stimulation regardless of HCV status (Fig. 2B and C), with minimal gamma interferon (IFN-γ) or IL-2 production. Interestingly, significant TNF-α production was observed in FoxP3+ T cells, although less than in FoxP3− T cells. FoxP3+ T cells were also mostly negative for Th2 or regulatory cytokines such as IL-4, IL-10, and TGF-β; however, IL-10 was slightly more frequently detected among FoxP3+ T cells than among FoxP3− CD4 T cells (means, 2.1% for CD25+ FoxP3+ versus 0.9% for CD25+ FoxP3− versus 0.3% for CD25−FoxP3−; P = 0.0025 by the Friedman test). Further cytokine analysis by cytokine bead array or enzyme-linked immunosorbent assay failed to show significant IL-10 and TGF-β production from CD4+CD25+ T cells sorted by magnetic beads and stimulated with PMA-ionomycin or HCV-derived proteins, even with longer durations of stimulation (data not shown). Notably, FoxP3− cells within sorted CD4+CD25+ T cells showed intermediate levels of IFN-γ, IL-2, and TNF-α production compared to FoxP3− T cells within sorted CD4+CD25− T cells, consistent with partial cytokine suppression by FoxP3+ T cells contained within the sorted CD4+CD25+ T-cell subset.
Finally, comparing the global gene expression profiles of FACS-sorted CD4+CD25high T cells (>90% pure) from four HCV-infected and four uninfected subjects using Affymetrix Human Genome U133 Plus 2.0 Array analysis, we found only 5/54,675 probe sets that differed by more than twofold, with a 36.3% false-discovery rate (Table 1). Collectively, these data indicate that CD25high FoxP3+ T cells from HCV-infected and uninfected subjects are indistinguishable in their phenotypes, cytokine profiles, and levels of gene expression.
TABLE 1.
Genes differentially expressed in CD4+CD25high T cells sorted from HCV-infected and uninfected subjects using Affymetrix Human Genome U133 Plus 2.0 Array GeneChipa
| Affymetrix identification | Gene title | Gene designation | Fold change HCV+/HCV− | False-discovery rate (%) |
|---|---|---|---|---|
| 218016_s_at | RNA polymerase III (DNA directed) polypeptide E (80 kDa) gene | POLR3E | 2 | 0 |
| 228077_at | Hypothetical protein MGC3207 gene | MGC3207 | 0.5 | 36.3 |
| 212296_at | Proteasome (prosome, macropain) 26S subunit non-ATPase 14 gene | PSMD14 | 0.5 | 36.3 |
| 232793_at | Immunoglobulin superfamily member 4 gene | IGSF4 | 0.5 | 36.3 |
| 206363_at | v-maf musculoaponeurotic fibrosarcoma oncogene homolog (avian) | MAF | 0.4 | 36.3 |
Only 5/54,675 probe sets showed significant differential expression between CD4+CD25high T cells sorted from four HCV-infected and four uninfected subjects by at least twofold and with a 36.3% false-discovery rate.
CD4+CD25high T cells are hypoproliferative and suppress CD4+CD25− cell proliferation in a dose-dependent manner following anti-CD3/anti-CD28 and PHA stimulation.
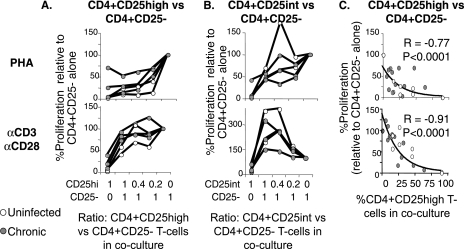
The suppressive capacities of FACS-sorted CD4+CD25high T cells from HCV-infected and uninfected subjects against autologous CD4+CD25− responder T cells were tested at various regulator/responder ratios. As shown in Fig. 3A, CD4+CD25high T cells from HCV-infected and uninfected subjects were hypoproliferative in isolation and inhibited proliferation of autologous CD4+CD25− T cells in a dose-dependent manner upon PHA as well as CD3/CD28 stimulation. By contrast, CD4+CD25int T cells were less suppressive than CD4+CD25high T cells and even enhanced proliferation markedly following CD3/CD28 stimulation (Fig. 3B). Relative T-cell proliferation in each coculture showed a tight inverse correlation with coculture CD4+CD25high T-cell content, as calculated from the purity of sorted CD4+CD25high T cells (Fig. 3C). Thus, CD4+CD25high T cells from HCV-infected and uninfected subjects displayed similar levels of dose-dependent suppression of CD4+CD25− T cells upon polyclonal stimulation.
FIG. 3.
CD4+CD25high T cells from HCV-infected and uninfected subjects display similar levels of dose-dependent suppression following polyclonal stimulation. (A and B) Proliferative capacities of CD4+CD25− responder cells alone and with autologous FACS-sorted CD4+CD25+ T-cell subsets following PHA or anti (α)-CD3/anti-CD28 stimulation at CD25+/CD25− T-cell ratios of 1:0, 1:1, 0.4:1, 0.2:1, and 0:1. The y axis shows the SIs from CD25+/CD25− cocultures divided by the SI from CD4+CD25− T cells stimulated alone. The graphs represent data from three chronically HCV-infected and two uninfected control subjects. (C) Percent proliferation (relative to that for CD4+CD25− responder T cells stimulated alone) shows a tight inverse correlation with the actual CD4+CD25high T-cell content in each coculture. Values were calculated based on the purity of the FACS-sorted CD4+CD25high subset. (Top) PHA. (Bottom) Anti-CD3/anti-CD28.
Increased FoxP3+ T cells in PBMC of HCV-seropositive subjects following HCV-specific stimulation in vitro.
To determine if FoxP3+ T cells can be expanded in an antigen-specific manner, PBMC of HCV-seropositive persons were cultured with recombinant HCV or control proteins, high-dose rIL-2, and anti-CD28 as previously reported for polyclonal Treg expansion (20, 60). As shown in Fig. 4, FoxP3 expression was detected in divided (i.e., CFSE-low) as well as undivided (CFSE-high) CD4 T cells from HCV-seropositive subjects following in vitro stimulation. While robust cell division was observed in all cultures with high-dose rIL-2 and CD28 costimulation, increased expansion of CFSE-low FoxP3+ T cells was detected in some patients following in vitro stimulation with HCV antigens compared to that with negative control SOD. For example, in subject C107, FoxP3+ Tregs expanded vigorously with HCV NS3/4 (5.3%) or NS5 (6.1%) but not SOD (1.8%). FoxP3+ Tregs could also be expanded by HCV-specific stimulation from HCV-resolved subjects, as shown for R58 (Fig. 4A), but not in HCV-seronegative controls (data not shown). Interestingly, tetanus toxoid also promoted FoxP3+ Treg expansion, suggesting that antigen-specific Treg expansion may extend beyond HCV. Antigen-specific FoxP3+ Treg expansion tended to increase in the presence of recombinant TGF-β in subjects C98 (all antigens), C107 (NS5 and tetanus toxoid), and R58 (NS5). Conversely, Treg expansion was reduced by TGF-β inhibition in subjects C107 (NS3/4, NS5) and R58 (NS3/4, NS5, tetanus toxoid), consistent with a role for TGF-β in FoxP3+ Treg expansion in vitro.
FIG. 4.
HCV-specific expansion of FoxP3+ Tregs in vitro from HCV-seropositive persons. (A) Antigen-specific expansion with CFSE dilution and FoxP3 expression (gated on CD4 T cells) by FACS in CFSE-labeled PBMC following stimulation with SOD, recombinant HCV NS3/4 and NS5 proteins, and tetanus toxoid in the presence of 1,000 U/ml rIL-2 and 10 μg/ml of soluble anti-CD28 alone (−TGF-β), with 10 ng/ml TGF-β (+TGFβ) or 1 μM SD-208, a type I receptor kinase inhibitor which inhibits TGF-β signaling (i-TGFβ). Results are shown for three representative HCV-seropositive subjects: C98 and C107, with chronic infection, and R58, with spontaneous HCV clearance. Numbers in the upper left quadrants represent percentages for FoxP3+ CFSElow CD4 cells (i.e., expanded FoxP3+ CD4+ T cells). Increased FoxP3+ CFSElow cell frequencies at least 1% above the background for SOD are underlined. (B) Percentages of IFN-γ and TNF-α production by intracellular cytokine staining following HCV NS3/4 and PMA-ionomycin stimulation of FoxP3+ and FoxP3− CD4 T cells in PBMC cultures stimulated for 14 days with recombinant HCV NS3/4 protein with rIL-2 and anti-CD28. (C) Phenotypes of FoxP3+ and FoxP3− CD4 T cells in 14-day Treg cultures expanded with HCV NS3/4 protein, high-dose rIL-2, and anti-CD28.
HCV-expanded FoxP3+ Tregs displayed an anergic cytokine profile without HCV-specific IFN-γ and TNF-α expression (Fig. 4B), suggesting that they are not FoxP3+ effector T cells. They also did not express IL-2 or IL-10 (data not shown). Finally, HCV-expanded FoxP3+ Tregs resembled natural Tregs in their phenotype, with increased CD25, CTLA4, HLA-DR, CD45RO, and GITR expression compared to FoxP3− CD4 T cells (Fig. 4C). Collectively, these results show that virus-specific FoxP3+ Tregs can be expanded by antigen-specific stimulation in vitro.
HCV-specific FoxP3+ Tregs demonstrated by class II MHC/peptide tetramer binding.
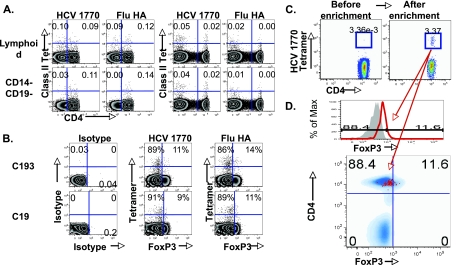
The antigen specificity of expanded FoxP3+ Tregs was examined more directly by using class II peptide tetramers specific for DRB1*04-restricted CD4 T-cell epitopes derived from HCV (NS3 1248, NS4 1770) and influenza virus HA. Antigen specificities for these class II tetramers were confirmed with peptide-specific short-term T-cell lines (Fig. 5A and B). As shown in Fig. 5C, NS3 1248- and NS4 1770-specific class II tetramer+ CD4 T cells could be expanded from PBMC of HCV-seropositive persons with our Treg expansion method using antigen, high-dose rIL-2, and CD28 costimulation. For example, recombinant HCV NS3/4 protein promoted the expansion of HCV-specific but not influenza virus-specific class II tetramer+ T cells (0.8% for HCV 1248, 2.9% for HCV 1770, and 0.1% for influenza virus HA). Importantly, FoxP3 was expressed in 44% of expanded HCV 1770-specific CD4 T cells, with a further increase to 90% in the presence of TGF-β. Antigen-specific FoxP3+ Treg induction was not limited to HCV since FoxP3 was also expressed in Flu HA tetramer+ CD4 T cells with antigen-specific expansion in vitro (Fig. 5D).
FIG. 5.
HCV specificity of expanded FoxP3+ Tregs demonstrated by MHC-II peptide tetramers. (A) Antigen specificities for HLA DRB1*04-restricted class II tetramers bound to influenza virus hemagglutinin (Flu HA), HCV NS3 1248, and HCV NS4 1770 epitopes in short-term peptide-stimulated T-cell lines are shown. (B) Specific binding of class II tetramers is shown by staining an HCV NS3/4-stimulated T-cell line with isotype antibody, HCV NS4 1770 tetramer, and influenza virus HA-specific tetramer. An HCV NS3/4-specific T-cell line binds HCV 1770 but not influenza virus HA tetramer. (C) Expansion of HCV-specific FoxP3+ CD4 T cells in vitro. CFSE-labeled PBMC from a DRB1*04+ HCV-seropositive subject (R19) were stimulated with recombinant HCV NS3/4 protein, with the expansion of FoxP3+ CD4 T cells (left panels) and HCV-specific (but not influenza virus HA-specific) DRB1*04-restricted class II tetramer+ CD4 T cells (middle three panels). Circled gates indicate class II tetramer+ CFSElow CD4 T cells that divided following antigenic stimulation. FACS plots on the far right show FoxP3 expression in the gated HCV 1770-specific CFSElow CD4 T cells. (D) FoxP3 expression by influenza virus HA tetramer+ CD4 T cells is shown in CFSE-labeled PBL following antigenic influenza virus HA peptide stimulation (gated on CD4 T cells on the upper graph) is shown. (Bottom) FoxP3 expression by 51% of gated CFSElow influenza virus tetramer (Tet)+ CD4 T cells in an overlay with total CD4.
Finally, the presence of HCV-specific FoxP3+ Tregs was examined directly ex vivo using class II tetramers in freshly isolated PBMC. As shown in Fig. 6A, exclusion of CD14+ monocytes and CD19+ B cells reduced the nonspecific background staining for non-CD4 T cells while maintaining specific binding to CD4 T cells in DRB1*04+ subjects (Fig. 6A). Notably, FoxP3 expression was detected at a low level in a subset of HCV 1770 tetramer+ CD4 T cells in HLA DRB1*04+ HCV-seropositive subjects directly ex vivo (Fig. 6B) or following further enrichment (Fig. 6C and D) as reported previously (17, 33). However, FoxP3 was also expressed in influenza virus HA-specific CD4 T cells (Fig. 6B), consistent with the notion that FoxP3 expression is a global characteristic of antigen-specific CD4 T cells.
FIG. 6.
Detection of antigen-specific FoxP3+ CD4 T cells ex vivo. (A) Staining strategy to reduce nonspecific background staining for class II tetramers. CD4 and class II tetramer staining characteristics for freshly isolated PBL in subjects with and without the HLA DRB1*04 allele are shown. Top graphs represent 7-amino-actinomycin-negative cells with lymphoid gate based on forward and side scatter characteristics. Bottom graphs represent lymphoid cells with further exclusion of CD14+ and/or CD19+ cells. Flu HA, influenza virus HA. (B) FoxP3 expression in antigen-specific CD4 T cells ex vivo. FoxP3 expression relative to HCV 1770 and influenza virus-specific class II tetramer staining is shown for two patients with chronic HCV infection (C193 and C19). Percentages show relative FoxP3 expression among tetramer+ cells. (C) Enrichment of HCV NS4 1770-specific class II tetramer+ CD4 T cells by magnetic beads using freshly isolated PBMC from an HCV-seropositive subject (17, 33), gating on CD4+ CD14− CD19− Viaprobe− lymphoid cells. The multicolor FACS plots show that an obvious cluster of tetramer+ CD4 T cells can be seen after (3.37%) but not before (0.00336%) tetramer enrichment (tetramer+ CD4+; boxes). (D) FoxP3 expression in gated HCV NS4 1770-specific class II tetramer+ CD4 T cells following enrichment. The upper histogram of tetramer+ CD4+ T cells shows that 11.6% of gated HCV 1770-specific tetramer+ CD4+ T cells (red line) are FoxP3+, based on the cutoff below which 99.8% of the isotype antibody-stained control sample (gray shaded region) is negative. The lower FACS plot shows FoxP3 expression by gated tetramer+ CD4+ T cells overlaid onto a density plot for CD14− CD19− 7-amino-actinomycin-negative lymphocytes. Specifically, 11 events were positive for FoxP3, compared to 84 events negative for FoxP3.
Dose-dependent suppression of HCV-specific CD8 T cells by HCV-specific CD25+ Tregs.
To confirm that HCV-expanded FoxP3+ T cells are indeed Tregs with immunity-suppressive function. we tested their capacity to suppress HCV-specific CD8 T cells. This involved the selection of HCV-seropositive subject R23, who had HCV NS3-specific CD8 T cells that were readily detectable by class I MHC/peptide tetramers ex vivo (Fig. 7A) as well as efficient HCV NS3/4-specific FoxP3+ Treg expansion in vitro (Fig. 7B, top) and therefore yielded sufficient CD4+CD25+ (eTregs) and control CD4+CD25− (eCD25−) cells after magnetic bead sorting (Fig. 7B, bottom). The sorted eTregs and eCD25− T cells were added to autologous peripheral blood lymphocytes (PBL) and stimulated with HCV-derived overlapping 15-mer peptide pools to activate HCV-specific eTregs as well as effector CD4 and CD8 T cells.
FIG. 7.
HCV-specific CD8 T-cell suppression by in vitro-expanded HCV-specific CD4+CD25+ T cells. (A) HCV NS3 1406-specific CD8 T cells demonstrated directly ex vivo by class I tetramer staining in HCV-seropositive HLA-A2+ subject R23. Efficient antigen-specific expansion occurred following 7 days of in vitro stimulation with overlapping NS3-derived HCV peptides. (B) The top three FACS plots demonstrate preferential expansion of HCV-specific FoxP3+ CD4+ Tregs by day 14 following in vitro stimulation with recombinant NS3/4 protein, 1,000 U/ml rIL-2, and 10 μg/ml soluble anti-CD28 alone (NS3/4, 27.2% FoxP3+ CD4+) and with added TGF-β (NS3/4 plus TGF-β, 51.4% FoxP3+ CD4+), compared to control SOD (7.5% FoxP3+ CD4+). The bottom three graphs show FoxP3+ CD25+ T-cell content in autoMACS-sorted CD4+CD25+ (eTregs) and CD4+CD25− (eCD25−) subsets from the expanded Treg cultures. (C) CD8+ T-cell expansion is shown by histograms representing CFSE dilution on day 7 in PBMC stimulated alone or with added eTregs isolated from the NS3/4-stimulated Treg cultures in panel B. The stimulating condition is indicated above each set of graphs. The ratios between eTreg and PBL are indicated below the graphs. (D) HCV-specific CD8+ T-cell expansion in PBL was directly monitored by a class I tetramer specific for HCV NS3 1406-specific CD8 T cells in the presence of NS3/4-expanded eTregs at various eTreg/PBL ratios (0:1, 0:25:1, and 1:1). Assays using eTregs expanded with TGF-β are indicated by 1:0.25T and 1:1T. The graphs at the extreme right represent assays in which CD4+CD25− T cells are sorted from expanded Treg culture (eCD25−) and added at 1:1 ratio to PBL before antigenic stimulation with the NS3 peptide (top) or with anti-CD3/anti-CD28 stimulation (bottom).
As shown in Fig. 7C, HCV-specific CD8+ T-cell expansion was suppressed in a dose-dependent manner with the addition of HCV-specific eTregs. Comparable levels of suppression were noted for eTregs expanded with and without TGF-β. The reduction in CD8 T-cell expansion was less dramatic when PBMC/eTreg cocultures were stimulated with NS5B peptides or anti-CD3/anti-CD28. Since these eTregs were initially expanded with NS3/4 protein, this suggests that antigen-specific Tregs may suppress more efficiently when stimulated in an antigen-specific manner. Finally, HCV-specific CD8 T-cell suppression by HCV-specific Tregs was confirmed by class I MHC/peptide tetramer staining, which showed a dose-dependent suppression of HCV NS3 1406-specific CD8 T-cell expansion in the presence of eTregs (Fig. 7D). HCV 1406-specific CD8 T-cell expansion was not efficiently suppressed by the negative control eCD25− T cells. These results suggest that HCV-specific FoxP3+ Tregs can be expanded from HCV-seropositive persons with antigen-specific regulatory function.
DISCUSSION
CD25+ Tregs represent 5 to 10% of circulating CD4 T cells in mice and healthy humans (6, 51). Hypoproliferative and suppressive in vitro, these low-frequency T cells nonetheless mediate immune tolerance to self- and nonself antigens in vivo (27, 44, 49, 51). Tregs also regulate immunity to infectious pathogens, as shown in small-animal models including murine herpesvirus (58), Friend leukemia virus (19, 45), and Leishmania major (7) models. While the role of Tregs in human infections cannot be as directly assessed, HCV presents a compelling scenario for Treg involvement given its high rate of persistence with increased CD4+CD25+ T-cell frequencies, ongoing necroinflammatory liver disease, and virus-specific effector T-cell dysfunction (10, 12, 48, 54). In this study, we asked (i) whether true Tregs (rather than activated T cells) are upregulated in HCV-infected subjects using the specific marker FoxP3, (ii) whether FoxP3+ Tregs in HCV-infected subjects differ from those in uninfected subjects, and (iii) whether HCV-specific FoxP3+ Tregs can be induced, thus providing virus-specific immune regulation.
Both CD4+CD25high and FoxP3+ T cells were significantly upregulated in HCV-infected subjects in our study. FoxP3 was expressed in CD25high rather than CD25int CD4 T cells regardless of HCV infection, resulting in a tight correlation between FoxP3+ and CD25high CD4 T-cell frequencies (R = +0.76; P = 0.007). Thus, HCV infection was associated with a preferential increase in FoxP3+ Tregs rather than activated CD25+ effector T cells. However, there was no significant difference between FoxP3+ Tregs from HCV-infected and uninfected subjects ex vivo. First, FoxP3+ CD25high T cells displayed an activation phenotype similar to that for natural Tregs regardless of HCV status: mostly positive for CD45RO, CD62L, HLA-DR, and CTLA4 but not CD127 (18, 32, 50). Second, FoxP3+ Tregs from HCV-infected and uninfected subjects both displayed an anergic cytokine profile, with little to no expression of regulatory cytokines IL-10 and TGF-β. Therefore, FoxP3+ Tregs in our subjects differed from IL-10-producing Tr1 cells or TGF-β-producing Th3 cells with IL-10- or TGF-β-dependent immune regulation (4, 12, 30), previously described in HCV infection (12, 35, 63). Third, minimal differences were found in global gene expression analysis comparing FACS-sorted CD4+CD25high T cells from HCV-infected and uninfected subjects. Finally, the T-cell-suppressive capacities of FoxP3+ Tregs for HCV-infected and uninfected patients were similar on a per cell basis. Collectively, these results show that FoxP3+ Tregs rather than activated CD25+ effector T cells are upregulated in HCV infection.
The remarkable similarity between CD25+ Tregs from HCV-infected and uninfected subjects raises the possibility that natural Tregs are expanded in HCV infection. However, FoxP3 expression and suppressive function can also be induced de novo in CD4+CD25− T cells following CD3/CD28 or antigenic stimulation, particularly in the setting of TGF-β or IFN-γ (16, 65, 66). Based on combined analysis of T-cell turnover and clonality, it has been suggested that a proportion of FoxP3+ Tregs include an adaptive population that differentiates from rapidly dividing memory CD4 T cells rather than natural Tregs (1, 64). The progressive increase in memory and activation marker expression from CD25− to CD25+ FoxP3− and CD25+ FoxP3+ CD4 T cells in our subjects may be consistent with a continuum between memory and regulatory T cells. Consistent with the notion of adaptive Tregs, HCV-specific FoxP3+ Tregs could be expanded in vitro in an antigen-specific manner from HCV-seropositive persons. HCV-specific FoxP3+ Tregs were also detected ex vivo, albeit in limited cases with only low levels of FoxP3 expression. Along these lines, antigen-specific expansion of FoxP3− memory/effector T cells as well as FoxP3+ regulatory T cells has been reported for CD8 T cells (9). Treg induction may be enhanced by HCV gene products with immune regulatory capacities as well as increased circulating levels of TGF-β (2, 41, 46), an immune regulatory cytokine which is known to enhance adaptive Treg induction (16, 65, 66). Although Treg frequency is not upregulated ex vivo in HCV-recovered subjects (54), HCV-specific Tregs could also be expanded in vitro from HCV-recovered subjects (e.g., R58 and R23), suggesting that they may be maintained at a low frequency in vivo but expand upon antigenic stimulation, similar to memory T cells. While FoxP3 can be expressed by activated human T cells (3), HCV-specific FoxP3+ T cells in our study were functional Tregs based on their ability to suppress effector CD8 T-cell proliferation. FoxP3 expression was also detected in CD4 T cells specific for non-HCV antigens (e.g., influenza virus HA or tetanus toxoid), suggesting that immune regulation via antigen-specific Tregs may extend beyond HCV. While antigen-specific Tregs have been reported in the context of T-cell receptor-transgenic animals or de novo induction (37, 60, 65, 68), this is the first direct demonstration of antigen-specific FoxP3+ Tregs using class II tetramers in human infection to our knowledge.
Induction of virus-specific Tregs can provide more-targeted immune regulation at the site of infection and inflammation, similar to the greater protection against autoimmune diabetes in nonobese diabetic mice by Tregs specific for pancreatic islet antigen than by polyclonally expanded Tregs (37, 38). Since CD25+ Tregs require activation through the T-cell receptor to become suppressive (61), antigen-specific Tregs may become suppressive in vivo only after encountering antigen-presenting cells expressing their cognate antigens (e.g., in lymphoid tissue during immune induction or in HCV-infected liver). As the effector T cells home to the site of antigen expression, activation of antigen-specific Tregs (e.g., by HCV or even influenza virus) in close proximity to the effector T cells will provide local rather than global immune regulation to limit immune-mediated damage while promoting viral persistence.
The ratio between CD25high and CD25int T cells correlated positively with viremia in HCV-infected patients (P = 0.009), suggesting that the balance between Tregs and effector T cells determines the level of viremia. Similar correlation was not observed for serum liver transaminase levels, perhaps due to generally well-preserved liver function among our study subjects. Nevertheless, the accelerated liver disease progression in HCV-infected patients with HIV coinfection (39, 57) suggests that an HIV-associated CD4+ T-cell defect may limit Treg-mediated immune regulation and tilt the balance toward pathogenetic effector T-cell response. In this respect, adoptive transfer of antigen-specific Tregs may be a useful immunotherapeutic strategy to limit the immune-mediated damage in HCV infection, if combined with antiviral strategies to reduce HCV replication. Further studies are needed to examine the role of Tregs in liver disease pathogenesis and to develop therapeutic approaches to control the balance between Tregs and effector T cells to enhance viral clearance while limiting liver inflammation.
In conclusion, functionally active FoxP3+ Tregs are upregulated ex vivo in HCV persistence. FoxP3+ Tregs in HCV-infected patients are phenotypically, functionally, and genetically indistinguishable from FoxP3+ Tregs in uninfected persons, and they include HCV-specific FoxP3+ Tregs that provide more-targeted virus-specific immune regulation. We also provide a novel demonstration of HCV-specific FoxP3+ Tregs using class II tetramers. These findings provide new insights into antigen-specific immune regulation during viral infection with potential therapeutic application.
Acknowledgments
We thank Paul Hallberg, Hank Pletcher, and Jonni Moore at the Flow Cytometry and Cell Sorting Resource Laboratory of the University of Pennsylvania Abramson Cancer Center and the BSL3 Cell Sorting Core Facility at the Children's Hospital of Philadelphia and John Baldwin at the University of Pennsylvania Microarray facility for their support and technical expertise; Mary E. Valiga for patient recruitment; Sutharsan Ganesan for technical assistance; and all subjects who participated in this study. We are grateful to Linda Higgins of Scios Inc. for the SD208.
This work was supported with the resources and the use of facilities at the Philadelphia VA Medical Research, NIH/NIDDK Center of Molecular Studies in Digestive and Liver Diseases, P30DK50306, and its Molecular Biology and Cell Culture Core Facilities and NIH Public Health Service Research grant M01-RR00040. Funding support was provided through NIH grants AI47519 and AA12849, the AGA Fiterman and Elsevier Awards, and the Viral Hepatitis Research Foundation of Japan. D.A.P. is a Medical Research Council (United Kingdom) Senior Clinical Fellow.
Footnotes
Published ahead of print on 12 March 2008.
REFERENCES
- 1.Akbar, A. N., M. Vukmanovic-Stejic, L. S. Taams, and D. C. Macallan. 2007. The dynamic co-evolution of memory and regulatory CD4+ T cells in the periphery. Nat. Rev. Immunol. 7231-237. [DOI] [PubMed] [Google Scholar]
- 2.Alatrakchi, N., C. S. Graham, J. Van der Vliet, K. E. Sherman, M. A. Exley, and M. J. Koziel. 2007. Hepatitis C virus (HCV)-specific CD8+ cells produce transforming growth factor β that can suppress HCV-specific T-cell responses. J. Virol. 815882-5892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allan, S. E., S. Q. Crome, N. K. Crellin, L. Passerini, T. S. Steiner, R. Bacchetta, M. G. Roncarolo, and M. K. Levings. 2007. Activation-induced FOXP3 in human T effector cells does not suppress proliferation or cytokine production. Int. Immunol. 19345-354. [DOI] [PubMed] [Google Scholar]
- 4.Asseman, C., S. Mauze, M. W. Leach, R. L. Coffman, and F. Powrie. 1999. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J. Exp. Med. 190995-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baecher-Allan, C., J. A. Brown, G. J. Freeman, and D. A. Hafler. 2001. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 1671245-1253. [DOI] [PubMed] [Google Scholar]
- 6.Baecher-Allan, C., V. Viglietta, and D. A. Hafler. 2002. Inhibition of human CD4+CD25+high regulatory T cell function. J. Immunol. 1696210-6217. [DOI] [PubMed] [Google Scholar]
- 7.Belkaid, Y., C. A. Piccirillo, S. Mendez, E. M. Shevach, and D. L. Sacks. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420502-507. [DOI] [PubMed] [Google Scholar]
- 8.Bennett, C. L., J. Christie, F. Ramsdell, M. E. Brunkow, P. J. Ferguson, L. Whitesell, T. E. Kelly, F. T. Saulsbury, P. F. Chance, and H. D. Ochs. 2001. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat. Genet. 2720-21. [DOI] [PubMed] [Google Scholar]
- 9.Billerbeck, E., H. E. Blum, and R. Thimme. 2007. Parallel expansion of human virus-specific FoxP3- effector memory and de novo-generated FoxP3+ regulatory CD8+ T cells upon antigen recognition in vitro. J. Immunol. 1791039-1048. [DOI] [PubMed] [Google Scholar]
- 10.Boettler, T., H. C. Spangenberg, C. Neumann-Haefelin, E. Panther, S. Urbani, C. Ferrari, H. E. Blum, F. von Weizsacker, and R. Thimme. 2005. T cells with a CD4+ CD25+ regulatory phenotype suppress in vitro proliferation of virus-specific CD8+ T cells during chronic hepatitis C virus infection. J. Virol. 797860-7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brunkow, M. E., E. W. Jeffery, K. A. Hjerrild, B. Paeper, L. B. Clark, S. A. Yasayko, J. E. Wilkinson, D. Galas, S. F. Ziegler, and F. Ramsdell. 2001. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat. Genet. 2768-73. [DOI] [PubMed] [Google Scholar]
- 12.Cabrera, R., Z. Tu, Y. Xu, R. J. Firpi, H. R. Rosen, C. Liu, and D. R. Nelson. 2004. An immunomodulatory role for CD4+CD25+ regulatory T lymphocytes in hepatitis C virus infection. Hepatology 401062-1071. [DOI] [PubMed] [Google Scholar]
- 13.Chang, K. M. 2003. Immunopathogenesis of hepatitis C virus infection. Clin. Liver Dis. 789-105. [DOI] [PubMed] [Google Scholar]
- 14.Chang, K. M. 2005. Regulatory T cells and the liver: a new piece of the puzzle. Hepatology 41700-702. [DOI] [PubMed] [Google Scholar]
- 15.Chang, K. M., R. Thimme, J. J. Melpolder, D. Oldach, J. Pemberton, J. Moorhead-Loudis, J. G. McHutchison, H. J. Alter, and F. V. Chisari. 2001. Differential CD4+ and CD8+ T-cell responsiveness in hepatitis C virus infection. Hepatology 33267-276. [DOI] [PubMed] [Google Scholar]
- 16.Chen, W., W. Jin, N. Hardegen, K. J. Lei, L. Li, N. Marinos, G. McGrady, and S. M. Wahl. 2003. Conversion of peripheral CD4+CD25− naive T cells to CD4+CD25+ regulatory T cells by TGF-β induction of transcription factor Foxp3. J. Exp. Med. 1981875-1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Day, C. L., N. P. Seth, M. Lucas, H. Appel, L. Gauthier, G. M. Lauer, G. K. Robbins, Z. M. Szczepiorkowski, D. R. Casson, R. T. Chung, S. Bell, G. Harcourt, B. D. Walker, P. Klenerman, and K. W. Wucherpfennig. 2003. Ex vivo analysis of human memory CD4 T cells specific for hepatitis C virus using MHC class II tetramers. J. Clin. Investig. 112831-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dieckmann, D., H. Plottner, S. Berchtold, T. Berger, and G. Schuler. 2001. Ex vivo isolation and characterization of CD4+CD25+ T cells with regulatory properties from human blood. J. Exp. Med. 1931303-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dittmer, U., H. He, R. J. Messer, S. Schimmer, A. R. Olbrich, C. Ohlen, P. D. Greenberg, I. M. Stromnes, M. Iwashiro, S. Sakaguchi, L. H. Evans, K. E. Peterson, G. Yang, and K. J. Hasenkrug. 2004. Functional impairment of CD8+ T cells by regulatory T cells during persistent retroviral infection. Immunity 20293-303. [DOI] [PubMed] [Google Scholar]
- 20.Earle, K. E., Q. Tang, X. Zhou, W. Liu, S. Zhu, M. L. Bonyhadi, and J. A. Bluestone. 2005. In vitro expanded human CD4+CD25+ regulatory T cells suppress effector T cell proliferation. Clin. Immunol. 1153-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fontenot, J. D., M. A. Gavin, and A. Y. Rudensky. 2003. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 4330-336. [DOI] [PubMed] [Google Scholar]
- 22.Franzese, O., P. T. Kennedy, A. J. Gehring, J. Gotto, R. Williams, M. K. Maini, and A. Bertoletti. 2005. Modulation of the CD8+-T-cell response by CD4+ CD25+ regulatory T cells in patients with hepatitis B virus infection. J. Virol. 793322-3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu, J., D. Xu, Z. Liu, M. Shi, P. Zhao, B. Fu, Z. Zhang, H. Yang, H. Zhang, C. Zhou, J. Yao, L. Jin, H. Wang, Y. Yang, Y. X. Fu, and F. S. Wang. 2007. Increased regulatory T cells correlate with CD8 T-cell impairment and poor survival in hepatocellular carcinoma patients. Gastroenterology 1322328-2339. [DOI] [PubMed] [Google Scholar]
- 24.Grakoui, A., N. H. Shoukry, D. J. Woollard, J. H. Han, H. L. Hanson, J. Ghrayeb, K. K. Murthy, C. M. Rice, and C. M. Walker. 2003. HCV persistence and immune evasion in the absence of memory T cell help. Science 302659-662. [DOI] [PubMed] [Google Scholar]
- 25.Hoofnagle, J. H. 1997. Hepatitis C: the clinical spectrum of disease. Hepatology 2615S-20S. [DOI] [PubMed] [Google Scholar]
- 26.Hutchinson, S. L., L. Wooldridge, S. Tafuro, B. Laugel, M. Glick, J. M. Boulter, B. K. Jakobsen, D. A. Price, and A. K. Sewell. 2003. The CD8 T cell coreceptor exhibits disproportionate biological activity at extremely low binding affinities. J. Biol. Chem. 27824285-24293. [DOI] [PubMed] [Google Scholar]
- 27.Jonuleit, H., E. Schmitt, H. Kakirman, M. Stassen, J. Knop, and A. H. Enk. 2002. Infectious tolerance: human CD25+ regulatory T cells convey suppressor activity to conventional CD4+ T helper cells. J. Exp. Med. 196255-260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaplan, D. E., K. Sugimoto, F. Ikeda, J. Stadanlick, M. Valiga, K. Shetty, K. R. Reddy, and K. M. Chang. 2005. T-cell response relative to genotype and ethnicity during antiviral therapy for chronic hepatitis C. Hepatology 411365-1375. [DOI] [PubMed] [Google Scholar]
- 29.Leung, S. Y., A. Niimi, A. Noble, T. Oates, A. S. Williams, S. Medicherla, A. A. Protter, and K. F. Chung. 2006. Effect of transforming growth factor-beta receptor I kinase inhibitor 2,4-disubstituted pteridine (SD-208) in chronic allergic airway inflammation and remodeling. J. Pharmacol. Exp. Ther. 319586-594. [DOI] [PubMed] [Google Scholar]
- 30.Levings, M. K., R. Sangregorio, C. Sartirana, A. L. Moschin, M. Battaglia, P. C. Orban, and M. G. Roncarolo. 2002. Human CD25+CD4+ T suppressor cell clones produce transforming growth factor beta, but not interleukin 10, and are distinct from type 1 T regulatory cells. J. Exp. Med. 1961335-1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang, T. J., B. Rehermann, L. B. Seeff, and J. H. Hoofnagle. 2000. Pathogenesis, natural history, treatment, and prevention of hepatitis C. Ann. Intern. Med. 132296-305. [DOI] [PubMed] [Google Scholar]
- 32.Liu, W., A. L. Putnam, Z. Xu-Yu, G. L. Szot, M. R. Lee, S. Zhu, P. A. Gottlieb, P. Kapranov, T. R. Gingeras, B. F. de St Groth, C. Clayberger, D. M. Soper, S. F. Ziegler, and J. A. Bluestone. 2006. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 2031701-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lucas, M., A. Ulsenheimer, K. Pfafferot, M. H. Heeg, S. Gaudieri, N. Gruner, A. Rauch, J. T. Gerlach, M. C. Jung, R. Zachoval, G. R. Pape, W. Schraut, T. Santantonio, H. Nitschko, M. Obermeier, R. Phillips, T. J. Scriba, N. Semmo, C. Day, J. N. Weber, S. Fidler, R. Thimme, A. Haberstroh, T. F. Baumert, P. Klenerman, and H. M. Diepolder. 2007. Tracking virus-specific CD4+ T cells during and after acute hepatitis C virus infection. PLoS ONE 2e649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luhn, K., C. P. Simmons, E. Moran, N. T. Dung, T. N. Chau, N. T. Quyen, L. T. Thao, T. Van Ngoc, N. M. Dung, B. Wills, J. Farrar, A. J. McMichael, T. Dong, and S. Rowland-Jones. 2007. Increased frequencies of CD4+CD25high regulatory T cells in acute dengue infection. J. Exp. Med. 204979-985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.MacDonald, A. J., M. Duffy, M. T. Brady, S. McKiernan, W. Hall, J. Hegarty, M. Curry, and K. H. Mills. 2002. CD4 T helper type 1 and regulatory T cells induced against the same epitopes on the core protein in hepatitis C virus-infected persons. J. Infect. Dis. 185720-727. [DOI] [PubMed] [Google Scholar]
- 36.Manigold, T., E. C. Shin, E. Mizukoshi, K. Mihalik, K. K. Murthy, C. M. Rice, C. A. Piccirillo, and B. Rehermann. 2006. Foxp3+CD4+CD25+ T cells control virus-specific memory T cells in chimpanzees that recovered from hepatitis C. Blood 1074424-4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Masteller, E. L., Q. Tang, and J. A. Bluestone. 2006. Antigen-specific regulatory T cells—ex vivo expansion and therapeutic potential. Semin. Immunol. 18103-110. [DOI] [PubMed] [Google Scholar]
- 38.Masteller, E. L., M. R. Warner, Q. Tang, K. V. Tarbell, H. McDevitt, and J. A. Bluestone. 2005. Expansion of functional endogenous antigen-specific CD4+CD25+ regulatory T cells from nonobese diabetic mice. J. Immunol. 1753053-3059. [DOI] [PubMed] [Google Scholar]
- 39.Merriman, N. A., S. B. Porter, C. M. Brensinger, K. R. Reddy, and K. M. Chang. 2006. Racial difference in mortality among U.S. veterans with HCV/HIV coinfection. Am. J. Gastroenterol. 101760-767. [DOI] [PubMed] [Google Scholar]
- 40.Murali-Krishna, K., J. D. Altman, M. Suresh, D. J. Sourdive, A. J. Zajac, J. D. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8177-187. [DOI] [PubMed] [Google Scholar]
- 41.Nelson, D. R., R. P. Gonzalez-Peralta, K. Qian, Y. Xu, C. G. Marousis, G. L. Davis, and J. Y. Lau. 1997. Transforming growth factor-beta 1 in chronic hepatitis C. J. Viral Hepat. 429-35. [DOI] [PubMed] [Google Scholar]
- 42.Novak, E. J., A. W. Liu, G. T. Nepom, and W. W. Kwok. 1999. MHC class II tetramers identify peptide-specific human CD4+ T cells proliferating in response to influenza A antigen. J. Clin. Investig. 104R63-R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ono, M., J. Shimizu, Y. Miyachi, and S. Sakaguchi. 2006. Control of autoimmune myocarditis and multiorgan inflammation by glucocorticoid-induced TNF receptor family-related proteinhigh, Foxp3-expressing CD25+ and CD25− regulatory T cells. J. Immunol. 1764748-4756. [DOI] [PubMed] [Google Scholar]
- 44.Powrie, F., and K. J. Maloy. 2003. Immunology. Regulating the regulators. Science 2991030-1031. [DOI] [PubMed] [Google Scholar]
- 45.Robertson, S. J., R. J. Messer, A. B. Carmody, and K. J. Hasenkrug. 2006. In vitro suppression of CD8+ T cell function by Friend virus-induced regulatory T cells. J. Immunol. 1763342-3349. [DOI] [PubMed] [Google Scholar]
- 46.Roulot, D., H. Durand, T. Coste, J. Rautureau, A. D. Strosberg, R. Benarous, and S. Marullo. 1995. Quantitative analysis of transforming growth factor beta 1 messenger RNA in the liver of patients with chronic hepatitis C: absence of correlation between high levels and severity of disease. Hepatology 21298-304. [PubMed] [Google Scholar]
- 47.Rouse, B. T., and S. Suvas. 2004. Regulatory cells and infectious agents: detentes cordiale and contraire. J. Immunol. 1732211-2215. [DOI] [PubMed] [Google Scholar]
- 48.Rushbrook, S. M., S. M. Ward, E. Unitt, S. L. Vowler, M. Lucas, P. Klenerman, and G. J. Alexander. 2005. Regulatory T cells suppress in vitro proliferation of virus-specific CD8+ T cells during persistent hepatitis C virus infection. J. Virol. 797852-7859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sakaguchi, S. 2004. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22531-562. [DOI] [PubMed] [Google Scholar]
- 50.Seddiki, N., B. Santner-Nanan, J. Martinson, J. Zaunders, S. Sasson, A. Landay, M. Solomon, W. Selby, S. I. Alexander, R. Nanan, A. Kelleher, and B. Fazekas de St Groth. 2006. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J. Exp. Med. 2031693-1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shevach, E. M. 2002. CD4+ CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2389-400. [DOI] [PubMed] [Google Scholar]
- 52.Shevach, E. M., R. S. McHugh, C. A. Piccirillo, and A. M. Thornton. 2001. Control of T-cell activation by CD4+ CD25+ suppressor T cells. Immunol. Rev. 18258-67. [DOI] [PubMed] [Google Scholar]
- 53.Shoukry, N. H., A. Grakoui, M. Houghton, D. Y. Chien, J. Ghrayeb, K. A. Reimann, and C. M. Walker. 2003. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J. Exp. Med. 1971645-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sugimoto, K., F. Ikeda, J. Stadanlick, F. A. Nunes, H. J. Alter, and K. M. Chang. 2003. Suppression of HCV-specific T cells without differential hierarchy demonstrated ex-vivo in persistent HCV infection. Hepatology 381437-1448. [DOI] [PubMed] [Google Scholar]
- 55.Sugimoto, K., D. E. Kaplan, F. Ikeda, J. Ding, J. Schwartz, F. A. Nunes, H. J. Alter, and K. M. Chang. 2005. Strain-specific T-cell suppression and protective immunity in patients with chronic hepatitis C virus infection. J. Virol. 796976-6983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sugimoto, K., J. Stadanlick, F. Ikeda, C. Brensinger, E. E. Furth, H. J. Alter, and K. M. Chang. 2003. Influence of ethnicity in the outcome of hepatitis C virus infection and cellular immune response. Hepatology 37590-599. [DOI] [PubMed] [Google Scholar]
- 57.Sulkowski, M. S., and D. L. Thomas. 2003. Hepatitis C in the HIV-infected person. Ann. Intern. Med. 138197-207. [DOI] [PubMed] [Google Scholar]
- 58.Suvas, S., U. Kumaraguru, C. D. Pack, S. Lee, and B. T. Rouse. 2003. CD4+CD25+ T cells regulate virus-specific primary and memory CD8+ T cell responses. J. Exp. Med. 198889-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takaki, A., M. Wiese, G. Maertens, E. Depla, U. Seifert, A. Liebetrau, J. L. Miller, M. P. Manns, and B. Rehermann. 2000. Cellular immune responses persist and humoral responses decrease two decades after recovery from a single-source outbreak of hepatitis C. Nat. Med. 6578-582. [DOI] [PubMed] [Google Scholar]
- 60.Tang, Q., K. J. Henriksen, M. Bi, E. B. Finger, G. Szot, J. Ye, E. L. Masteller, H. McDevitt, M. Bonyhadi, and J. A. Bluestone. 2004. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J. Exp. Med. 1991455-1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thornton, A. M., and E. M. Shevach. 2000. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J. Immunol. 164183-190. [DOI] [PubMed] [Google Scholar]
- 62.Uhl, M., S. Aulwurm, J. Wischhusen, M. Weiler, J. Y. Ma, R. Almirez, R. Mangadu, Y. W. Liu, M. Platten, U. Herrlinger, A. Murphy, D. H. Wong, W. Wick, L. S. Higgins, and M. Weller. 2004. SD-208, a novel transforming growth factor beta receptor I kinase inhibitor, inhibits growth and invasiveness and enhances immunogenicity of murine and human glioma cells in vitro and in vivo. Cancer Res. 647954-7961. [DOI] [PubMed] [Google Scholar]
- 63.Ulsenheimer, A., J. T. Gerlach, N. H. Gruener, M. C. Jung, C. A. Schirren, W. Schraut, R. Zachoval, G. R. Pape, and H. M. Diepolder. 2003. Detection of functionally altered hepatitis C virus-specific CD4 T cells in acute and chronic hepatitis C. Hepatology 371189-1198. [DOI] [PubMed] [Google Scholar]
- 64.Vukmanovic-Stejic, M., Y. Zhang, J. E. Cook, J. M. Fletcher, A. McQuaid, J. E. Masters, M. H. Rustin, L. S. Taams, P. C. Beverley, D. C. Macallan, and A. N. Akbar. 2006. Human CD4+ CD25hi Foxp3+ regulatory T cells are derived by rapid turnover of memory populations in vivo. J. Clin. Investig. 1162423-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walker, M. R., B. D. Carson, G. T. Nepom, S. F. Ziegler, and J. H. Buckner. 2005. De novo generation of antigen-specific CD4+CD25+ regulatory T cells from human CD4+CD25− cells. Proc. Natl. Acad. Sci. USA 1024103-4108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang, Z., J. Hong, W. Sun, G. Xu, N. Li, X. Chen, A. Liu, L. Xu, B. Sun, and J. Z. Zhang. 2006. Role of IFN-gamma in induction of Foxp3 and conversion of CD4+ CD25− T cells to CD4+ Tregs. J. Clin. Investig. 1162434-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wildin, R. S., F. Ramsdell, J. Peake, F. Faravelli, J. L. Casanova, N. Buist, E. Levy-Lahad, M. Mazzella, O. Goulet, L. Perroni, F. Bricarelli, G. Byrne, M. McEuen, S. Proll, M. Appleby, and M. E. Brunkow. 2001. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat. Genet. 2718-20. [DOI] [PubMed] [Google Scholar]
- 68.Yamazaki, S., T. Iyoda, K. Tarbell, K. Olson, K. Velinzon, K. Inaba, and R. M. Steinman. 2003. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J. Exp. Med. 198235-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yao, Z. Q., D. T. Nguyen, A. I. Hiotellis, and Y. S. Hahn. 2001. Hepatitis C virus core protein inhibits human T lymphocyte responses by a complement-dependent regulatory pathway. J. Immunol. 1675264-5272. [DOI] [PubMed] [Google Scholar]
- 70.Ziegler, S. F. 2006. FOXP3: of mice and men. Annu. Rev. Immunol. 24209-246. [DOI] [PubMed] [Google Scholar]