Abstract
Cytotoxic T-lymphocyte (CTL) responses frequently select for immunodeficiency virus mutations that result in escape from CTL recognition with viral fitness costs. The replication in vivo of such viruses carrying not single but multiple escape mutations in the absence of the CTL pressure has remained undetermined. Here, we have examined the replication of simian immunodeficiency virus (SIV) with five gag mutations selected in a macaque possessing the major histocompatibility complex haplotype 90-120-Ia after its transmission into 90-120-Ia-negative macaques. Our results showed that even such a “crippled” SIV infection can result in persistent viral replication, multiple reversions, and AIDS progression.
Virus-specific CD8+ cytotoxic T-lymphocyte (CTL) responses exert a suppressive effect on human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV) replication (1, 10, 15, 21, 27). Under the CTL pressure, viral mutations resulting in viral escape from CTL recognition are frequently selected for, with viral fitness costs (2, 5, 8, 9, 12, 16, 19, 20, 24, 25, 26, 28). The transmission of the virus carrying a CTL escape mutation with lower viral fitness between major histocompatibility complex class I (MHC-I)-mismatched individuals can result in reversion of the mutation due to the absence of the CTL pressure (7, 14, 16, 17, 18). Such CTL escape mutations and their reversions have been suggested to be involved in viral evolution (3, 11, 13, 23).
We have developed a prophylactic vaccine using a Sendai virus vector expressing SIVmac239 Gag and shown its protective efficacy against SIVmac239 challenge in a group of Burmese rhesus macaques (Macaca mulatta) possessing MHC-I haplotype 90-120-Ia (20). In these vaccinated macaques that are controlling SIVmac239 replication, Gag206-216 epitope-specific CTL responses exerted strong selective pressure on the virus, and rapid selection of a mutant escaping from this CTL was observed at week 5 postchallenge. The virus, SIVmac239Gag216S, with this CTL escape mutation, GagL216S, leading to a substitution from leucine (L) to serine (S) at amino acid (aa) 216 in Gag showed lower replicative ability than the wild type (14, 20). Two of these vaccinees (macaques V3 and V5) showed an accumulation of additional viral CTL escape mutations in gag during the period of viral control and then the reappearance of plasma viremia around week 60 after SIVmac239 challenge (12). The SIV carrying these multiple CTL escape mutations showed lower replicative ability in vitro than the SIV carrying the single GagL216S mutation.
How such viruses with multiple CTL escape mutations replicate and evolve in the absence of the CTL pressure has not yet been well determined, while the reversion of CTL escape mutations has previously been shown by the transmission of viruses with single escape mutations (7, 14, 18). In the present study, we have examined the replication, in the absence of the CTL pressure in 90-120-Ia-negative macaques, of the SIV with multiple gag CTL escape mutations that were accumulated in a 90-120-Ia-positive macaque.
The induction of Gag206-216-specific CTL, Gag241-249-specific CTL, and Gag373-380-specific CTL responses has previously been observed after SIVmac239 challenge in 90-120-Ia-positive macaques (12). The 90-120-Ia-positive vaccinees V5 and V3 showed rapid selection of the GagL216S mutation (Gag206-216 CTL escape mutation) and then of an additional two mutations resulting in escape from Gag241-249-specific CTL and Gag373-380-specific CTL recognition, respectively, during the period of viral control. These were a Gag241-249 CTL escape mutation leading to a GagD244E (aspartic acid [D] to glutamic acid [E] at aa 244 in Gag) substitution and a Gag373-380 CTL escape mutation leading to GagA373T (alanine [A] to threonine [T] at aa 373) in vaccinee V5 or GagV375A (valine [V] to A at aa 375) or GagP376S (proline [P] to S at aa 376) in vaccinee V3. Viruses at the reappearance of viremia had one or two additional mutations in gag, GagI247L (isoleucine [I] to L at aa 247) and GagA312V (A to V at aa 312) in vaccinee V5 or GagP172S (P to S at aa 172) or GagV145A (V to A at aa 145) in vaccinee V3. All of these mutations except for the Gag373-380 CTL escape mutations resulted in amino acid changes in the Gag CA. We constructed molecular clones of SIVs with these gag mutations (12). The SIVs with three CTL escape mutations (Gag206-216, Gag241-249, and Gag373-380 CTL escape mutations) were referred to as group Q SIV mutants, and the SIVs with four or five gag mutations selected at the reappearance of viremia as group R SIV mutants. These group Q and R SIV mutants both showed lower replicative ability in vitro than SIVmac239Gag216S, while in the competition assay between groups Q and R, the viral replicative ability was not significantly affected by the GagP172S or GagV145A mutation but was reduced by the addition of the GagI247L and GagA312V mutations (12). These results do not support the possibility of compensation for loss of viral fitness from these mutations (4, 6). In the present study, we have examined the in vivo replication of the SIV carrying five gag mutations, GagL216S, GagD244E, GagI247L, GagA312V, and GagA373T, selected in macaque V5 at the reappearance of viremia, which was assumed to show the lowest replicative ability among group Q and R SIV mutants. The macaques were maintained in accordance with the guidelines for animal experiments performed at the National Institute of Infectious Diseases (22).
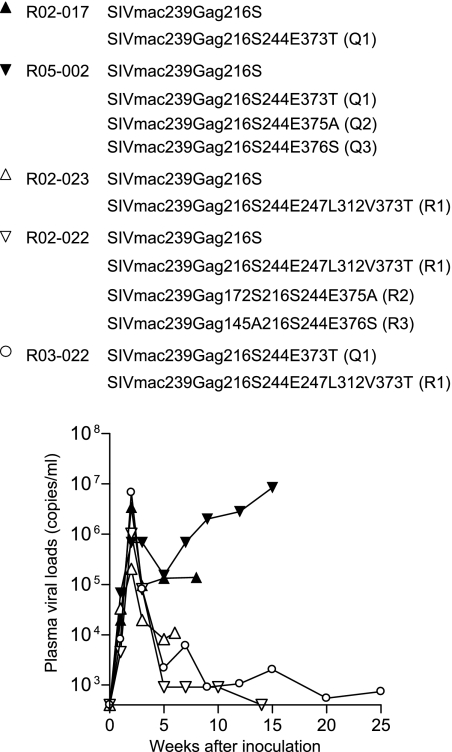
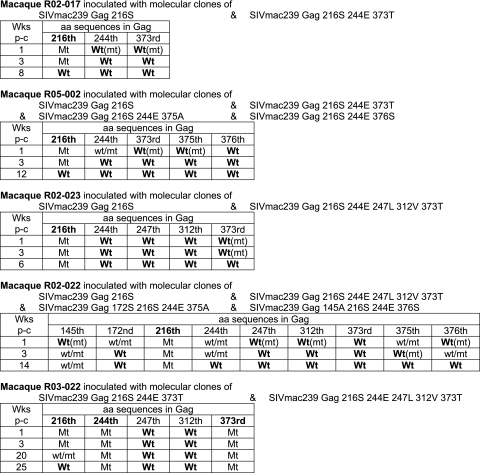
We first compared the in vivo replication abilities of the SIV with a single GagL216S mutation and the SIVs with multiple CTL escape mutations in 90-120-Ia-negative macaques (Fig. 1). In the competition between SIVmac239Gag216S and group Q SIV mutants, macaque R02-017 was coinoculated intramuscularly with molecular-clone DNAs of SIVmac239Gag216S and SIVmac239Gag216S244E373T and macaque R05-002 with molecular-clone DNAs of SIVmac239Gag216S and all three group Q SIV mutants. The results of the analysis of plasma viral gag genome sequences (Fig. 2) showed selection of SIVmac239Gag216S; i.e., all the mutations other than GagL216S became undetectable in 3 weeks postinoculation, indicating lower replicative abilities in vivo of group Q SIV mutants than of SIVmac239Gag216S, as indicated previously by in vitro competition (12). Further analysis revealed reversion of the selected GagL216S mutation to the wild-type sequence in a few months.
FIG. 1.
Plasma viral loads of macaques used for in vivo competition assay (SIV gag RNA copies/ml plasma) after inoculation with SIV molecular-clone DNAs. Animals received 10 mg in total of DNAs consisting of an equal amount of each DNA; i.e., macaques R02-017, R02-023, and R03-022 were inoculated with 5 mg of each DNA, and macaques R05-002 and R02-022 with 2.5 mg of each DNA. Plasma viral loads were determined as described previously (20).
FIG. 2.
Dominant viral genome sequences in competition assay. A gag DNA fragment was amplified from plasma RNA by reverse transcription and nested PCR and sequenced as described previously (20). The amino acid sequences at the positions where mutations were included in the inoculums are shown. Q and R groups of SIV mutants are described in the text. Wt, only the wild-type sequence was detected; Wt(mt), the wild-type sequence was dominant but the mutant was detectable (the mutant/wild-type ratio was less than 1/4); wt/mt, the wild type and the mutant were detected equally; Mt(wt), the mutant was dominant but the wild type was detectable (the wild-type/mutant ratio was less than 1/4); Mt, only the mutant was detected. Other than the residues indicated in this figure, no dominant mutation resulting in an amino acid change was detected in the gag region in macaque R02-017, R05-002, R02-023, or R02-022, but macaque R03-022 showed one amino acid change resulting in a GagV375M substitution at weeks 20 and 25. p-c, postchallenge.
In the competition between SIVmac239Gag216S and group R SIV mutants, macaque R02-023, coinoculated with molecular clone DNAs of SIVmac239Gag216S and SIVmac239Gag216S244E247L312V373T, showed selection of the former (Fig. 2). This macaque was euthanized at week 6 before exhibiting reversion of the GagL216S mutation. In macaque R02-022, coinoculated with molecular clone DNAs of SIVmac239Gag216S and all three group R SIV mutants, almost all mutations other than GagL216S became undetectable rapidly but the GagV145A mutation was detected even at week 14. The GagL216S mutation was still dominant without reversion at week 14, and plasma viremia became undetectable after week 14 in this macaque. Both cases indicated a lower replicative ability in vivo of SIVmac239Gag216S244E247L312V373T than of SIVmac239Gag216S.
Additionally, macaque R03-022, coinoculated with the molecular-clone DNAs of SIVmac239Gag216S244E373T and SIVmac239Gag216S244E247L312V373T, showed selection of the former (Fig. 2), indicating a lower replicative ability in vivo of SIVmac239Gag216S244E247L312V373T than of SIVmac239Gag216S244E373T. In this macaque, reversion of the GagL216S mutation was observed in 6 months, while the GagD244E and GagA373T mutations were still dominant without reversion.
Next, we inoculated 90-120-Ia-negative macaques with the SIV carrying multiple gag CTL escape mutations that was selected in 90-120-Ia-positive macaque V5 (Fig. 3). The SIV carrying five gag mutations, GagL216S, GagD244E, GagI247L, GagA312V, and GagA373T, that was dominant at the reappearance of viremia in macaque V5, was propagated on rhesus macaque peripheral blood mononuclear cells to prepare the SIVmac239Gag216S244E247L312V373T challenge stock for macaques R05-001 and R06-016. Sequencing analysis confirmed no gag mutation except for the five mutations in the challenge virus. These two macaques were challenged intravenously with 1,000 50% tissue culture infective dose of SIVmac239Gag216S244E247L312V373T. Both of them showed persistent viremia, although the levels of set-point plasma viral loads were low in macaque R06-016. Macaque R05-001, maintaining high viral loads, showed typical signs of AIDS, such as a reduction in peripheral CD4+ T-cell counts, diarrhea, and general weakness, and was euthanized approximately 2 years postchallenge. Autopsy revealed postpersistent generalized lymphadenopathy conditions and pneumocystis pneumonia. This macaque showed reversion of the GagD244E mutation in a few months, followed by reversion of the GagL216S, GagI247L, and GagA312V mutations in a year postchallenge, while the GagA373T mutation remained dominant without reversion until euthanasia (Fig. 4). In contrast, macaque R06-016, with lower viral loads, showed no reversion of the five mutations. In the chronic phase, these two macaques showed additional Gag amino acid changes, including GagI140V (I to V at aa 140) and GagV375M (V to methionine [M] at aa 375) that were detected in both. Some of these mutations may contribute to the recovery of viral fitness.
FIG. 3.
Plasma viral loads (SIV gag RNA copies/ml plasma) in macaques after challenge with SIV carrying five gag mutations.
FIG. 4.
Dominant viral genome sequences after challenge with SIV carrying five gag mutations. The amino acid sequences at the residues where mutations were included in the inoculums and dominant amino acid changes at other residues in gag are shown. In the column of other residues, the predominant mutations with detectable wild-type sequence are shown in parentheses. Wt, Wt(mt), wt/mt, Mt(wt), Mt, and p-c are defined in the Fig. 2 legend.
To see the possibility of transmission of the viruses carrying the five gag mutations in the context of the polyclonal, V5-derived SIVs, macaques R05-024 and R05-025 were inoculated with plasma obtained from macaque V5 in the chronic phase of SIVmac239 infection (Fig. 3). For the challenge, plasma was obtained from macaque V5 at weeks 81, 87, 92, 100, and 113 post-SIVmac239 challenge and 0.2 ml of each was intravenously inoculated into these two macaques. In the challenge SIV plasma, the five gag mutations (GagL216S, GagD244E, GagI247L, GagA312V, and GagA373T) and GagV145A were dominant, and additional gag mutations were detected in the MA- and NC-coding regions. In macaque R05-024, exhibiting low viral loads, the SIV GagL216S and GagD244E mutations remained dominant, while reversion of the GagI247L, GagA312V, and GagA373T mutations was observed (Fig. 4). Macaque R05-025, exhibiting high viral loads, developed AIDS and was euthanized at week 18 postchallenge. Autopsy revealed lymphoatrophy and cytomegalovirus infection. This macaque showed rapid reversion of the SIV GagI247L and GagA312V mutations but maintained the GagL216S, GagD244E, and GagA373T mutations until euthanasia.
In samples from these four macaques challenged with SIVmac239Gag216S244E247L312V373T or V5-derived plasma, we examined the virus-specific CD8+ T-cell responses around 3 months postinfection by flow cytometric analysis of antigen-specific gamma interferon induction (data not shown) as described previously (14, 20). Analyses using vesicular stomatitis virus G-pseudotyped SIV-infected cells as a stimulator revealed SIV-specific CD8+ T-cell responses in macaques R05-001, R06-016, and R05-024, but not in macaque R05-025, which may have contributed to the rapid AIDS progression in this animal. Macaque R05-024, exhibiting lower viral loads and rapid selection of a gag mutation resulting in an I257K (I to lysine [K] at aa 257) substitution, showed CD8+ T-cell responses specific for the Gag245-269 peptide mixture (a mixture of Gag245-260, Gag250-265, and Gag255-269 peptides), suggesting a possibility of this mutation for viral escape from strong CTL pressure. None of these four macaques showed CD8+ T-cell responses specific for the Gag206-225 (a mixture of Gag206-220 and Gag210-225 peptides), Gag206-225.216S (Gag206-220.216S and Gag210-225.216S), Gag232-255 (Gag232-246, Gag236-250, and Gag240-255), Gag232-255.244E, Gag236-255.244E247L, Gag362-385 (Gag362-377, Gag367-381, and Gag371-385), or Gag362-385.373T peptide mixture, indicating that CTL responses were not involved in the reversion or nonreversion at residue 216, 244, 247, or 373 in these macaques.
The in vivo competition assay in the present study showed loss of viral fitness from the addition of the GagD244E and GagA373T mutations into SIVmac239Gag216S and further loss of viral fitness from additional GagI247L and GagA312V mutations. The reversion of GagD244E in macaque R05- 001, GagA373T in macaque R05-024, and GagI247L and GagA312V in macaques R05-024 and R05-025 (Fig. 4) supports this notion. However, reversion was not observed in all the mutations after challenge with SIV carrying the five gag mutations. Challenge with SIVmac239Gag216S carrying the single GagL216S mutation has shown its reversion in 3 months (14), whereas the reversion of the GagL216S mutation was delayed or not observed after challenge with the SIV carrying five gag mutations. This may be due to the predominant selection of the reversion of other mutations or to lower viral replication efficiency in the latter case. Compensatory mutations can also be involved in this delay or nonreversion, but no additional gag mutation was observed in the early phase in macaque R06-016. The possibility of a contribution to this delay by GagI140V in macaque R05-001 and GagV145A in macaques R05-024 and R05-025 may be considered, while significant recovery of viral fitness by the latter mutation has not been observed (12).
It has been suggested that a reduction in viral fitness by CTL escape mutations may contribute to HIV/SIV control (19, 20, 28). Pressure by multiple epitope-specific CTLs may result in the selection of HIV/SIV with diminished replicative ability because of accumulating multiple escape mutations. The inefficient viral replication in macaques R02-022 and R03-022 (Fig. 1) and two of four macaques in the second experiment (Fig. 3) may reflect such a lower replicative ability of the mutant SIVs, but conversely, the results of the present study also showed efficient viral replication in macaques R05-001 and R05-025, indicating that the transmission of even such “crippled” HIV/SIV carrying multiple CTL escape mutations can result in persistent viral replication and AIDS progression. It remains unclear what host factors determined the viral replication efficiency in vivo in our study, while macaques with higher viral loads (R02-017, R05-002, R05-001, and R05-025) showed the first reversion earlier than those with lower viral loads (R02-022, R03-022, R06-016, and R05-024), suggesting an association of reversion with viral loads. Earlier reversion may result in the recovery of viral fitness, leading to higher viral loads, or conversely, higher viral loads may accelerate reversion.
Thus, our results suggest that in the transmission of HIV accumulating CTL escape mutations at the cost of viral fitness between MHC-mismatched individuals, even such crippled HIV infection can finally result in AIDS progression. Previous studies on SIVs with single CTL escape mutations showed their rapid reversion, but the present study on SIV with multiple CTL escape mutations indicates that the reversion of all the mutations was not required for the establishment of persistent viral replication or for the onset of disease. Furthermore, it suggests a possibility that CTL escape mutations resulting in viral fitness costs may not always revert rapidly even in the absence of CTL pressure after their transmission into MHC-mismatched hosts and can be transmitted further to other hosts. These results provide an important insight into HIV pathogenicity and evolution in human individuals with divergent MHC polymorphisms.
Acknowledgments
This work was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology, grants from the Japan Health Sciences Foundation, and grants from the Ministry of Health, Labor, and Welfare in Japan.
The animal experiments were conducted through the Cooperative Research Program in Tsukuba Primate Research Center, National Institute of Biomedical Innovation, with the help of the Corporation for Production and Research of Laboratory Primates. We thank F. Ono, A. Hiyaoka, K. Oto, H. Akari, K. Terao, Y. Yasutomi, M. Yasunami, A. Kimura, K. Ishikawa, T. Nakasone, K. Mori, N. Yamamoto, T. Kurata, Y. Nagai, and A. Nomoto for their help.
Footnotes
Published ahead of print on 12 March 2008.
REFERENCES
- 1.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 686103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borrow, P., H. Lewicki, X. Wei, M. S. Horwitz, N. Peffer, H. Meyers, J. A. Nelson, J. E. Gairin, B. H. Hahn, M. B. Oldstone, and G. M. Shaw. 1997. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTL) during primary infection demonstrated by rapid selection of CTL escape virus. Nat. Med. 3205-211. [DOI] [PubMed] [Google Scholar]
- 3.Brander, C., and B. D. Walker. 2003. Gradual adaptation of HIV to human host populations: good or bad news? Nat. Med. 91359-1362. [DOI] [PubMed] [Google Scholar]
- 4.Crawford, H., J. G. Prado, A. Leslie, S. Hué, I. Honeyborne, S. Reddy, M. van der Stok, Z. Mncube, C. Brander, C. Rousseau, J. I. Mullins, R. Kaslow, P. Goepfert, S. Allen, E. Hunter, J. Mulenga, P. Kiepiela, B. D. Walker, and P. J. R. Goulder. 2007. Compensatory mutation partially restores fitness and delays reversion of escape mutation within the immunodominant HLA-B*5703-restricted Gag epitope in chronic human immunodeficiency virus type 1 infection. J. Virol. 818346-8351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez, C. S., I. Stratov, R. De Rose, K. Walsh, C. J. Dale, M. Z. Smith, M. B. Agy, S. L. Hu, K. Krebs, D. I. Watkins, D. H. O'Connor, M. P. Davenport, and S. J. Kent. 2005. Rapid viral escape at an immunodominant simian-human immunodeficiency virus cytotoxic T-lymphocyte epitope exacts a dramatic fitness cost. J. Virol. 795721-5731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Friedrich, T. C., C. A. Frye, L. J. Yant, D. H. O'Connor, N. A. Kriewaldt, M. Benson, L. Vojnov, E. J. Dodds, C. Cullen, R. Rudersdorf, A. L. Hughes, N. Wilson, and D. I. Watkins. 2004. Extra-epitopic compensatory substitutions partially restore fitness to simian immunodeficiency virus variants that escape from an immunodominant cytotoxic T-lymphocyte response. J. Virol. 782581-2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Friedrich, T. C., E. J. Dodds, L. J. Yant, L. Vojnov, R. Rudersdorf, C. Cullen, D. T. Evans, R. C. Desrosiers, B. R. Mothe, J. Sidney, A. Sette, K. Kunstman, S. Wolinsky, M. Piatak, J. Lifson, A. L. Hughes, N. Wilson, D. H. O'Connor, and D. I. Watkins. 2004. Reversion of CTL escape-variant immunodeficiency viruses in vivo. Nat. Med. 10275-281. [DOI] [PubMed] [Google Scholar]
- 8.Goulder, P. J., and D. I. Watkins. 2004. HIV and SIV CTL escape: implications for vaccine design. Nat. Rev. Immunol. 4630-640. [DOI] [PubMed] [Google Scholar]
- 9.Goulder, P. J. R., R. E. Phillips, R. A. Colbert, S. McAdam, G. Ogg, M. A. Nowak, P. Giangrande, G. Luzzi, B. Morgana, A. Edwards, A. J. McMichael, and S. Rowland-Jones. 1997. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat. Med. 3212-217. [DOI] [PubMed] [Google Scholar]
- 10.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaslow, R. A., M. Carrington, R. Apple, L. Park, A. Muñoz, A. J. Saah, J. J. Goedert, C. Winkler, S. J. O'Brien, C. Rinaldo, R. Detels, W. Blattner, J. Phair, H. Erlich, and D. L. Mann. 1996. Influence of combinations of human major histocompatibility complex genes on the course of HIV-1 infection. Nat. Med. 2405-411. [DOI] [PubMed] [Google Scholar]
- 12.Kawada, M., H. Igarashi, A. Takeda, T. Tsukamoto, H. Yamamoto, S. Dohki, M. Takiguchi, and T. Matano. 2006. Involvement of multiple epitope-specific cytotoxic T-lymphocyte responses in vaccine-based control of simian immunodeficiency virus replication in rhesus macaques. J. Virol. 801949-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kiepiela, P., A. J. Leslie, I. Honeyborne, D. Ramduth, C. Thobakgale, S. Chetty, P. Rathnavalu, C. Moore, K. J. Pfafferott, L. Hilton, P. Zimbwa, S. Moore, T. Allen, C. Brander, M. M. Addo, M. Altfeld, I. James, S. Mallal, M. Bunce, L. D. Barber, J. Szinger, C. Day, P. Klenerman, J. Mullins, B. Korber, H. M. Coovadia, B. D. Walker, and P. J. R. Goulder. 2004. Dominant influence of HLA-B in mediating the potential co-evolution of HIV and HLA. Nature 432769-775. [DOI] [PubMed] [Google Scholar]
- 14.Kobayashi, M., H. Igarashi, A. Takeda, M. Kato, and T. Matano. 2005. Reversion in vivo after inoculation of a molecular proviral DNA clone of simian immunodeficiency virus with a cytotoxic-T-lymphocyte escape mutation. J. Virol. 7911529-11532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 684650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leslie, A. J., K. J. Pfafferott, P. Chetty, R. Draenert, M. M. Addo, M. Feeney, Y. Tang, E. C. Holmes, T. Allen, J. G. Prado, M. Altfeld, C. Brander, C. Dixon, D. Ramduth, P. Jeena, S. A. Thomas, A. St. John, T. A. Roach, B. Kupfer, G. Luzzi, A. Edwards, G. Taylor, H. Lyall, G. Tudor-Williams, V. Novelli, J. Martinez-Picado, P. Kiepiela, B. D. Walker, and P. J. R. Goulder. 2004. HIV evolution: CTL escape mutation and reversion after transmission. Nat. Med. 10282-289. [DOI] [PubMed] [Google Scholar]
- 17.Li, B., A. D. Gladden, M. Altfeld, J. M. Kaldor, D. A. Cooper, A. D. Kelleher, and T. M. Allen. 2007. Rapid reversion of sequence polymorphisms dominates early human immunodeficiency virus type 1 evolution. J. Virol. 81193-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loh, L., C. J. Batten, J. Petravic, M. P. Davenport, and S. J. Kent. 2007. In vivo fitness costs of different Gag CD8 T-cell escape mutant simian-human immunodeficiency viruses for macaques. J. Virol. 815418-5422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinez-Picado, J., J. G. Prado, E. E. Fry, K. Pfafferott, A. Leslie, S. Chetty, C. Thobakgale, I. Honeyborne, H. Crawford, P. Matthews, T. Pillay, C. Rousseau, J. I. Mullins, C. Brander, B. D. Walker, D. I. Stuart, P. Kiepiela, and P. Goulder. 2006. Fitness cost of escape mutations in p24 Gag in association with control of human immunodeficiency virus type 1. J. Virol. 803617-3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matano, T., M. Kobayashi, H. Igarashi, A. Takeda, H. Nakamura, M. Kano, C. Sugimoto, K. Mori, A. Iida, T. Hirata, M. Hasegawa, T. Yuasa, M. Miyazawa, Y. Takahashi, M. Yasunami, A. Kimura, D. H. O'Connor, D. I. Watkins, and Y. Nagai. 2004. Cytotoxic T lymphocyte-based control of simian immunodeficiency virus replication in a preclinical AIDS vaccine trial. J. Exp. Med. 1991709-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matano, T., R. Shibata, C. Siemon, M. Connors, H. C. Lane, and M. A. Martin. 1998. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J. Virol. 72164-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Institute of Infectious Diseases. 2007. Guides for animal experiments performed at National Institute of Infectious Diseases. National Institute of Infectious Diseases, Tokyo, Japan. (In Japanese.)
- 23.O'Connor, D. H., A. B. McDermott, K. C. Krebs, E. J. Dodds, J. E. Miller, E. J. Gonzalez, T. J. Jacoby, L. Yant, H. Piontkivska, R. Pantophlet, D. R. Burton, W. M. Rehrauer, N. Wilson, A. L. Hughes, and D. I. Watkins. 2004. A dominant role for CD8+-T-lymphocyte selection in simian immunodeficiency virus sequence variation. J. Virol. 7814012-14022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peyerl, F. W., D. H. Barouch, W. W. Yeh, H. S. Bazick, J. Kunstman, K. J. Kunstman, S. M. Wolinsky, and N. L. Letvin. 2003. Simian-human immunodeficiency virus escape from cytotoxic T-lymphocyte recognition at a structurally constrained epitope. J. Virol. 7712572-12578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phillips, R. E., S. Rowland-Jones, D. F. Nixon, F. M. Gotch, J. P. Edwards, A. O. Ogunlesi, J. G. Elvin, J. A. Rothbard, C. R. Bangham, C. R. Rizza, and A. J. McMichael. 1991. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature 354453-459. [DOI] [PubMed] [Google Scholar]
- 26.Price, D. A., P. J. Goulder, P. Klenerman, A. K. Sewell, P. J. Easterbrook, M. Troop, C. R. Bangham, and R. E. Phillips. 1997. Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proc. Natl. Acad. Sci. USA 941890-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283857-860. [DOI] [PubMed] [Google Scholar]
- 28.Schneidewind, A., M. A. Brockman, R. Yang, R. I. Adam, B. Li, S. L. Gall, C. R. Rinaldo, S. L. Craggs, R. L. Allgaier, K. A. Power, T. Kuntzen, C.-S. Tung, M. X. LaBute, S. M. Mueller, T. Harrer, A. J. McMichael, P. J. R. Goulder, C. Aiken, C. Brander, A. D. Kelleher, and T. M. Allen. 2007. Escape from the dominant HLA-B27-restricted cytotoxic T-lymphocyte response in Gag is associated with a dramatic reduction in human immunodeficiency virus type 1 replication. J. Virol. 8112382-12393. [DOI] [PMC free article] [PubMed] [Google Scholar]