Abstract
Hepatitis C virus (HCV) is an enveloped positive-stranded RNA hepatotropic virus. HCV pseudoparticles infect liver-derived cells, supporting a model in which liver-specific molecules define HCV internalization. Three host cell molecules have been reported to be important entry factors or receptors for HCV internalization: scavenger receptor BI, the tetraspanin CD81, and the tight junction protein claudin-1 (CLDN1). None of the receptors are uniquely expressed within the liver, leading us to hypothesize that their organization within hepatocytes may explain receptor activity. Since CD81 and CLDN1 act as coreceptors during late stages in the entry process, we investigated their association in a variety of cell lines and human liver tissue. Imaging techniques that take advantage of fluorescence resonance energy transfer (FRET) to study protein-protein interactions have been developed. Aequorea coerulescens green fluorescent protein- and Discosoma sp. red-monomer fluorescent protein-tagged forms of CD81 and CLDN1 colocalized, and FRET occurred between the tagged coreceptors at comparable frequencies in permissive and nonpermissive cells, consistent with the formation of coreceptor complexes. FRET occurred between antibodies specific for CD81 and CLDN1 bound to human liver tissue, suggesting the presence of coreceptor complexes in liver tissue. HCV infection and treatment of Huh-7.5 cells with recombinant HCV E1-E2 glycoproteins and anti-CD81 monoclonal antibody modulated homotypic (CD81-CD81) and heterotypic (CD81-CLDN1) coreceptor protein association(s) at specific cellular locations, suggesting distinct roles in the viral entry process.
Hepatitis C virus (HCV) is an enveloped positive-stranded RNA virus whose principal reservoir for replication is believed to be hepatocytes within the liver. Viruses initiate infection by attaching to molecules or receptors at the cell surface. Expression and localization of such receptors are often important determinants of a cell's susceptibility to infection and of viral tropism for a particular tissue. The development of retroviral pseudoparticles bearing HCV E1-E2 glycoproteins (gps) (i.e., HCVpp) (5, 24, 38) and an infectious system generating HCV particles in cell culture (HCVcc) (51, 75, 83) has allowed studies on the mechanism of HCV entry and replication.
HCVpp primarily infect liver-derived cells (5, 38, 63), supporting a model in which molecules expressed specifically within the liver act as receptors for the virus and help define HCV tropism. Current evidence suggests that at least three host cell molecules are important for HCV entry in vitro: scavenger receptor class B member I (SR-BI) (6, 33, 39, 64), the tetraspanin CD81 (6, 38, 51, 59), and the tight junction (TJ) protein claudin-1 (CLDN1) (25). HCV gps have been reported to interact with SR-BI and CD81 (reviewed in reference 19). Other factors, such as glycosaminoglycans (2, 3) and low-density-lipoprotein receptor (57), have been implicated in HCV entry, although their role is less well established (reviewed in reference 74). In vivo, SR-BI is present within steroidogenic tissue, macrophages, and liver (44); CD81 is in most tissues (50); and CLDN1 is present in many tissues but is present at high levels in the liver (29). Since these molecules are not uniquely expressed in the liver, their organization or stoichiometry within hepatocytes may explain their viral receptor activity.
SR-BI is a member of the scavenger receptor family and is the major receptor for high-density lipoprotein (44). Antibodies specific for SR-BI have been reported to inhibit HCV infection and overexpression of SR-BI promotes viral infection (6, 14, 33, 39, 80). Experiments to validate an essential role for SR-BI in HCV entry have proven difficult, since all cell types studied to date express SR-BI and since small interfering RNA silencing has been reported to have variable effects on HCVpp infectivity (6, 47, 73, 80).
CD81 is a member of the tetraspanin family of proteins, and experiments demonstrating that expression of CD81 in the CD81-negative HepG2 hepatoma cell line confers viral infectivity support a critical role for CD81 in the viral entry process (6, 27, 54, 81). Recombinant forms of CD81 and antibodies specific for CD81 inhibit infectivity after viral adsorption to the target cell, suggesting that CD81 does not confer an ability for the virus to attach but acts as a coreceptor during the internalization process (20, 27). CLDN1 is a member of the integral membrane protein family which is involved in the formation of TJs (30). Functional studies have failed to demonstrate a direct interaction between the HCV gps and CLDN1, which may reflect a requirement for the virus to bind its receptors in a defined sequence or the low sensitivity of current cell-based methods. Mutagenesis and antibody-blocking studies with tagged versions of CLDN1 suggest that the first extracellular loop is essential during late stage(s) of the HCV entry process (25). The exact role(s) played by the HCV (co)receptors in the viral entry process is unclear.
CLDNs are critical components of TJs that regulate the paracellular permeability of endothelial and epithelial cells and establish cell polarity. CLDN polymerization is critical for establishing the membranous strands that form TJs (43); however, the molecular structure and organization of TJs are unclear. CLDN proteins associate in the plasma membrane (PM) of a single cell and between opposing cells via interactions between their extracellular loops (60). Several TJ proteins have been reported to act as primary receptors for a range of viruses; such proteins include junctional adhesion molecule for reovirus (4) and feline calicivirus (53) and coxsackie and adenovirus receptor for coxsackievirus and adenovirus (8). Recent work detailing the complex mechanism(s) underlying coxsackievirus group B virus (CBV) highlight the dynamic properties of intercellular junctions (21, 22). CBV binds to a primary receptor, decay-accelerating factor (DAF), expressed on the luminal surface of polarized intestinal epithelial cells. CBV interaction with DAF initiates a signaling cascade that triggers an actin-dependent relocalization of the virion-DAF complex to the lateral cell junctions, where coxsackie and adenovirus receptor is located, and endocytosis can occur (22). We previously reported that CLDN1 localized predominantly at the apical (canalicular) surface of hepatocytes in healthy liver tissue, consistent with its location at TJs; however, CLDN1 was also detected at the basolateral domain of hepatocytes (63). CLDN1 colocalized with CD81 at apical and basolateral domains and with SR-BI specifically at basolateral sites, supporting a model where receptor complexes are expressed at the site of HCV entry into the parenchyma via the sinusoidal blood (58).
To understand how these molecules coordinate HCV entry, it is important to study their association in primary liver tissue and in model hepatoma cells in which expression can be modulated and the effect(s) on viral entry assessed. Imaging techniques that take advantage of fluorescence resonance energy transfer (FRET) between fluorescent proteins to study protein-protein interactions have been developed. Cell-based FRET technology has been used to study protein compartmentalization (35), signaling protein complexes (58), structural organization and conformation of kinesin-1 (13), and the effect of human immunodeficiency virus (HIV) gps on CD4 and CCR-5 receptor complex formation (1, 31, 69, 78). We demonstrate FRET between receptor-specific antibodies bound to hepatocytes in liver tissue and between fluorescently tagged CD81 and CLDN1 in cell lines, demonstrating that a subpopulation of CD81 and CLDN1 associate. FRET was independent of the cellular permissiveness to support HCV entry. Treatment of Huh-7.5 hepatoma cells with recombinant HCV E1-E2 gps did not modulate the frequency of FRET between CD81 and CLDN1, suggesting that coreceptor complexes exist in the absence of viral proteins. HCV infection and treatment of hepatoma cells with the neutralizing anti-CD81 monoclonal antibody (MAb) reduced the frequency of FRET between CD81-CD81 associations but not the frequency of CD81-CLDN1 associations, highlighting potential antigenic and functional differences between homotypic and heterotypic coreceptor protein complexes in the viral entry process.
MATERIALS AND METHODS
Cell lines and reagents.
Huh-7.5 cells (11), HepG2, and 293T cells were propagated in Dulbecco's modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 1% nonessential amino acids. T84 cells were propagated in a 1:1 mixture of F12 and DMEM with 10% FBS. Huh-7.5, HepG2, and 293T cells were kindly provided by Charles Rice (Rockefeller University, NY), and T84 was provided by Chris Tselepis (University of Birmingham).
The primary antibodies used were anti-CLDN1, JAY.8 (Invitrogen, CA), and 1C5-D9 (Novus); anti-CLDN4 (Invitrogen, CA); anti-CD81 M38 (F. Berditchevski, University of Birmingham, United Kingdom); anti-E2 1/39 (26) and anti-NS5A 9E10 (C. Rice, Rockefeller University). Secondary labeled antibodies were obtained from Invitrogen (Alexa Fluor 488 goat anti-mouse immunoglobulin G [IgG], Alexa Fluor 488 goat anti-mouse IgG2a, Alexa Fluor 633 goat anti-mouse IgG, Alexa Fluor 633 goat anti-mouse IgG1, Alexa Fluor 488 goat anti-rabbit IgG, Alexa Fluor 633 goat anti-rabbit IgG, Alexa Fluor 488 goat anti-human IgG, Alexa Fluor 633 goat anti-human IgG, tetramethyl rhodamine isothiocyanate [TRITC] goat anti-mouse IgG, and TRITC goat anti-rabbit IgG).
HCV strain HCV-1 (genotype 1a) E2715 (the subscript refers to the final amino acid of the expressed protein within the HCV polyprotein [45]) and E1-E2 were expressed in CHO cells and extracted from extracellular supernatants or intracellular lysates, respectively. Antigens were purified using Galanthus nivalis lectin (Sigma, United Kingdom) chromatography and fast-flow S-Sepharose cation-exchange chromatography (Pharmacia) to >90% purity, as previously reported (18).
HCVpp/HCVcc genesis and infection.
Pseudoviruses expressing luciferase or enhanced green fluorescent protein (eGFP) reporters were generated by the following protocols. 293T cells were transfected with a 1:1 ratio of plasmids carrying HIV provirus expressing luciferase and HCV strain H77 E1-E2 envelope gps, murine leukemia virus (MLV) gp, or empty vector (Env−pp), as previously described (38). Alternatively, 293T cells were cotransfected with plasmids carrying HIV provirus expressing eGFP (CSGW) (10), HIV gag-pol, and HCV strain H77 gps or empty vector in a 1:1:4 ratio as previously described (27). Supernatants were harvested 48 h posttransfection, pooled, and filtered. Virus-containing medium was added to target cells plated at 1.5 × 104 cells/cm2 and incubated for 8 h. Unbound virus was removed, and the cells were fed again with their respective growth media and incubated at 37°C. After 72 h, infections were terminated and firefly luciferase activity in lysed cells was measured or cellular eGFP was quantified by flow cytometry. Specific pseudotype infectivity was calculated by subtracting the mean Env−pp signal from the HCVpp or MLVpp signals.
HCVcc JFH-1 was generated as previously described (51, 70). Briefly, RNA was transcribed in vitro from full-length genomes by using the Megascript T7 kit (Ambion, Austin, TX) and electroporated into Huh-7.5 cells. At 72 h and 96 h postelectroporation, supernatants were collected, pooled, and stored immediately at −80°C. Virus-containing medium was added to target cells plated at 1.5 × 104 cells/cm2 and incubated for 1 h. Unbound virus was removed replaced with 3% FBS-DMEM, and the cells were incubated at 37°C. After 72 h, infected cells were visualized by staining methanol-fixed cells for NS5A expression using the anti-NS5A 9E10 MAb and Alexa Fluor-conjugated anti-mouse IgG (Invitrogen, CA).
Generation of AcGFP- and DsRed-tagged CD81, CLDN1, and CLDN4.
To generate pTRIP lentiviral vectors expressing Aequorea coerulescens GFP (AcGFP)-CD81 (g.CD81TRIP), Discosoma sp. red-monomer fluorescent protein (DsRed)-CD81 (r.CD81TRIP) and DsRed-CLDN1 (r.CLDN1TRIP), the AcGFP and DsRed open reading frames were cloned into preexisting constructs encoding CD81 and CLDN1 (27). A C-terminal deletion mutant of CLDN1 (r.CLDN1ΔC) was generated using the method described by Evans and colleagues and expressed in pTRIP (25). pBABE vectors encoding CLDN1 and CLDN4 were modified in a similar manner to generate g.CLDN1BABE, r.CLDN1BABE, and r.CLDN4BABE (82).
SDS-PAGE and Western blotting.
Huh-7.5 and 293T-CLDN1TRIP cells were plated at 1.5 × 104 cells/cm2 and the following day lysed in 1% Brij-97, 10 mM Tris (pH 7.5), 150 mM NaCl, 1 mM CaCl2, and 1 mM MgCl2, containing protease inhibitors (Complete medium; Roche). Lysates were clarified by centrifugation (20,000 × g, 30 min), and protein concentrations were determined by using protein assay reagent (Pierce) according to the manufacturer's instructions. Defined concentrations of cell lysates were separated by 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to polyvinylidene difluoride membranes for incubation with an anti-CLDN1 (JAY.8) antibody (1 μg/ml; 1 h) and after washing to remove primary antibody with a secondary horseradish peroxidase-conjugated donkey anti-rabbit antibody (GE Healthcare). Bound antibody was detected by enhanced chemiluminescence (Geneflow).
LSCM.
Huh-7.5, HepG2.CD81, 293T.CLDN1, and T84 cells were grown on 22-mm-diameter borosilicate glass coverslips (Fisher Scientific, United Kingdom) and fixed in 3% paraformaldehyde (for anti-CD81 M38) or ice-cold methanol (for anti-CLDN1 1C5-D9). Cells were permeabilized for 30 min in 0.05% saponin and 0.5% bovine serum albumin (BSA) in phosphate-buffered saline (PBS) and incubated with M38 or 1C5-D9 for 1 h at room temperature. Cells were washed three times in PBS-saponin-BSA before labeled secondary Abs were added for 1 h at room temperature. Cells were washed three times in PBS-saponin-BSA before being counterstained with DAPI (4′,6-diamidino-2-phenylindole; Invitrogen) in PBS for 5 min. Coverslips were mounted (ProLong Gold antifade; Invitrogen) on glass slides, and cells were imaged by laser scanning confocal microscopy (LSCM) with a Meta-Head confocal microscope (Zeiss) and a 63× 1.2-numerical-aperture (NA) water immersion objective. Cells expressing tagged forms of CD81 and CLDN1 were stained with specific antibodies and visualized with anti-mouse IgG coupled to Alexa Fluor 633. Direct and indirect fluorescent signals were quantified by measuring the pixel intensity at cell-cell junctions (CJs) and intracellular and PM locations within 50 cells.
Formalin-fixed paraffin-embedded tissue blocks were obtained from hepatectomy specimens of patients undergoing liver transplant due to cirrhosis induced by HCV. Healthy livers were obtained from surplus donor tissues obtained at liver transplantation. Informed consent from each patient/donor at the University Hospital Birmingham was obtained prior to experiments, along with regional ethics committee approval. Representative 3-μm sections were cut from formalin-fixed tissue, placed onto charged slides, and incubated for 1 h at 60°C. Sections were dewaxed, rehydrated, and subjected to an agitated low-temperature epitope retrieval technique as previously described (62). Sections were mounted onto a Shandon sequencer and incubated with primary antibodies specific for CD81 (M38) and CLDN1 (JAY.8) in PBS-0.05% Tween (PBS-Tween) for 1 h. After a PBS-Tween wash, anti-mouse Alexa Fluor 488 and anti-rabbit TRITC-labeled secondary antibodies (Invitrogen) were applied for 30 min. Following a further wash, sections were counterstained with hematoxylin and mounted. Confocal images were collected using by LSCM (as detailed above) with a 63× 1.2-NA water immersion objective. Background and autofluorescence of tissue samples were corrected throughout.
Measurement of FRET between AcGFP- and DsRed-tagged CD81 and CLDN1.
Huh-7.5, 293T, and T84 cells were transduced to coexpress AcGFP- and DsRed-tagged proteins (g.CD81, r.CD81, g.CLDN1, r.CDLN1, r.CLDN1ΔC, and r.CLDN4) and grown on 22-mm-diameter borosilicate glass coverslips (Fisher Scientific, United Kingdom). Images were collected using a Meta LSCM (model LSM510; Zeiss), and areas of protein colocalization (100% pixel overlap) were identified by using the Colocalization Finder plugin (34) and ImageJ software (http://rsb.info.nih.gov/ij/). Proteins within the regions of interest were assessed for FRET.
The efficiency of FRET (EFRET) was obtained by measuring the fluorescence intensities of the donor fluorophore with (Fda; before photobleaching) and without (Fd; after photobleaching) the acceptor fluorophore and using the equation EFRET = 1 − (Fda/Fd). The EFRET value depends on the separation distance, r, between the donor and the acceptor. Hence, for any observed value of EFRET, a value of r can be inferred from the equation EFRET = Ro6/(Ro6 + r6) (see reference 32), where Ro is the Förster distance (28), a constant defined as the distance between the donor and acceptor molecules when their efficiency of energy transfer is 50%. This equation can be rearranged to give r = Ro(1/EFRET − 1)1/6. For AcGFP and DsRed fluorophores, this distance is reported to be 4.73 nm (Clontech). FRET does not occur between fluorophores that are >10-nm apart (Fda = Fd), giving an EFRET value of 0. The percentage of fields where FRET occurs (%FRET) is an indicator of the frequency of protein-protein association.
To determine the EFRET, we used an approach based on the photobleaching FRET methods described by Zal and Gascoigne (79). Currently available confocal microscopes offer tunable lasers which allow the simultaneous photobleaching of donor and acceptor molecules. EFRET values were determined using the donor fluorescence quenching/increased acceptor emission method, which combines sensitized and gradual acceptor photobleaching methods to measure Fda and Fd (12, 17, 56, 72). Images of cells expressing donor or acceptor fluorescent proteins were collected with all laser permutations to determine the degree of residual spectral bleed-through (see Fig. S1 in the supplemental material). Fluorophore lifetimes in cells expressing AcGFP- or DsRed-tagged proteins were measured by photobleaching at low and high laser powers. As expected, both fluorophores showed an exponential decay over long time periods at a high laser power, whereas a low laser power resulted in a linear decay over the relatively short time periods used in cell imaging (see Fig. S2 in the supplemental material). Measurement of fluorophore lifetime in all regions of protein colocalization allowed us to account for the relative abundances of the donor and the acceptor and to correct for photobleaching in a site-specific manner (72).
Energy transfer from a donor fluorophore to an acceptor can be used to infer their separation. However, several parameters, including cross talk between fluorophores due to spectral overlap, the relative contribution of FRET and non-FRET energies to the measured fluorescence intensity, and the impact of relative donor and acceptor concentrations on overall FRET energy, must be considered (reviewed in reference 9). AcGFP and DsRed proteins fluoresce as monomers, display a 30% overlap in their excitation and emission spectra, and are ideally suited for FRET studies (65). To minimize spectral bleed-through, we utilized the meta-head function of the microscope at the following wavelengths (λ): for AcGFP, excitation λ 488 nm and emission λ 520 nm, and for DsRed, excitation λ 561 nm and emission λ 600 nm (see Fig. S1 and S3 in the supplemental material). Analysis of donor and acceptor photobleaching in several cell lines demonstrated similar levels of cross talk for AcGFP and DsRed. Providing these two parameters are constant, FRET values can be inferred over a wide range of fluorophore concentrations (donor/acceptor ratios of 1:10 to 10:1) (9, 17). Quantification of CD81 and CLDN1/CLDN4 in transduced cells showed an approximately threefold range in expression levels.
Cell treatments.
Huh-7.5 cells expressing g.CD81/r.CD81 or g.CD81/r.CLDN1 were grown on 22-mm2 round coverslips for FRET analysis or in 48-well tissue culture plates for HCVpp or HCVcc infection. Cells were treated at 37°C for 1 h with increasing concentrations of HCV-1 E2715 or E1-E2 in PBS and anti-CD81 M38 or control antibody. Cells were fixed immediately in ice-cold methanol prior to confocal imaging and FRET analysis. Replicate wells were stained with anti-E2 MAb 1/39 to visualize cell-bound antigens. In parallel experiments, cells were treated with the agents for 1 h at 37°C and infected with HCVpp, MLVpp, Env−pp, or JFH-1 for 1 h at 37°C. Unbound virus and agents were removed by washing, and the cells were cultured for 72 h and assessed for viral infection.
Statistics.
Differences between FRET distances were assessed using a nonparametric Kruskal-Wallis test and Dunn's multiple-comparison test. The relationship between fluorophore fluorescence intensity and distance was assessed by linear regression. Differences in %FRET observed between samples were compared by Fisher's exact test. Corrections for multiple sampling (Bonferroni method) were used when appropriate. Statistical analyses were carried out with the ImageJ program or using the Prism 4 package (GraphPad Software, CA), with probabilities as described in the figure legends and table footnotes.
RESULTS
CD81 and CLDN1 cellular localization.
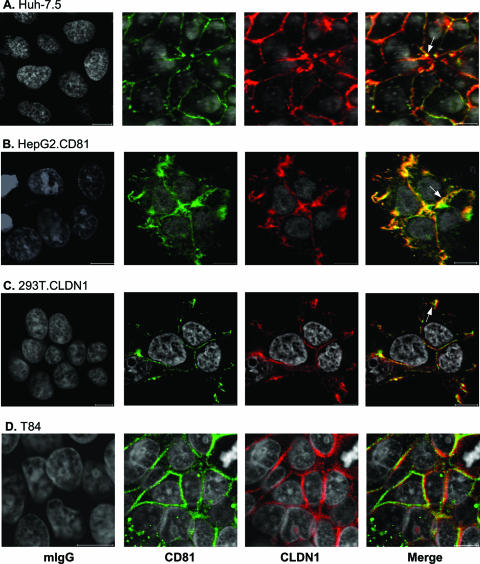
The cellular expression of CD81 and CLDN1 was studied by LSCM. Huh-7.5 cells support efficient HCV entry and express CD81 and CLDN1 at the PM, with some punctate intracellular CD81 observed (Fig. 1A). CD81 and CLDN1 colocalize in Huh-7.5 cells (Fig. 1A). Comparable patterns of CD81-CLDN1 colocalization were observed in permissive hepatoma (HepG2.CD81) and epithelial (293T.CLDN1) cell lines and a nonpermissive colorectal carcinoma T84 cell line (Fig. 1B to D), suggesting that receptor colocalization per se does not predict cellular permissiveness for HCV entry.
FIG. 1.
CD81 and CLDN1 colocalization. Huh-7.5 (A), HepG2.CD81 (B), 293T.CLDN1 (C), and T84 cells (D) were grown on poly-l-lysine-treated glass coverslips and stained with normal mouse IgG (mIgG) or antibodies specific for CD81 (M38) and CLDN1 (1C5-D9). Bound antibodies were visualized using the Alexa Fluor 488 anti-mouse IgG1 (M38; green) and Alexa Fluor 633 anti-mouse IgG2a (1C5-D9; red). LSCM images were obtained using a 63× 1.2-NA objective (the scale bar represents 10 μm). Areas of CD81-CLDN1 colocalization at CJs are labeled with an arrow. Cells were inoculated with HCVpp to define their permissiveness for viral entry. HCV-specific infectivity levels (expressed in relative light units [RLU]) were as follows: for Huh-7.5 cells, 139 × 104 RLU; for HepG2-CD81 cells, 9 × 104 RLU; for 293T-CLDN1 cells, 14 × 104 RLU; and for T84 cells, 0.2 × 104 RLU.
Characterization of AcGFP- and DsRed-tagged CD81 and CLDN1 proteins.
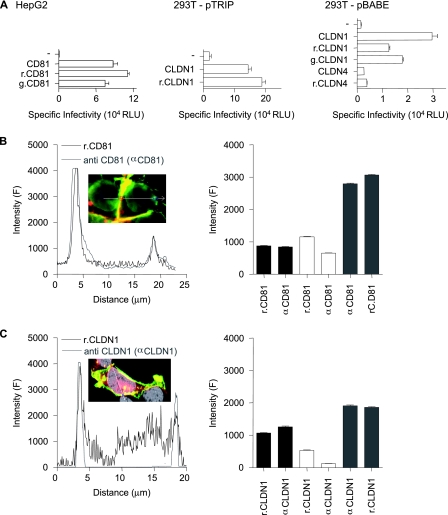
CD81 and CLDN1 fusion proteins with AcGFP and DsRed at their N termini were constructed in pTRIP and pBABE retroviral vectors for FRET studies. To ascertain if AcGFP or DsRed fluorophores modulate protein activity, HepG2 (CD81 negative) and 293T (CLDN1 negative) cells were transduced to express the parental and tagged proteins and screened for viral receptor activity and protein localization with specific antibodies. Parental and tagged versions of CD81 and CLDN1 permitted comparable levels of HCVpp entry into cells (Fig. 2A). 293T cells transduced with pBABE-CLDN1 were approximately fivefold less permissive for HCVpp entry than were cells transduced with pTRIP-CLDN1, which most likely reflects the reduced levels of CLDN expression observed in pBABE-transduced cells (Fig. 3D). MLVpp infected all cells with similar efficiencies independent of CD81 or CLDN expression (data not shown). As a control, 293T cells were transduced with the related CLDN family member CLDN4, and both parental and r.CLDN4 failed to support HCV entry (Fig. 2A), despite levels of PM CLDN4 expression comparable to that observed for CLDN1 (Fig. 3A).
FIG. 2.
Characterization of fluorescently N terminus-tagged CD81 and CLDN1. (A) HepG2 and 293T cells were transduced with retroviral vector pTRIP or pBABE expressing CD81, r.CD81, g.CD81, CLDN1, r.CLDN1, g.CLDN1, CLDN4, or r.CLDN4 and infected with HCVpp-H77, MLVpp, or Env−pp. Data are expressed as levels of specific infectivity and represent the mean luciferase levels (relative light units [RLU]) determined from replicate infections, with the Env−pp value subtracted (270 RLU for HepG2 cells and 360 RLU for 293T cells). HepG2 cells expressing r.CD81 (B) or 293T cells expressing r.CLDN1 (C) were stained with antibodies specific for CD81 (anti-CD81 [αCD81]) or CLDN1 (anti-CLDN1 [αCLDN1]), respectively. Linear profiling of the fluorescence signal emitted by the tagged protein (black line) and the indirect fluorescence signal from antibody staining (gray line) is shown. The mean fluorescence intensities from fluorescently tagged proteins (r.CD81 and r.CLDN1) and from antibody-stained (anti-CD81 and anti-CLDN1) receptors were obtained by profiling 50 cells. Regions were defined as the nonjunctional PM (black bar), intracellular junctions (white bar), and CJs (gray bar). All cells were imaged under the same conditions, and the data are expressed as arbitrary fluorescence units (F). The data from a single experiment are presented and are representative of two further experiments.
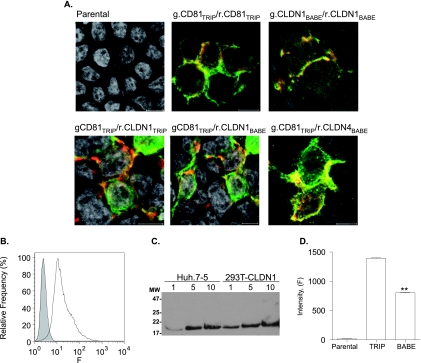
FIG. 3.
Expression and colocalization of fluorescently N terminus-tagged CD81, CLDN1, and CLDN4. (A) Confocal images of parental and 293T cells transduced with retroviral vector pTRIP or pBABE expressing g.CD81/r.CD81, g.CD81/r.CLDN1, or g.CD81/r.CLDN4. (B) Flow cytometric analysis of parental (gray, filled histogram) and TRIP-CLDN1-transduced (white histogram) 293T cells stained with anti-CLDN1 (JAY.8). Irrelevant-isotype-matched IgG control-stained parental and transduced cells with mean fluorescence intensities of 8 and 6, respectively. (C) One, 5, and 10 μg of Huh-7.5 and 293T-CLDN1TRIP total cell lysates were separated on a nonreducing 12% SDS-PAGE gel, the proteins were transferred to a polyvinylidene difluoride membrane and probed with anti-CLDN1 (JAY.8), and bound antibody was visualized with horseradish peroxidase-conjugated anti-rabbit IgG and enhanced chemiluminescence. (D) Fluorescence intensities of parental and TRIP-r.CLDN1- or BABE-r.CLDN1-transduced 293T cells. Fifty cells were imaged under the same conditions, and data were expressed as arbitrary fluorescence units (F). The difference in fluorescence between the transduced cell populations was evaluated using the Mann-Whitney method (**, P < 0.01).
Quantification of r.CD81 and r.CLDN1 expression by direct enumeration of the fluorescent tag or indirectly via receptor specific antibodies identified intracellular forms of r.CLDN1 that were not recognized by anti-CLDN1 antibodies which may represent unfolded or immature forms of tagged CLDN1 (Fig. 2C). CD81 and CLDN1 demonstrated a preferential localization at CJs and tagged proteins expressed at the PM were recognized by receptor-specific antibodies (Fig. 2B and C). Consequently, FRET experiments will focus on PM-expressed forms of the coreceptors.
Validation and FRET measurement of CD81 and CLDN1 protein interactions.
To study CD81 and CLDN1 interaction(s), we utilized a cell-based FRET method to investigate protein association(s). FRET is a process in which energy is transferred from an excited donor to an acceptor molecule and can occur only when the donor and acceptor are less than 10 nm apart (28). We utilized a gradual acceptor photobleaching FRET methodology in which donor and acceptor fluorescence intensities are monitored throughout the period of photobleaching (9, 17, 32, 72, 79). This method provides photobleaching coefficients for both fluorophores and allows us to determine the frequency of colocalized molecules where FRET occurs and the amount of energy transferred (EFRET). EFRET values allow the distance(s) between donor and acceptor proteins to be estimated. Although the relative orientations of AcGFP and DsRed in the fusion proteins are unknown, if we assume that proteins expressed in the same cell background are under the same constraints, it is possible to estimate distances between tagged molecules.
CD81 is reported to form homodimers in the formation of higher-order oligomeric structures characteristic of the tetraspanin web (41). We therefore measured FRET between AcGFP (donor)- and DsRed (acceptor)-tagged CD81 proteins expressed in Huh-7.5, 293T, and T84 cells. g.CD81 and r.CD81 colocalized (Fig. 3A), and FRET was detected with varying efficiencies across cell types (42% to 84%), with estimated distances in the range of 4.2 to 6.9 nm (Table 1). The variable frequencies of FRET may reflect the presence of untagged endogenous receptors which may compete with tagged receptors for protein association (69). Due to the low frequency of transduction of HepG2 cells, we were unable to ascertain g.CD81-r.CD81 FRET values in a cellular background lacking CD81 expression.
TABLE 1.
FRET between fluorescently N terminus-tagged CD81, CLDN1, and CLDN4a
| Donor | Acceptor | Cell type | Ntote | %FRET | Distance (nm) |
|---|---|---|---|---|---|
| Same protein type | |||||
| g.CD81TRIPb | r.CD81TRIP | Huh-7.5 | 364 | 82 | 6.0 ± 1.8 |
| r.CD81TRIP | 293T | 102 | 69 | 5.8 ± 3.8 | |
| r.CD81TRIP | 293T-CLDN1 | 108 | 56 | 4.9 ± 2.9 | |
| r.CD81TRIP | T84 | 159 | 54 | 5.6 ± 2.6 | |
| g.CLDN1BABEc | r.CLDN1BABE | 293T | 227 | 52 | 5.4 ± 1.6 |
| Different protein typed | |||||
| g.CD81TRIP | r.CLDN1TRIP | 293T | 142 | 59 | 6.2 ± 2.1 |
| r.CLDN1BABE | 293T | 147 | 58 | 5.8 ± 1.6 | |
| r.CLDN1TRIP | 293T-CLDN1 | 153 | 15* | 1.1 ± 1.9*** | |
| g.CLDN1BABE | r.CD81TRIP | 293T | 245 | 47 | 5.8 ± 2.4 |
| g.CD81TRIP | r.CLDN4BABE | 293T | 459 | 15* | 7.4 ± 1.8 |
Areas of fluorescent protein colocalization were selected and imaged by confocal microscopy. All tests were performed using the Mann-Whitney test (*, P < 0.05; ***, P < 0.001).
Huh-7.5, 293T, 293T-CLDN1TRIP and T84 cells were transduced with pTRIP vectors expressing g.CD81 and r.CD81.
293T cells were transduced with pBABE vectors expressing g.CD81 and r.CLDN1.
293T or 293T-CLDN1TRIP cells were transduced with pTRIP and pBABE vectors expressing g.CD81, r.CD81, g.CLDN1, r.CLDN1, or r.CLDN4 as indicated.
Ntot, the number of colocalized regions analyzed.
CLDN1 is reported to form dimers and higher-order oligomeric structures (43, 60), and we therefore investigated g.CLDN1-r.CLDN1 FRET in CLDN-null 293T cells. CLDN1 localized predominantly to CJs and FRET occurred between 52% of tagged CLDN1 molecules (Fig. 3A and Table 1). We measured FRET between g.CD81-r.CLDN1 in 293T cells in the presence and absence of untagged competitor CLDN1. pTRIP lentivirus efficiently transduced >95% of 293T cells to express CLDN1 (Fig. 3B), and the level of expression was comparable to the endogenous levels observed in Huh-7.5 cells (Fig. 3C). FRET occurred between 59% of colocalized g.CD81 and r.CLDN1 in naïve 293T cells, and this percentage was reduced to 15% in the presence of untagged CLDN1, with an increase in the estimated distance(s) between associating molecules (Table 1). In contrast, CLDN1 expression had no significant effect on %FRET between g.CD81 and r.CD81 (Table 1). Exchanging the donor and acceptor fluorophores maintained comparable levels of FRET between colocalized CD81 and CLDN1 (Table 1). We noted that CLDN1 expression levels in 293T pTRIP-CLDN1-transduced cells were significantly higher than the levels for those transduced with pBABE-CLDN1 (Fig. 3D), allowing us to study the effect(s) of CLDN1 expression on FRET association with CD81. Comparable frequencies of FRET were observed between g.CD81 and r.CLDN1 expressed from pTRIP or pBABE (Table 1). To assess the specificity of FRET between CD81 and CDLN1, we investigated FRET between CD81 and the inactive HCV receptor CLDN4 in 293T cells, which lack endogenous CLDN1/CLDN4 expression. CD81 colocalized with CLDN4 and showed a low level of FRET (Fig. 3A and Table 1), comparable to that seen between g.CD81 and r.CLDN1 in 293T cells expressing untagged CLDN1, suggesting a baseline of 15% FRET for nonspecific protein interactions.
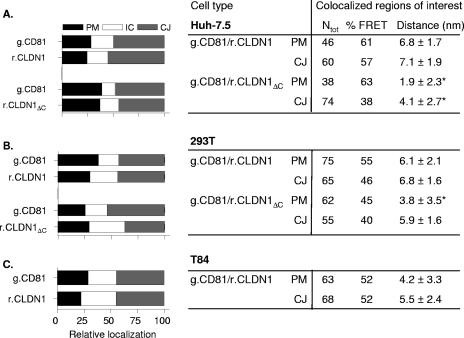
To investigate whether differences in FRET between g.CD81 and r.CLDN1 exist between permissive (Huh-7.5 and 293T) and nonpermissive (T84) targets, the cells were transduced to express the tagged proteins, and FRET analyses were completed. Since CD81 and CLDN1 preferentially localize at CJs in contrast to nonopposing regions of the PM, we quantified protein localization and compared FRETs at these cellular locations (Fig. 4). FRET occurred between g.CD81 and r.CLDN1 in all three cell lines, with comparable frequencies and distance estimates at both PM and CJs (Fig. 4). These data suggest that CD81 associates with CLDN1 in permissive and nonpermissive cell types.
FIG. 4.
Localization and FRET between fluorescently N terminus-tagged CD81 and CLDN1. The localizations of g.CD81, r.CLDN1, and r.CLDN1ΔC in Huh-7.5 (A), 293T (B), and T84 (C) cells were assessed by quantifying the fluorescence intensity at the nonjunctional PM (black bar), intracellular junctions (IC; white bar), and CJs (gray bar) of 50 transduced cells. Data are presented as the relative localization of the tagged protein in each cell line. A number of regions (Ntot) where CD81 and CLDN1 colocalize at the PM or CJs were selected for FRET analysis. The %FRET and the estimated distance between fluorophores (nm) are shown. The data presented are from a single experiment and is representative of two further experiments. *, P < 0.05.
The C terminus of CLDN1 has been reported to mediate interactions with junction adhesion molecule and zonula occludens-1 in the formation of TJ protein complexes (36, 71). To investigate if the C-terminal region of CLDN1 is important for associating with CD81, we generated an r.CLDN1 lacking the C-terminal region (r.CLDN1ΔC) and investigated its localization, association with CD81, and viral receptor activity. r.CLDN1ΔC colocalized with g.CD81 in Huh-7.5 and 293T at the PM and CJs. A greater quantity of intracellular protein was noted in 293T cells compared to full-length r.CLDN1, suggesting that the C-terminal region may have some localizing properties in 293T that are not apparent in Huh-7.5 cells (Fig. 4A and B). The %FRET between g.CD81 and r.CLDN1ΔC in Huh-7.5 and 293T cells was comparable to that observed for g.CD81 and r.CLDN1; however, the distance estimate(s) between CD81 and CLDN1ΔC at the PM was reduced compared to those at the CJs (Fig. 4A and B). Deletion of the C-terminal region reduced viral receptor activity by approximately 50%, with HCVpp infecting 293T cells expressing r.CLDN1 and r.CLDN1ΔC having specific infectivities of 26 × 104 and 13 × 104 RLU, respectively. In contrast, 293T cells expressing both forms of tagged CLDN1 were equally susceptible to infection by MLVpp (data not shown). These data suggest that the C-terminal region of CLDN1 is not critical for an association with CD81 in nonpolarized Huh-7.5 and 293T cells; however, this motif may regulate the distance(s) between CD81-CLDN1 complexes at the PM and may contribute to the reduced viral receptor activity.
Measurement of indirect FRET between CD81-CLDN1 in healthy and HCV-infected liver tissues.
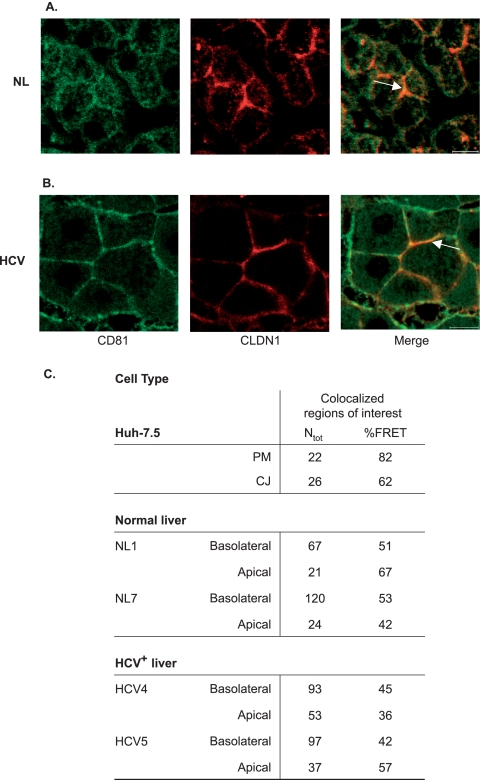
To investigate if our observations of FRET between CD81 and CLDN1 in cell lines are consistent with protein association in hepatocytes within liver tissue, we labeled liver biopsy specimens with CD81 and CLDN1 receptor-specific antibodies bearing fluorophores Alexa Fluor 488 and TRITC and assessed whether FRET occurred. For a control for this indirect method of FRET measurement, we stained Huh-7.5 cells with the same antibodies and measured FRET. The autofluorescence of liver tissue was subtracted from the specific antibody-dependent fluorescence. CD81 and CLDN1 showed some level of colocalization in hepatocytes within healthy (Fig. 5A) and HCV-infected (Fig. 5B) liver tissues. FRET was detected between receptor-specific antibodies bound to healthy (NL1 and NL7) and HCV-infected (HCV4 and HCV5) liver tissues, confirming CD81-CLDN1 association(s) (Fig. 5C).
FIG. 5.
Association of CD81 and CLDN1 in healthy and HCV-infected liver tissues. Healthy (NL; panel A) and HCV-infected (HCV; panel B) liver tissues were stained with antibodies specific for CD81 (M38) and CLDN1 (JAY.8) and visualized with anti-mouse Ig Alexa Fluor 488 (CD81; green) and anti-rabbit TRITC (CLDN1; red). Colocalized regions of interest at the apical and basolateral regions (indicated by arrows) were imaged by confocal microscopy and selected for FRET analysis. As a control, Huh-7.5 cells were stained with the same antibodies. The scale bar is 10 μm. (C) The number of regions analyzed (Ntot) and the %FRET are shown. Autofluorescence and background signals on the primary liver cells were corrected by using built-in software (Zeiss).
Hepatocytes are polarized cells with their PMs separated by TJs into apical (canalicular) and basolateral (sinusoidal) domains (76). As previously reported, CLDN1 expression was increased at the basolateral surface of hepatocytes in HCV-infected samples (Fig. 5B) (63). In healthy liver tissue, CD81 colocalized with CLDN1 preferentially at apical regions (Fig. 5A); however, this pattern was not apparent in the infected tissue, with proteins colocalizing at both cellular domains (Fig. 5B). %FRET values for anti-CD81 and anti-CLDN1 were comparable at apical and basolateral domains (Fig. 5C). In summary, these data suggest that CD81 and CLDN1 colocalize at both apical and basolateral domains of hepatocytes and that FRET occurs between specific antibodies at both sites, supporting an association between CD81 and CLDN1 in primary liver tissue.
Effect of HCV gps on CD81-CLDN1 association.
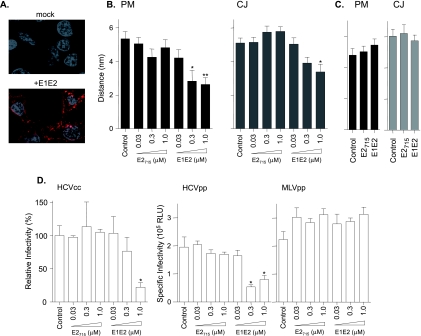
HCV encodes two gps, E1 and E2, which are critical for viral entry into cells (reviewed in reference 19). To investigate whether HCV gps modulate CD81-CLDN1 association(s), we utilized recombinant preparations of truncated E2715 and E1-E2 as a model for studying virus interaction with cell-surface-expressed coreceptors. E2715 and E1-E2 bound to Huh-7.5 cells, demonstrating a punctate staining pattern at the cell surface, with no detectable internalization (Fig. 6A). Huh-7.5 cells expressing g.CD81/r.CLDN1 were incubated with increasing concentrations of E2715 and E1-E2 for 1 h at 37°C, and the %FRET between CD81 and CLDN1 was assessed. E2715 had no effect on %FRET or CD81-CLDN1 estimated distances at PMs or CJs (Fig. 6B). In contrast, E1-E2 induced a dose-dependent decrease in the estimated distance between CD81-CLDN1 at both the PM and CJs with no significant change in %FRET (Fig. 6B). To investigate whether E2715 or E1-E2 modulates CD81-CD81 association, Huh-7.5 cells expressing g.CD81/r.CD81 were incubated with a saturating concentration of the gps. Neither antigen had an effect on CD81-CD81%FRET or distances (Fig. 6C). To investigate whether E1-E2 affects HCV entry, Huh-7.5 cells were incubated with recombinant gps for 1 h at 37°C and infected with HCVcc strain JFH-1 or HCVpp for 1 h in the presence or absence of the gps. E2715 had no effect on viral infectivity, whereas E1-E2 reduced HCVcc and HCVpp infection in a dose-dependent manner (Fig. 6D). Treatment of cells with E2715 or E1-E2 had no effect on MLVpp infectivity (Fig. 6D). These data demonstrate that HCV gps do not increase CD81-CLDN1 association in Huh-7.5 cells. However, the interaction of cells with E1-E2, but not E2715, reduced the estimated distance between CD81-CLDN1 proteins and HCV entry, suggesting that the stoichiometry of the coreceptors may be important during HCV entry.
FIG. 6.
Effect of HCV gps on FRET between fluorescently N terminus-tagged CD81 and CLDN1 and viral infectivity. (A) Huh-7.5 cells were incubated with mock or E1-E2 gps at 37°C for 1 h, and bound protein was visualized with anti-E2 1/39 and anti-rat TRITC (red). (B) Huh-7.5 cells expressing g.CD81/r.CLDN1 were incubated with increasing concentrations of E2715 or E1-E2 (0.03 to 1 μM) for 1 h at 37°C and fixed in ice-cold methanol, and areas of colocalization were selected for FRET analysis. FRET-inferred distances between CD81 and CLDN1 at the nonjunctional PM and CJs were determined. %FRET was unchanged by E2715 or E1-E2 treatments (data not shown); however, E1-E2 reduced the estimated distance between g.CD81 and r.CLDN1 (**, P < 0.05 [Dunn's test]). (C) Huh-7.5 cells expressing g.CD81/r.CD81 were incubated with a saturating concentration (1.0 μM) of E2715 or E1-E2 for 1 h at 37°C and fixed in ice-cold methanol, and areas of colocalization at the nonjunctional PM (black bars) and CJs (gray bars) were selected for FRET analysis. E2715 or E1-E2 treatment(s) had no detectable effect on %FRET and distance(s) between g.CD81 and r.CD81 in comparison to control values (data not shown). (D) Control or E2715- or E1-E2-treated cells were infected for 1 h with HCVcc JFH-1, HCVpp-H77, or MLVpp. Unbound virus was removed by washing, and cells were incubated for 72 h. For HCVcc JFH-1, results are the means from three replicate infections and expressed as relative infectivities compared to the infection of control cells; for HCVpp and MLVpp, specific infectivities are shown and represent the mean luciferase levels (relative light units [RLU]) determined from three replicate infections, with the average Env−pp value subtracted (420 RLU). Incubation with E1-E2 significantly reduced HCVcc and HCVpp relative infectivities (*, P < 0.05; **, P < 0.01 [Dunn's test]). The data presented are from a single experiment and are representative of two independent experiments.
Effect of anti-CD81 treatment on CD81-CD81 and CD81-CLDN1 association.
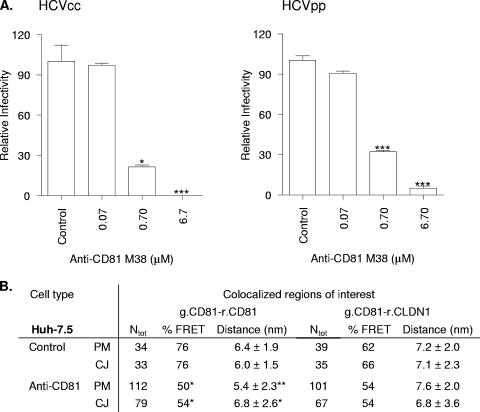
Several laboratories have reported that treatment of hepatoma cells with anti-CD81 MAbs inhibits HCV entry; however, the underlying mechanism of action is unknown. To assess whether the neutralizing CD81-specific MAb M38 modulates CD81 protein association(s), we studied the effect(s) of M38 on FRET between g.CD81-r.CD81 and g.CD81-r.CLDN1 in Huh-7.5 cells. Treatment of Huh-7.5 cells with MAb M38 inhibited HCVcc JFH-1 and HCVpp infection in a dose-dependent manner (Fig. 7A). A saturating concentration of M38 (6.7 μM) was incubated with Huh-7.5 cells expressing g.CD81/r.CD81 or g.CD81/r.CLDN1 for 1 h at 37°C, and the %FRET was determined. M38 reduced the %FRET between g.CD81 and r.CD81 by 30% at both the PM and CJs (Fig. 7B), suggesting that a proportion of tagged molecules were no longer in close enough proximity (<10 nm) for FRET to occur. In contrast, M38 had no effect on %FRET between g.CD81 and r.CLDN1 (Fig. 7B), suggesting differences in the reactivity or effect(s) of M38 binding to CD81 when associated with CD81 or CLDN1.
FIG. 7.
Effect of anti-CD81 on FRET between fluorescently N terminus-tagged CD81 and CLDN1 and viral infectivity. (A) Huh-7.5 cells were incubated with increasing concentrations of anti-CD81 MAb (M38) for 1 h at 37°C. Control and M38-treated cells were infected with HCVcc strain JFH-1 or HCVpp for 1 h, unbound virus was removed by washing, and cells were incubated at 37°C for 72 h. M38 significantly reduced HCVcc and HCVpp infectivity (*, P < 0.05 [Dunn's test]). Data are expressed as relative infectivities compared to control untreated cells and represent the means from three replicate infections. (B) Huh-7.5 cells expressing g.CD81/r.CD81 or g.CD81/r.CLDN1 were incubated with anti-CD81 M38 (2 μM) or isotype-matched control MAb for 1 h at 37°C at a concentration shown to saturate cell-surface-expressed CD81 by flow cytometry. Cells were fixed, and regions of colocalization at the nonjunctional PM and CJs were imaged by confocal microscopy. The numbers of colocalized regions analyzed (Ntot), the %FRET values, and the estimated distances between fluorescent proteins are shown. M38 significantly reduced the %FRET and estimated distances between CD81-CD81 (*, P < 0.05; **, P < 0.01; ***, P < 0.001 [Fisher's exact test and Mann Whitney test]).
Effect of HCV infection on CD81-CD81 and CD81-CLDN1 FRET.
To assess whether HCV infection alters coreceptor protein association(s), Huh-7.5 cells expressing g.CD81/r.CD81 or g.CD81/r.CLDN1 were infected with HCVcc JFH-1, and FRET was measured. HCV-infected cells were identified by expression of the nonstructural viral protein NS5A, and regions of CD81-CD81 and CD81-CLDN1 colocalization were selected for FRET analysis. HCV infection had no effect on the expression levels of the tagged coreceptors. Infection significantly reduced %FRET between CD81-CD81 at the PM and CJs and reduced the estimated distance between the molecules specifically at the PM (Table 2). In contrast, the %FRET and estimated distances between CD81-CLDN1 were unaltered following HCV infection.
TABLE 2.
Effect of HCV infection on FRET between fluorescently N terminus-tagged CD81 and CLDN1a
| Cell type | Colocalized region | Ntotb | %FRET | Distance (nm) |
|---|---|---|---|---|
| g.CD81/r.CD81 | ||||
| Uninfected | PM | 49 | 80 | 6.11 ± 2.04 |
| CJ | 44 | 84 | 6.19 ± 2.64 | |
| NS5A-positive | PM | 54 | 46 | 1.54 ± 2.70c |
| CJ | 156 | 64 | 5.10 ± 3.08 | |
| g.CD81/r.CLDN1 | ||||
| Uninfected | PM | 51 | 57 | 6.72 ± 2.49 |
| CJ | 47 | 60 | 6.98 ± 2.67 | |
| NS5A-positive | PM | 65 | 52 | 6.24 ± 3.44 |
| CJ | 121 | 58 | 5.58 ± 2.76 |
Huh-7.5 cells expressing g.CD81/r.CD81 or g.CD81/r.CLDN1 were infected with HCVcc JFH-1. At 72 h postinfection, naïve and infected cells were fixed and stained for NS5A, and regions of fluorescent protein colocalization at the PM and CJs were imaged by confocal microscopy for FRET analysis.
Ntot, the number of colocalized regions analyzed.
HCV infection significantly decreased the estimated distance between g.CD81 and r.CD81 at the PM (P < 0.001 [Mann-Whitney test]).
DISCUSSION
Confocal imaging and FRET analysis between fluorescence-tagged CD81 and CLDN1 molecules expressed in various cell types demonstrate protein association, consistent with the formation of coreceptor complexes. FRET between cell-surface-expressed g.CD81 (donor) and r.CLDN1 (acceptor) occurred in Huh-7.5 cells, 293T cells transduced to express CLDN1, and T84 colorectal carcinoma cells, suggesting that coreceptor localization and association is not unique to cells which support HCV entry. This is in contrast to a recent report by Yang and colleagues (77), who demonstrated minimal cell surface CLDN1 expression in nonpermissive HeLa cells, leading the authors to conclude that CLDN1 and CD81 localization predicts cellular permissiveness to HCV entry. In our experience, transduction of some cell types (HeLa and WIF-B) to express exogenous CLDN1 leads to an accumulation of the protein within cells and no detectable cell surface expression, consistent with an inability to support HCVpp entry (data not shown). However, interpreting data from cells in which CLDN1 demonstrates incorrect localization is fraught with difficulties. In contrast, the identification of nonpermissive cells with native patterns of CLDN1 and CD81 localization supports the conclusion that coreceptor localization and FRET-defined association does not predict cellular permissiveness to HCV infection.
The C-terminal region of CLDN1 is not critical for its association with CD81 in Huh-7.5 and 293T cells (Table 1), suggesting that the form of CLDN1 interacting with CD81 is not complexed with other TJ protein constituents. However, Huh-7.5 and 293T cells do not polarize or form TJs in cell culture (C. J. Mee, unpublished observations) and are not suitable for the study of the effects of polarization on CD81 and CLDN1 association and receptor activity. Recent experiments demonstrated that HCVpp and HCVcc can infect the colorectal adenocarcinoma Caco-2 cell line independent of the polarized nature of the cells, suggesting that CLDN coreceptor activity may be independent of its TJ function (55). Attempts to study CD81-CLDN1 FRET association in polarized Caco-2 cells have been technically challenging and have provided inconclusive data. Future experiments will need to address the role of cell polarization on CD81-CLDN1 association(s) and their sensitivity to inhibitors of various signaling pathways.
To investigate whether HCV gps promote CD81 and CLDN1 association, we used recombinant soluble E2715 and E1-E2 to model virus-receptor interactions. Both gp preparations bound to Huh-7.5 cells according to confocal imaging (Fig. 6A) and flow cytometry (data not shown). Neither antigen increased the %FRET between CD81 and CLDN1, suggesting that receptor complexes preexist within cells and that their formation or stability is not dependent upon or promoted by interaction(s) with the viral gps. This is in contrast to reports demonstrating that association between the HIV receptors CD4 and CCR-5 is dependent on the viral envelope gp (1, 31, 69, 78). The interaction of E1-E2, but not E2715, with Huh-7.5 cells reduced the estimated distance between CD81 and CLDN1 and inhibited HCV infection (Fig. 6). In contrast, saturating concentrations of E2715 and E1-E2 had no effect on %FRET or estimated distances between CD81-CD81, suggesting that the viral gps modulate CD81 association with CLDN1. We are unable to ascertain if this effect is regulated at the level of E1-E2 binding, since we cannot discriminate between E1-E2 binding to CD81 in association with CD81 and with CLDN1. The exact role of each gp in mediating receptor-dependent attachment and fusion of viral and cell membranes is unknown. A recent report demonstrating the presence of potential fusion peptides in both E1 and E2 (48) lends further support to the model that both gps are required for productive viral entry. There are several interpretations of the inhibitory effect(s) of E1-E2 on HCV infection. First, the gp-dependent reduction in CD81-CLDN1 FRET distances may reflect an induced change in the stoichiometry or distance between coreceptor molecules that is necessary for efficient HCV entry. Second, gp occupation of the CD81-CLDN1 complex may sterically block HCV interaction. Third, gp binding may lead to an internalization of receptor complexes. At the present, it is difficult to discriminate between these alternatives; however, current data do not support a gp-dependent internalization of the E1-E2-receptor complex (Fig. 6A).
Several publications have suggested that SR-BI may be the primary receptor defining HCV attachment to target cells (reviewed in reference 74). Our attempts to generate and express AcGFP- or DsRed-tagged SR-BI for FRET studies resulted in a series of molecules that accumulated within the cytoplasm and fail to express at the PM (Joe Grove, unpublished data). More-recent experiments with SR-BI fusion proteins with eGFP and mCherry added to the N terminus demonstrate PM expression; however, these fluorophores are not suitable for FRET studies, as they require dimerization to fluoresce. Future experiments will seek to investigate the localization and association of SR-BI with CD81 and CLDN1 in polarized and nonpolarized cell culture systems (55).
The principal site of HCV replication is believed to be hepatocytes within the liver. Hepatocytes are polarized, with TJs separating their PM into apical and basolateral domains (76). FRET occurred between hepatocyte-bound antibodies specific for CD81 and CLDN1 in healthy and HCV-infected liver tissues, suggesting that CD81 associates with CLDN1 at apical and basolateral surfaces (Fig. 5). However, the heterogeneity of coreceptor expression in hepatocytes and the limitations of indirect FRET methodology make comparison of CD81-CLDN1 FRET efficiencies between liver samples difficult to interpret (63).
Tetraspanins are four-transmembrane proteins that typically reside at the cell surface and assemble with themselves and other proteins to form tetraspanin-enriched microdomains (reviewed in reference 50). Multiple regions within the extracellular and transmembrane domains of CD81 have been reported to be important for oligomerization (15, 16, 41). Partner proteins for tetraspanins include integrins, Ig superfamily proteins, G protein-coupled receptors, and signaling enzymes (7, 37, 68). CLDN proteins similarly contain four transmembrane domains; however, the sequences and functions of the CLDN and tetraspanin families are quite distinct. CLDNs have been reported to form homophilic and heterophilic interactions at TJs between apposing cells (23, 61). The estimated distances between CD81-CLDN1 and CLDN1-CLDN1 of 5.5 to 7.0 nm at CJs are likely to reflect intracellular protein associations, as both donor and acceptor fluorophores are located intracellularly, and the average thickness of two opposing PMs is within the order of 10 to 12 nm (40).
Approaches to investigating tetraspanin-protein interactions have generally utilized mass spectroscopic analysis of immunoprecipitates from detergent-lysed cell preparations (49, 52, 66, 67). However, several reports suggest that microdomains isolated from detergent-lysed cells may not reflect the organization of protein-protein complexes in intact cells (reviewed in reference 46). Kovalenko et al. recently developed a cysteine cross-linking method to identify tetraspanin-protein associations and reported an interaction between CD9 and CD81 with CLDN1 (42). Given the reported interaction between CD81 and CD9 (reviewed in reference 37), we cannot discriminate whether the FRET-determined association(s) between CD81 and CLDN1 is direct or mediated via CD9. However, Huh-7.5 cells express minimal levels of CD9, transduction to overexpress CD9 has no effect on HCV entry, and treatment with anti-CD9 antibodies has no effect on HCV infection, suggesting that CD9 has no role in the HCV entry process (33, 81; A. Jennings, unpublished data). Our experiments to coprecipitate CD81 and CLDN1 from Huh-7.5 and 293T cells have been inconclusive and may reflect the specific association of the coreceptors at the PM, where intracellular protein interactions may mask the detection of protein associations at the cell surface. It is important to note that techniques to cross-link cell surface proteins involve the chilling of cells on ice and that recent experiments demonstrate that this low-temperature treatment reduces coreceptor expression at the PM, making these experiments difficult to interpret (M. Farquhar, unpublished data).
Treatment of Huh-7.5 cells with the neutralizing anti-CD81 MAb M38 reduced the %FRET between CD81-CD81 complexes and had a negligible effect on the %FRET between CD81 and CLDN1 (Fig. 7), suggesting that M38 can discriminate between CD81 in association with CD81 and that in association with CLDN1. Further investigation will require the generation of fluorescently labeled M38 to study its interaction with CD81 in discrete protein complexes. Current data suggest that anti-CD81 antibodies may inhibit HCV infection through mechanisms more complex than simple blocking of receptor-virus interactions, involving the reorganization of CD81 in the PM.
In summary, we have demonstrated that FRET occurs between CD81- and CLDN1-tagged molecules in cultured cells, consistent with protein complex association. The colocalization of CD81 and CLDN1 in liver tissue (76) and indirect FRET between receptor-specific antibodies presented here lend further support for the presence of receptor complexes in polarized cells that localize beyond the apically positioned TJs. Recent experiments studying coreceptor localization in polarized Caco-2 cells demonstrate CLDN1 expression beyond the lateral apical cell junctions, in agreement with our observations with human liver tissue (55, 63). These data support a model in which HCV may utilize forms of CLDN1 that are not associated with TJs. Perturbation of %FRET and estimated distances between CD81-CD81 and CD81-CLDN1 with HCV E1-E2 gps, anti-CD81 MAb M38, and HCV infection suggest that these complexes may have distinct role(s) in the viral entry process and offer new targets for antiviral intervention.
Supplementary Material
Acknowledgments
We thank Takaji Wakita for JFH-1, Charles Rice for Huh-7.5 cells and anti-NS5A 9E10 MAb, Adrian Thrasher for CSGW, Paul Bieniasz for HIV gag-pol plasmid, and Fedor Berditchevski for anti-CD81 MAb M38. We thank Joe Grove and Adam Jennings for permission to cite their unpublished work.
This work was supported by PHS grant AI50798, the MRC, and the Wellcome Trust.
Footnotes
Published ahead of print on 12 March 2008.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Baker, A. M., A. Sauliere, G. Gaibelet, B. Lagane, S. Mazeres, M. Fourage, F. Bachelerie, L. Salome, A. Lopez, and F. Dumas. 2007. CD4 interacts constitutively with multiple CCR5 at the plasma membrane of living cells: a vrFRAP approach. J. Biol. Chem. 28235163-35168. [DOI] [PubMed] [Google Scholar]
- 2.Barth, H., C. Schafer, M. I. Adah, F. Zhang, R. J. Linhardt, H. Toyoda, A. Kinoshita-Toyoda, T. Toida, T. H. Van Kuppevelt, E. Depla, F. Von Weizsacker, H. E. Blum, and T. F. Baumert. 2003. Cellular binding of hepatitis C virus envelope glycoprotein E2 requires cell surface heparan sulfate. J. Biol. Chem. 27841003-41012. [DOI] [PubMed] [Google Scholar]
- 3.Barth, H., E. Schnober, F. Zhang, R. Linhardt, E. Depla, B. Boson, F. Cosset, A. Patel, H. Blum, and T. Baumert. 2006. Viral and cellular determinants of the hepatitis C virus envelope-heparan sulfate interaction. J. Virol. 8010579-10590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barton, E. S., J. C. Forrest J. L. Connolly, J. D. Chappell, Y. Liu, F. J. Schnell, A. Nusrat, C. A. Parkos, and T. S. Dermody. 2001. Junction adhesion molecule is a receptor for reovirus. Cell 104441-451. [DOI] [PubMed] [Google Scholar]
- 5.Bartosch, B., J. Dubuisson, and F. L. Cosset. 2003. Infectious hepatitis C virus pseudo-particles containing functional E1-E2 envelope protein complexes. J. Exp. Med. 197633-642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bartosch, B., A. Vitelli, C. Granier, C. Goujon, J. Dubuisson, S. Pascale, E. Scarselli, R. Cortese, A. Nicosia, and F. L. Cosset. 2003. Cell entry of hepatitis C virus requires a set of co-receptors that include the CD81 tetraspanin and the SR-B1 scavenger receptor. J. Biol. Chem. 27841624-41630. [DOI] [PubMed] [Google Scholar]
- 7.Berditchevski, F. 2001. Complexes of tetraspanins with integrins: more than meets the eye. J. Cell Sci. 1144143-4151. [DOI] [PubMed] [Google Scholar]
- 8.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for coxsackie B viruses and adenoviruses 2 and 5. Science 2751320-1323. [DOI] [PubMed] [Google Scholar]
- 9.Berney, C., and G. Danuser. 2003. FRET or no FRET: a quantitative comparison. Biophys. J. 843992-4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Besnier, C., Y. Takeuchi, and G. Towers. 2002. Restriction of lentivirus in monkeys. Proc. Natl. Acad. Sci. USA 9911920-11925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blight, K. J., J. A. McKeating, and C. M. Rice. 2002. Highly permissive cell lines for subgenomic and genomic hepatitis C virus RNA replication. J. Virol. 7613001-13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bykova, E. A., X. D. Zhang, T. Y. Chen, and J. Zheng. 2006. Large movement in the C terminus of CLC-0 chloride channel during slow gating. Nat. Struct. Mol. Biol. 131115-1119. [DOI] [PubMed] [Google Scholar]
- 13.Cai, D., A. D. Hoppe, J. A. Swanson, and K. J. Verhey. 2007. Kinesin-1 structural organization and conformational changes revealed by FRET stoichiometry in live cells. J. Cell Biol. 17651-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Catanese, M., R. Graziani, T. von Hahn, M. Moreau, T. Huby, G. Paonessa, C. Santini, A. Luzzago, C. Rice, R. Cortese, A. Vitelli, and A. Nicosia. 2007. High-avidity monoclonal antibodies against the human scavenger class B type I receptor efficiently block hepatitis C virus infection in the presence of high-density lipoprotein. J. Virol. 818063-8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charrin, S., F. Le Naour, V. Labas, M. Billard, J. P. Le Caer, J. F. Emile, M. A. Petit, C. Boucheix, and E. Rubinstein. 2003. EWI-2 is a new component of the tetraspanin web in hepatocytes and lymphoid cells. Biochem. J. 373409-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charrin, S., F. Le Naour, M. Oualid, M. Billard, G. Faure, S. M. Hanash, C. Boucheix, and E. Rubinstein. 2001. The major CD9 and CD81 molecular partner. Identification and characterization of the complexes. J. Biol. Chem. 27614329-14337. [DOI] [PubMed] [Google Scholar]
- 17.Chen, H., H. L. Puhl III, S. V. Koushik, S. S. Vogel, and S. R. Ikeda. 2006. Measurement of FRET efficiency and ratio of donor to acceptor concentration in living cells. Biophys. J. 91L39-L41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choo, Q. L., G. Kuo, R. Ralston, A. Weiner, D. Chien, G. Van Nest, J. Han, K. Berger, K. Thudium, C. Kuo, et al. 1994. Vaccination of chimpanzees against infection by the hepatitis C virus. Proc. Natl. Acad. Sci. USA 911294-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cocquerel, L., C. Voisset, and J. Dubuisson. 2006. Hepatitis C virus entry: potential receptors and their biological functions. J. Gen. Virol. 871075-1084. [DOI] [PubMed] [Google Scholar]
- 20.Cormier, E., F. Tsamis, F. Kajumo, R. Durso, J. Gardner, and T. Dragic. 2004. CD81 is an entry coreceptor for hepatitis C virus. Proc. Natl. Acad. Sci. USA 1017270-7274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coyne, C. B., and J. M. Bergelson. 2005. CAR: a virus receptor within the tight junction. Adv. Drug Deliv. Rev. 57869-882. [DOI] [PubMed] [Google Scholar]
- 22.Coyne, C. B., and J. M. Bergelson. 2006. Virus-induced Abl and Fyn kinase signals permit coxsackievirus entry through epithelial tight junctions. Cell 124119-131. [DOI] [PubMed] [Google Scholar]
- 23.Daugherty, B. L., C. Ward, T. Smith, J. D. Ritzenthaler, and M. Koval. 2007. Regulation of heterotypic claudin compatibility. J. Biol. Chem. 28230005-30013. [DOI] [PubMed] [Google Scholar]
- 24.Drummer, H. E., A. Maerz, and P. Poumbourios. 2003. Cell surface expression of functional hepatitis C virus E1 and E2 glycoproteins. FEBS Lett. 546385-390. [DOI] [PubMed] [Google Scholar]
- 25.Evans, M., T. von Hahn, D. Tscherne, A. Syder, M. Panis, B. Wolk, T. Hatziioannou, J. McKeating, P. Bieniasz, and C. Rice. 2007. Claudin-1 is a hepatitis C virus co-receptor required for a late step in entry. Nature 446801-805. [DOI] [PubMed] [Google Scholar]
- 26.Flint, M., J. M. Thomas, C. M. Maidens, C. Shotton, S. Levy, W. S. Barclay, and J. A. McKeating. 1999. Functional analysis of cell surface-expressed hepatitis C virus E2 glycoprotein. J. Virol. 736782-6790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Flint, M., T. von Hahn, J. Zhang, M. Farquhar, C. Jones, P. Balfe, C. Rice, and J. McKeating. 2006. Diverse CD81 proteins support hepatitis C virus infection. J. Virol. 8011331-11342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Förster, T. 1948. Intermolecular energy migration and fluorescence. Ann. Physik 255-75. [Google Scholar]
- 29.Furuse, M., K. Fujita, T. Hiiragi, K. Fujimoto, and S. Tsukita. 1998. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J. Cell Biol. 1411539-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Furuse, M., H. Sasaki, K. Fujimoto, and S. Tsukita. 1998. A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J. Cell Biol. 143391-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaibelet, G., T. Planchenault, S. Mazeres, F. Dumas, F. Arenzana-Seisdedos, A. Lopez, B. Lagane, and F. Bachelerie. 2006. CD4 and CCR5 constitutively interact at the plasma membrane of living cells: a confocal fluorescence resonance energy transfer-based approach. J. Biol. Chem. 28137921-37929. [DOI] [PubMed] [Google Scholar]
- 32.Gordon, G. W., G. Berry, X. H. Liang, B. Levine, and B. Herman. 1998. Quantitative fluorescence resonance energy transfer measurements using fluorescence microscopy. Biophys. J. 742702-2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grove, J., T. Huby, Z. Stamataki, T. Vanwolleghem, P. Meuleman, M. Farquhar, A. Schwarz, M. Moreau, J. S. Owen, G. Leroux-Roels, P. Balfe, and J. A. McKeating. 2007. Scavenger receptor BI and BII expression levels modulate hepatitis C virus infectivity. J. Virol. 813162-3169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hachet-Haas, M., N. Converset, O. Marcha, H. Matthes, S. Gioria, J.-L. Galzi, and S. Lecat. 2006. FRET and colocalization analyzer—a method to validate measurements of sensitized emission FRET acquired by confocal microscopy and available as an ImageJ Plug-in. Microsc. Res. Tech. 69941-956. [DOI] [PubMed] [Google Scholar]
- 35.Hanson, M. R., and R. H. Kohler. 2001. GFP imaging: methodology and application to investigate cellular compartmentation in plants. J. Exp. Bot. 52529-539. [PubMed] [Google Scholar]
- 36.Heiskala, M., P. A. Peterson, and Y. Yang. 2001. The roles of claudin superfamily proteins in paracellular transport. Traffic 293-98. [DOI] [PubMed] [Google Scholar]
- 37.Hemler, M. E. 2003. Tetraspanin proteins mediate cellular penetration, invasion, and fusion events and define a novel type of membrane microdomain. Annu. Rev. Cell Dev. Biol. 19397-422. [DOI] [PubMed] [Google Scholar]
- 38.Hsu, M., J. Zhang, M. Flint, C. Logvinoff, C. Cheng-Mayer, C. Rice, and J. McKeating. 2003. Hepatitis C virus glycoproteins mediate pH-dependent cell entry of pseudotyped retroviral particles. Proc. Natl. Acad. Sci. USA 1007271-7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kapadia, S., H. Barth, T. Baumert, J. McKeating, and F. Chisari. 2007. Initiation of hepatitis C virus infection is dependent on cholesterol and cooperativity between CD81 and scavenger receptor B type I. J. Virol. 81374-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karakotchian, M., and I. S. Fraser. 2007. An ultrastructural study of microvascular inter-endothelial tight junctions in normal endometrium. Micron 38632-636. [DOI] [PubMed] [Google Scholar]
- 41.Kitadokoro, K., D. Bordo, G. Galli, R. Petracca, F. Falugi, S. Abrignani, G. Grandi, and M. Bolognesi. 2001. CD81 extracellular domain 3D structure: insight into the tetraspanin superfamily structural motifs. EMBO J. 2012-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kovalenko, O. V., X. H. Yang, and M. E. Hemler. 2007. A novel cysteine crosslinking method reveals a direct association between claudin-1 and tetraspanin CD9. Mol. Cell. Proteomics 61855-1867. [DOI] [PubMed] [Google Scholar]
- 43.Krause, G., L. Winkler, S. L. Mueller, R. F. Haseloff, J. Piontek, and I. E. Blasig. 2008. Structure and function of claudins. Biochim. Biophys. Acta 1778631-645. [DOI] [PubMed] [Google Scholar]
- 44.Krieger, M. 2001. Scavenger receptor class B type I is a multiligand HDL receptor that influences diverse physiological systems. J. Clin. Investig. 108793-797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuiken, C., K. Yusim, L. Boykin, and R. Richardson. 2005. The Los Alamos hepatitis C sequence database. Bioinformatics 21379-384. [DOI] [PubMed] [Google Scholar]
- 46.Lagerholm, B. C., G. E. Weinreb, K. Jacobson, and N. L. Thompson. 2005. Detecting microdomains in intact cell membranes. Annu. Rev. Phys. Chem. 56309-336. [DOI] [PubMed] [Google Scholar]
- 47.Lavillette, D., Y. Morice, G. Germanidis, P. Donot, A. Soulier, E. Pagkalos, G. Sakellariou, L. Intrator, B. Bartosch, J. Pawlotsky, and F. Cosset. 2005. Human serum facilitates hepatitis C virus infection, and neutralizing responses inversely correlate with viral replication kinetics at the acute phase of hepatitis C virus infection. J. Virol. 796023-6034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lavillette, D., E. Pecheur, P. Donot, J. Fresquet, J. Molle, R. Corbau, M. Dreux, F. Penin, and F. Cosset. 2007. Characterization of fusion determinants points to the involvement of three discrete regions of both E1 and E2 glycoproteins in the membrane fusion process of hepatitis C virus. J. Virol. 818752-8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Le Naour, F., M. Andre, C. Greco, M. Billard, B. Sordat, J. F. Emile, F. Lanza, C. Boucheix, and E. Rubinstein. 2006. Profiling of the tetraspanin web of human colon cancer cells. Mol. Cell Proteomics 5845-857. [DOI] [PubMed] [Google Scholar]
- 50.Levy, S., and T. Shoham. 2005. The tetraspanin web modulates immune-signalling complexes. Nat. Rev. Immunol. 5136-148. [DOI] [PubMed] [Google Scholar]
- 51.Lindenbach, B., M. Evans, A. Syder, B. Wolk, T. Tellinghuisen, C. Liu, T. Maruyama, R. Hynes, D. Burton, J. McKeating, and C. Rice. 2005. Complete replication of hepatitis C virus in cell culture. Science 309623-626. [DOI] [PubMed] [Google Scholar]
- 52.Little, K. D., M. E. Hemler, and C. S. Stipp. 2004. Dynamic regulation of a GPCR-tetraspanin-G protein complex on intact cells: central role of CD81 in facilitating GPR56-Galpha q/11 association. Mol. Biol. Cell 152375-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Makino, A., M. Shimojima, T. Miyazawa, K. Kato, Y. Tohya, and H. Akashi. 2006. Junctional adhesion molecule 1 is a functional receptor for feline calicivirus. J. Virol. 804482-4490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McKeating, J. A., L. Q. Zhang, C. Logvinoff, M. Flint, J. Zhang, J. Yu, D. Butera, D. D. Ho, L. B. Dustin, C. M. Rice, and P. Balfe. 2004. Diverse hepatitis C virus glycoproteins mediate viral infection in a CD81-dependent manner. J. Virol. 788496-8505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mee, C. J., J. Grove, H. J. Harris, K. Hu, P. Balfe, and J. A. McKeating. 2008. Effect of cell polarization on hepatitis C virus viral entry. J. Virol. 82461-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mekler, V. M. 1994. A photochemical technique to enhance sensitivity of detection of fluorescence resonance energy transfer. Photochem. Photobiol. 59615-620. [Google Scholar]
- 57.Molina, S., V. Castet, C. Fournier-Wirth, L. Pichard-Garcia, R. Avner, D. Harats, J. Roitelman, R. Barbaras, P. Graber, P. Ghersa, M. Smolarsky, A. Funaro, F. Malavasi, D. Larrey, J. Coste, J. Fabre, A. Sa-Cunha, and P. Maurel. 2007. The low-density lipoprotein receptor plays a role in the infection of primary human hepatocytes by hepatitis C virus. J. Hepatol. 46411-419. [DOI] [PubMed] [Google Scholar]
- 58.Oliveria, S. F., L. L. Gomez, and M. L. Dell'Acqua. 2003. Imaging kinase-AKAP79-phosphatase scaffold complexes at the plasma membrane in living cells using FRET microscopy. J. Cell Biol. 160101-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pileri, P., Y. Uematsu, S. Compagnoli, G. Galli, F. Falugi, R. Petracca, A. J. Weiner, M. Houghton, D. Rosa, G. Grandi, and S. Abrignani. 1998. Binding of hepatitis C virus to CD81. Science 282938-941. [DOI] [PubMed] [Google Scholar]
- 60.Piontek, J., L. Winkler, H. Wolburg, S. L. Muller, N. Zuleger, C. Piehl, B. Wiesner, G. Krause, and I. E. Blasig. 2008. Formation of tight junction: determinants of homophilic interaction between classic claudins. FASEB J. 22146-158. [DOI] [PubMed] [Google Scholar]
- 61.Pummi, K. P., A. M. Heape, R. A. Grenman, J. T. Peltonen, and S. A. Peltonen. 2004. Tight junction proteins ZO-1, occludin, and claudins in developing and adult human perineurium. J. Histochem. Cytochem. 521037-1046. [DOI] [PubMed] [Google Scholar]
- 62.Reynolds, G. M., L. J. Billingham, L. J. Gray, J. R. Flavell, S. Najafipour, J. Crocker, P. Nelson, L. S. Young, and P. G. Murray. 2002. Interleukin 6 expression by Hodgkin/Reed-Sternberg cells is associated with the presence of ‘B’ symptoms and failure to achieve complete remission in patients with advanced Hodgkin's disease. Br. J. Haematol 118195-201. [DOI] [PubMed] [Google Scholar]
- 63.Reynolds, G. M., H. Harris, A. Jennings, K. Hu, J. Grove, P. F. Lalor, D. H. Adams, P. Balfe, S. G. Hubscher, and J. A. McKeating. 2008. Hepatitis C virus receptor expression in normal and diseased liver tissue. Hepatology 47418-427. [DOI] [PubMed] [Google Scholar]
- 64.Scarselli, E., H. Ansuini, R. Cerino, R. M. Roccasecca, S. Acali, G. Filocamo, C. Traboni, A. Nicosia, R. Cortese, and A. Vitelli. 2002. The human scavenger receptor class B type I is a novel candidate receptor for the hepatitis C virus. EMBO J. 215017-5025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sekar, R. B., and A. Periasamy. 2003. Fluorescence resonance energy transfer (FRET) microscopy imaging of live cell protein localizations. J. Cell Biol. 160629-633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Stipp, C. S., T. V. Kolesnikova, and M. E. Hemler. 2001. EWI-2 is a major CD9 and CD81 partner and member of a novel Ig protein subfamily. J. Biol. Chem. 27640545-40554. [DOI] [PubMed] [Google Scholar]
- 67.Stipp, C. S., D. Orlicky, and M. E. Hemler. 2001. FPRP, a major, highly stoichiometric, highly specific CD81- and CD9-associated protein. J. Biol. Chem. 2764853-4862. [DOI] [PubMed] [Google Scholar]
- 68.Tarrant, J. M., L. Robb, A. B. van Spriel, and M. D. Wright. 2003. Tetraspanins: molecular organisers of the leukocyte surface. Trends Immunol. 24610-617. [DOI] [PubMed] [Google Scholar]
- 69.Toth, P. T., D. Ren, and R. J. Miller. 2004. Regulation of CXCR4 receptor dimerization by the chemokine SDF-1alpha and the HIV-1 coat protein gp120: a fluorescence resonance energy transfer (FRET) study. J. Pharmacol. Exp. Ther. 3108-17. [DOI] [PubMed] [Google Scholar]
- 70.Tscherne, D., C. Jones, M. Evans, B. Lindenbach, J. McKeating, and C. Rice. 2006. Time- and temperature-dependent activation of hepatitis C virus for low-pH-triggered entry. J. Virol. 801734-1741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Van Itallie, C. M., and J. M. Anderson. 2006. Claudins and epithelial paracellular transport. Annu. Rev. Physiol. 68403-429. [DOI] [PubMed] [Google Scholar]
- 72.Van Munster, E. B., G. J. Kremers, M. J. Adjobo-Hermans, and T. W. Gadella, Jr. 2005. Fluorescence resonance energy transfer (FRET) measurement by gradual acceptor photobleaching. J. Microsc. 218253-262. [DOI] [PubMed] [Google Scholar]
- 73.Voisset, C., N. Callens, E. Blanchard, A. Op De Beeck, J. Dubuisson, and N. Vu-Dac. 2005. High density lipoproteins facilitate hepatitis C virus entry through the scavenger receptor class B type I. J. Biol. Chem. 2807793-7799. [DOI] [PubMed] [Google Scholar]
- 74.von Hahn, T., and C. M. Rice. 2008. Hepatitis C virus entry. J. Biol. Chem. 2833689-3693. [DOI] [PubMed] [Google Scholar]
- 75.Wakita, T., T. Pietschmann, T. Kato, T. Date, M. Miyamoto, Z. Zhao, K. Murthy, A. Habermann, H. G. Krausslich, M. Mizokami, R. Bartenschlager, and T. J. Liang. 2005. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med. 11791-796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang, L., B. J. 2004. The maintenance and generation of membrane polarity in hepatocytes. Hepatology 39892-899. [DOI] [PubMed] [Google Scholar]
- 77.Yang, W., C. Qiu, N. Biswas, J. Jin, S. C. Watkins, R. C. Montelaro, C. B. Coyne, and T. Wang. 2008. Correlation of the tight junction-like distribution of claudin-1 to the cellular tropism of HCV. J. Biol. Chem. 2838643-8653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yi, L., J. Fang, N. Isik, J. Chim, and T. Jin. 2006. HIV gp120-induced interaction between CD4 and CCR5 requires cholesterol-rich microenvironments revealed by live cell fluorescence resonance energy transfer imaging. J. Biol. Chem. 28135446-35453. [DOI] [PubMed] [Google Scholar]
- 79.Zal, T., and N. R. Gascoigne. 2004. Photobleaching-corrected FRET efficiency imaging of live cells. Biophys. J. 863923-3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zeisel, M. B., S. Fafi-Kremer, I. Fofana, H. Barth, F. Stoll-Keller, M. Doffoel, and T. F. Baumert. 2007. Neutralizing antibodies in hepatitis C virus infection. World J. Gastroenterol. 134824-4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang, J., G. Randall, A. Higginbottom, P. Monk, C. M. Rice, and J. A. McKeating. 2004. CD81 is required for hepatitis C virus glycoprotein-mediated viral infection. J. Virol. 781448-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zheng, A., F. Yuan, Y. Li, F. Zhu, P. Hou, J. Li, X. Song, M. Ding, and H. Deng. 2007. Claudin-6 and claudin-9 function as additional coreceptors for hepatitis C virus. J. Virol. 8112465-12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhong, J., P. Gastaminza, G. Cheng, S. Kapadia, T. Kato, D. R. Burton, S. F. Wieland, S. L. Uprichard, T. Wakita, and F. V. Chisari. 2005. Robust hepatitis C virus infection in vitro. Proc. Natl. Acad. Sci. USA 1029294-9299. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.