Abstract
Many viruses encode proteins that inhibit the induction of programmed cell death at the mitochondrial checkpoint. Murine cytomegalovirus (MCMV) encodes the m38.5 protein, which localizes to mitochondria and protects human HeLa cells and fibroblasts from apoptosis triggered by proteasome inhibitors but not from Fas-induced apoptosis. However, the ability of this protein to suppress the apoptosis of murine cells and its role during MCMV infection have not been investigated previously. Here we show that m38.5 is expressed at early time points during MCMV infection. Cells infected with MCMVs lacking m38.5 showed increased sensitivity to cell death induced by staurosporine, MG132, or the viral infection itself compared to the sensitivity of cells infected with wild-type MCMV. This defect was eliminated when an m38.5 or Bcl-XL gene was inserted into the genome of a deletion mutant. Using fibroblasts deficient in the proapoptotic Bcl-2 family proteins Bak and/or Bax, we further demonstrated that m38.5 protected from Bax- but not Bak-mediated apoptosis and interacted with Bax in infected cells. These results consolidate the role of m38.5 as a viral mitochondrion-localized inhibitor of apoptosis and its functional similarity to the human cytomegalovirus UL37x1 gene product. Although the m38.5 gene is not homologous to the UL37x1 gene at the sequence level, m38.5 is conserved among rodent cytomegaloviruses. Moreover, the fact that MCMV-infected cells are protected from both Bak- and Bax-mediated cell death suggests that MCMV possesses an additional, as-yet-unidentified mechanism to block Bak-mediated apoptosis.
Programmed cell death (PCD) is a mechanism used by multicellular organisms to dispose of unwanted cells. This process is necessary for the shaping of an organism during development, for tissue homeostasis, and for defense against infectious agents. The removal of infected cells during viral infections is of particular importance, because viruses depend on the host cell for their replication. Therefore, it is not surprising that many viruses have evolved strategies to inhibit or delay the onset of PCD (7, 38, 44).
One way of initiating PCD is by the stimulation of so-called death receptors, such as the tumor necrosis factor (TNF) receptor and Fas, for instance, when immune effector cells recognize an infected cell. These death receptors can then activate a cascade of cellular proteases (the caspase cascade), which ultimately results in cell death (6). In addition to this extrinsic pathway to PCD, a cell can also sense the presence of a virus by itself and trigger a self-destruction program (7, 19). In both extrinsic and intrinsic pathways, mitochondria play an important role as integrators of diverse cell death-promoting and -inhibiting factors (22).
The Bcl-2 family consists of cellular proteins that govern a cell's decision to live or die at the mitochondrial checkpoint (22). These proteins are characterized by the presence of distinct Bcl-2 homology (BH) domains and can be divided into the anti- and proapoptotic family members. The proapoptotic family members Bax and Bak are key regulators of the apoptotic signaling pathway and contain BH domains 1 to 3 (46). By contrast, the antiapoptotic members of this family, such as Bcl-2 and Bcl-XL, usually contain all four BH domains (BH1 to BH4). The inhibition of antiapoptotic activity is mediated by the so-called BH3-only proteins, which share only the third BH domain with other family members. These proteins are activated as a consequence of intracellular damage, stress, or death receptor stimulation, which subsequently leads to the oligomerization of Bax and/or Bak at the mitochondrial outer membrane. This oligomerization causes the permeabilization of the membrane and the release of cytochrome c into the cytosol, where cytochrome c forms a complex with the adaptor protein Apaf-1 and participates in the activation of caspase-9 and caspase-3. The antiapoptotic proteins Bcl-2, Bcl-XL, Bcl-w, Mcl-1, and A1 antagonize this process by inhibiting the activation or the oligomerization of Bax and Bak. How exactly the BH3-only proteins activate Bax and Bak and how the Bcl-2-like proteins prevent this activation from happening have not been fully resolved and are in part still controversial (17).
To inhibit premature PCD, viruses express proteins that structurally and functionally resemble Bcl-2 (8, 47). Such proteins are encoded, for instance, by adenoviruses and gammaherpesviruses. Poxviruses also express mitochondrial cell death inhibitors, but these proteins show no homology in their amino acid sequences to the cellular Bcl-2-like proteins (14, 45). However, more recent investigations have revealed that they closely resemble Bcl-2 family proteins in their three-dimensional structures (23).
Cytomegaloviruses (CMVs), prototypes of the betaherpesviruses, do not encode sequence homologs of Bcl-2 in their genomes but still inhibit apoptosis at the mitochondrial checkpoint (3, 15, 37). The human CMV (HCMV) UL37x1 open reading frame (ORF) encodes a viral mitochondrion-localized inhibitor of apoptosis (vMIA) which inhibits the induction of PCD (15, 16). The UL37x1 vMIA protein was shown previously to block Bax- but not Bak-mediated apoptosis by binding and sequestering Bax at the mitochondrial membrane (4, 34). This finding was surprising, because many apoptotic stimuli (such as staurosporine [STS] and the stimulation of death receptors) can activate both Bax and Bak (46). The ability of vMIA to protect human HeLa cells and fibroblasts from Fas- or STS-induced PCD was proposed previously to result from the dominance of Bax over Bak in these cells. In murine fibroblasts, by contrast, Bax and Bak were proposed to be codominant, because apoptosis can be induced in knockout fibroblasts expressing either only Bax or only Bak. In these cells, vMIA protects from apoptosis only in the absence of Bak (4). Recently, Pauleau et al. demonstrated that the UL37x1 vMIA is likely to bind Bax preferentially when Bax is inserted into the mitochondrial membrane and that this binding function is independent of vMIA's ability to disrupt the mitochondrial network (32).
Interestingly, all primate CMVs seem to contain a vMIA gene, but no such gene in the genomes of rodent CMVs has been identified (29). However, a recent computational reevaluation of the murine CMV (MCMV) genome led to the discovery of a small ORF, termed the m38.5 ORF (9), which partially overlaps with the larger M38 ORF. The m38.5 ORF spans the region between nucleotide positions 51780 and 52367 of the MCMV genome (GenBank accession no. NC_004065) and was predicted previously to encode a protein of 196 amino acids (9, 28). This protein appears to be conserved among rodent CMVs since homologs of m38.5 are also present in both the English and Maastricht isolates of rat CMV (see Fig. 1A).
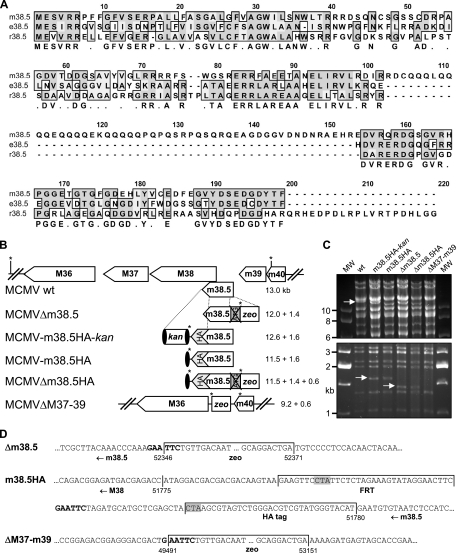
FIG. 1.
Evolutionary conservation and mutagenesis of MCMV m38.5. (A) Clustal W amino acid sequence alignment of MCMV Smith strain protein m38.5 (corresponding to nt 51780 to 52367; GenBank accession no. NC_004065), rat CMV English isolate protein e38.5 (corresponding to GenBank accession no. EU267790), and rat CMV Maastricht isolate protein r38.5 (corresponding to nt 35738 to 36235; GenBank accession no. NC_002512). Sequences were aligned using a Blossum matrix. Identical and similar amino acids are boxed and shaded in dark and light gray, respectively. The consensus sequence is shown below. (B) Arrangement of the M36 through m40 ORFs of wt MCMV and construction of m38.5 mutant viruses by BAC mutagenesis. The m38.5 gene was knocked out by deleting the ATG start codon and inserting a bacterial zeo gene, resulting in the MCMVΔm38.5 deletion mutant. A HA epitope tag sequence was attached to the 3′ end of the m38.5 gene by using a kan cassette flanked by FRT sites (black ovals) as a selectable marker. The kan cassette was removed by Flp recombinase. A second m38.5 knockout mutant, MCMVΔm38.5HA, was constructed on the basis of MCMV-m38.5HA. A larger deletion comprising M37 through m39 ORFs was generated by inserting a zeo cassette. EcoRI restriction sites are indicated by asterisks, and the sizes of the expected EcoRI fragments are listed. (C) EcoRI restriction patterns of wt and mutant MCMV BACs separated by agarose gel electrophoresis. The 13-kb fragment of wt MCMV and the 1.4- and 1.6-kb fragments of the mutant viruses are indicated by arrows. MW, molecular size standard; m38.5HA-kan, MCMV-m38.5HA-kan; m38.5HA, MCMV-m38.5HA; Δm38.5, MCMVΔm38.5; Δm38.5HA, MCMVΔm38.5HA; ΔM37-m39, MCMVΔM37-m39. (D) The mutated sites of all five mutant viruses were sequenced. Three sequences are shown, and the remaining sequences contained related or combined mutations. Numbers indicate nucleotide positions within the MCMV genome, and the inserted sequences are labeled. The FRT site is flanked by short linker sequences. EcoRI restriction sites are shown in bold. The stop codon of the m38.5 ORF (at the end of the HA tag sequence) and a stop codon within the FRT site that terminates the M38 ORF are shaded in gray.
The location of the m38.5 ORF within the MCMV genome is analogous to that of the UL37x1 ORF within the HCMV genome, but the m38.5 protein sequence shows little or no similarity to the sequence of the HCMV UL37x1 protein. Therefore, it appeared questionable whether the MCMV m38.5 protein exerts an antiapoptotic function similar to that of the UL37x1 vMIA protein. A study by McCormick et al. showed that the m38.5 protein localizes to mitochondria and protects transfected human fibroblasts and HeLa cells from cell death induced by proteasome inhibitors (28). However, m38.5 did not protect these cells from Fas-induced apoptosis, even though the UL37x1 protein was protective in the same assay. The exact nature of the antiapoptotic activity of m38.5 has remained largely unknown, particularly since the molecular mechanism by which proteasome inhibitors induce cell death is not fully understood. Moreover, the expression kinetics of m38.5 and its role during viral infection have not yet been investigated.
In this study, we analyzed the expression and function of m38.5 by using recombinant viruses in which the m38.5 gene was inactivated or replaced by genes with known functions. We showed that the m38.5 protein was expressed with early kinetics during viral infection and that the protein was required to protect MCMV-infected cells from STS- and MG132-mediated cell death, as well as from premature apoptosis induced by the viral infection itself. Using Bax- and Bak-deficient fibroblasts, we showed that m38.5 inhibited Bax- but not Bak-mediated apoptosis, likely as a result of an interaction with Bax. Moreover, the fact that MCMV-infected cells were resistant to both Bax- and Bak-mediated cell death indicates that MCMV encodes an additional mechanism that inhibits apoptosis induction via Bak.
MATERIALS AND METHODS
Cells and viruses.
Wild-type (wt) and recombinant MCMVs were grown in 10.1 mouse fibroblasts (18) according to standard procedures (10), and virus titers were determined using the median tissue culture infective dose method (27). Bak−/− and Bax−/− mouse fibroblast cell lines (48) were provided by Georg Häcker (Technical University Munich, Germany) with permission from David Huang (WEHI, Melbourne, Australia). NIH 3T3 fibroblasts (ATCC CRL-1658) and SVEC4-10 endothelial cells (ATCC CRL-2181) were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum with 100 U of penicillin/ml and 0.1 mg of streptomycin/ml. For analyses of growth kinetics, 106 cells per well were seeded into six-well dishes and infected with MCMV at the multiplicities of infection (MOIs) indicated below. Infectious medium was removed 2 h postinfection (hpi), cells were washed with phosphate-buffered saline, and 3 ml of fresh medium was added to each well. At the indicated time points, 100-μl aliquots of medium were collected and replaced with fresh medium. All growth kinetics experiments were done in triplicate, and the means and standard deviations were used for the diagrams.
Plasmids.
The m38.5 coding sequence (nucleotides [nt] 51780 to 52367 of the MCMV genome; GenBank accession no. NC_004065) was amplified by PCR and cloned with a hemagglutinin (HA) tag sequence attached to the 3′ end into pcDNA3 (Invitrogen) by using EcoRI and XhoI sites. The transfer vector pReplacer-m38.5 was made analogously to the previously described pReplacer-BclXL and pReplacer-UL37x1 constructs (21).
Construction of recombinant MCMVs.
All recombinant viruses were based on MCMV-GFP, a recombinant MCMV expressing the enhanced green fluorescent protein (GFP) (11). Mutations were introduced into the MCMV genome by using bacterial artificial chromosome (BAC) technology essentially as described before (13). Homologous recombination was carried out with Escherichia coli strain DY380 (49). For the tagging of the m38.5 gene, the HA tag sequence and a kanamycin resistance gene (kan) flanked by Flp recognition target (FRT) sites were amplified by PCR from pcDNA-m38.5HA-kan. PCR primers were designed in such a way that the resulting PCR product contained a 50-bp sequence homologous to the 3′ end of the m38.5 gene sequence and a 50-bp region homologous to the sequence downstream of the m38.5 stop codon. The kan cassette was removed by Flp recombinase as described previously (12). For the inactivation of the m38.5 gene, a sequence of 24 nt adjacent to the ATG start codon (nt 52347 to 52370) was deleted and replaced with a zeocin resistance gene (zeo) as described previously (12). Similarly, the region comprising M37, M38, m38.5, and m39 ORFs (nt 49492 to 53150) was deleted to obtain the MCMVΔM37-m39 mutant. Rescue mutants Rm38.5, RUL37x1, and RBcl-XL were constructed on the basis of MCMVΔM37-m39 by inserting sequences encoding HA-tagged versions of m38.5, UL37x1, and Bcl-XL proteins driven by a phosphoglycerate kinase promoter into the nonessential region of MCMV between the m02 and m06 genes by using the pReplacer system (21). Recombinant viruses were reconstituted by transfecting 10.1 fibroblasts with MCMV BACs by using PolyFect transfection reagent according to the protocol of the manufacturer (Qiagen). The recombinant MCMV Rm41 expressing a HA-tagged m41 protein (12) was constructed in our laboratory by Maren Syta (M. Syta and W. Brune, unpublished data).
Retroviral transduction.
The empty retroviral vector plasmid pRetroEBNA and the GFP-expressing derivative pRetroGFP were obtained from Tom Shenk (Princeton University, NJ) and have been used in previous studies (12). Sequences encoding HA-tagged Bcl-XL and m38.5 were inserted into pRetroEBNA to obtain pRetro-BclXL and pRetro-m38.5, respectively. Phoenix packaging cells were transfected with the plasmids. Supernatants were harvested after 48 h, passed through 0.45-μm-pore-size filters, and used for the transduction of fibroblasts in the presence of 4 μg of Polybrene/ml or stored at −80°C for later use.
Cell death assays.
Cells were seeded at a density of approximately 5 × 105 cells per 96-well plate and infected with MCMV at an MOI of 5. Unless stated otherwise, cell death was induced by adding STS (250 nM; Sigma), MG132 (10 μM; Calbiochem), or TNF-α (20 ng/ml; Promokine) 4 to 8 hpi. At the time points indicated in the figures, the medium was removed and cell viability was assessed by measuring mitochondrial activity with an MTT [3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] assay as described previously (21). Briefly, in the mitochondria of living cells, MTT is reduced into formazan, which can be measured photometrically. All viability experiments were done in quadruplicate. The pancaspase inhibitor z-VAD-fmk and the calpain inhibitor z-LLY-fmk were purchased from MBL International. To analyze nuclear DNA fragmentation as a late sign of apoptosis, cells were grown and infected on coverslips, fixed with 3% paraformaldehyde, and stained with a terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick-end-labeling (TUNEL) assay kit (Roche) containing tetramethylrhodamine-coupled dUTP. Nuclei were counterstained with 4′,6′-diamidino-2-phenylindole (DAPI). Fluorescence microscopy analyses were performed with a Zeiss Axiovert 200 microscope. Images were acquired with an AxioCamHRc camera and AxioVision software (Zeiss).
Analysis of immediate-early and early gene expression.
To allow immediate-early and early protein expression, cells were infected with MCMV in the presence of 250 μg of phosphonoacetic acid (PAA; Sigma)/ml. The selective expression of immediate-early proteins was achieved by infecting cells in the presence of 50 μg of cycloheximide (Sigma)/ml for 4 h. The medium was subsequently removed, and cells were incubated with medium containing 5 μg of actinomycin D (Sigma)/ml for another 4 h.
Western blotting and immunoprecipitation.
For Western blot analyses, cells were lysed in Triton buffer (10 mM Tris-HCl [pH 8], 140 mM NaCl, 1% Triton X-100) supplemented with a protease inhibitor cocktail (Roche). Proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and transferred onto a nitrocellulose membrane (Amersham). We used primary antibodies against the HA epitope (16B12; Covance Research Products), Bax (N-20; Santa Cruz), and β-actin (A5316; Sigma); CROMA101 and CROMA103 (provided by Stipan Jonjic, University of Rijeka, Croatia) against MCMV IE1 and E1 proteins, respectively; and 2E8.12A (provided by Lambert Loh, University of Saskatchewan, Canada) (25) against MCMV glycoprotein B. A horseradish peroxidase-conjugated secondary antibody (50447; DakoCytomation) and enhanced chemiluminescent reagents (Amersham) were used to visualize the proteins of interest.
For immunoprecipitations, MCMV-infected cells were lysed with a buffer containing 140 mM NaCl, 20 mM Tris-HCl (pH 7.6), 5 mM MgCl2, 1% Triton X-100, and protease inhibitors. After preclearing with protein G-Sepharose (GE Healthcare), m38.5 was precipitated with an anti-HA antibody and protein G-Sepharose. Precipitates were washed three times with buffer B (150 mM NaCl, 1 mM Tris-HCl [pH 7.6], 2 mM EDTA, 0.2% Triton X-100), twice with buffer C (500 mM NaCl, 1 mM Tris-HCl [pH 7.6], 2 mM EDTA, 0.2% Triton X-100), and once with buffer D (10 mM Tris-HCl, pH 7.6). Samples were then separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and subjected to Western blotting.
Nucleotide sequence accession number.
The rat CMV (English isolate) e38.5 gene sequence determined in this study has been deposited in GenBank under the accession number EU267790.
RESULTS
Construction of recombinant MCMVs with an altered m38.5 locus.
To study the role of m38.5 during MCMV infection, we constructed a set of recombinant viruses using the BAC technology. Specific mutagenesis of the m38.5 ORF was difficult, because the m38.5 ORF overlaps with the adjacent M38 ORF (Fig. 1B). First, a recombinant virus in which a HA epitope tag sequence was attached to the 3′ end of the m38.5 coding sequence was generated. A kanamycin (kan) cassette flanked by FRT sites was also inserted to facilitate the selection of recombinant MCMV BACs in E. coli, and this cassette was subsequently removed by Flp recombinase (Fig. 1B). Based on the genome of this recombinant virus (designated MCMV-m38.5HA) and on the wt MCMV genome, additional mutants were generated. The ATG start codon (the only ATG codon within the entire m38.5 ORF) was replaced with a bacterial zeocin resistance gene (zeo). In addition, a mutant MCMV with a larger deletion comprising the M37, M38, m38.5, and m39 ORFs was constructed (Fig. 1B). Recombinant BACs were digested with restriction enzymes and separated by gel electrophoresis in order to verify the expected changes in the restriction patterns (Fig. 1C). In addition, the mutated sites were analyzed by sequencing (Fig. 1D).
m38.5 is expressed with early kinetics.
A previous analysis of RNA transcripts expressed from the m38.5 coding region showed a very complex pattern with a number of overlapping transcripts (29), making it difficult to analyze m38.5 expression at the level of mRNA conclusively. Therefore, we decided to use recombinant viruses expressing a tagged m38.5 protein (Fig. 2A) in order to study the expression kinetics of the predicted m38.5 gene during viral infection. We could detect weak m38.5 protein expression as early as 4 hpi (Fig. 2B) but not at earlier time points, even with prolonged exposure of the film (data not shown). By contrast, the immediate-early protein IE1 was strongly expressed at 4 hpi (Fig. 2B) and could be detected weakly at 1 hpi upon prolonged exposure of the film (data not shown). On Western blots, the HA-tagged m38.5 protein had an apparent molecular mass of approximately 25 kDa, which corresponds to its predicted mass (Fig. 2A). No differences in the apparent molecular weights of m38.5 proteins expressed from plasmids and those expressed from the MCMV genome were detected (data not shown). The expression of m38.5 was not blocked by the addition of PAA, an inhibitor of viral DNA replication, suggesting that m38.5 is expressed with early kinetics (Fig. 2B). Moreover, m38.5 was not expressed at detectable levels after release from the cycloheximide block in the presence of actinomycin D, whereas the immediate-early protein IE1 was readily detected under the same experimental conditions (Fig. 2C). This finding provided additional support for the conclusion that m38.5 falls into the category of early proteins and thus differs from its putative analog in HCMV, the UL37x1 protein, which is expressed with immediate-early kinetics (16, 20, 42). In immunofluorescence experiments, we detected the HA-tagged m38.5 protein at mitochondria (data not shown), which is in agreement with the previously reported intracellular localization (28).
FIG. 2.
Kinetics of m38.5 expression as shown by Western blotting. (A) Lysates of cells infected with the indicated viruses were harvested 24 hpi and immunoblotted with an anti-HA antibody that recognized the tagged version of m38.5 with a size of approximately 25 kDa. The MCMV IE1 protein was detected with an IE1-specific antibody. m38.5HA-kan, MCMV-m38.5HA-kan; m38.5HA, MCMV-m38.5HA; Δm38.5HA, MCMVΔm38.5HA; Δm38.5, MCMVΔm38.5; ΔM37-m39, MCMVΔM37-m39. (B) Cells infected with MCMV-m38.5HA were harvested at the indicated hours postinfection. The MCMV proteins IE1 and E1 and the late glycoprotein B (gB) were detected with specific antibodies. The m38.5 protein was detected with an anti-HA antibody. Immediate-early and early proteins were expressed in the presence of PAA. M, mock-infected cells. (C) Cells were mock infected (M) or infected (I) with MCMV-m38.5HA for 8 h. Viral protein expression was blocked in the presence of cycloheximide (C) or actinomycin D (A). Immediate-early proteins were expressed selectively after release from the cycloheximide block after 4 h and incubation with actinomycin D for another 4 h (C▸A). In all experiments, β-actin served as a loading control.
The lack of m38.5 impairs viral replication and the survival of infected cells.
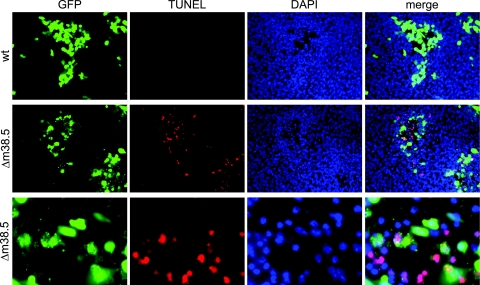
It has been shown previously for a number of viruses that the deletion of a suppressor of PCD can lead to the premature death of infected cells and thereby diminish viral replication (1, 5, 11, 12, 14, 33, 36, 45). Therefore, to determine if m38.5 has an antiapoptotic function, we tested if the lack of m38.5 results in increased apoptosis and decreased MCMV replication. Cells were infected with wt MCMV or the m38.5 gene deletion virus MCMVΔm38.5, and nuclear DNA fragmentation (as a sign of apoptosis) at day 3 after infection at an MOI of 0.01 was visualized by a TUNEL assay. In MCMVΔm38.5-infected foci, many cells stained positive for DNA fragmentation, but only a few TUNEL assay-positive cells were found in cell populations infected with wt virus (Fig. 3). At a higher magnification, membrane blebbing and the release of vesicles (putative apoptotic bodies) could be observed in many of the MCMVΔm38.5-infected cells (Fig. 3, third row) but only rarely in cells infected with the wt control virus (data not shown). The same phenotype was seen with the independently constructed m38.5 knockout virus MCMVΔm38.5HA. This result indicated that the observed phenotype was caused by the intended mutation at the m38.5 locus and not by an adventitious mutation that might have occurred elsewhere in the viral genome.
FIG. 3.
MCMVΔm38.5 induces nuclear DNA fragmentation. Murine 10.1 fibroblasts were infected at an MOI of 0.01 with wt MCMV or MCMVΔm38.5 (Δm38.5), both of which express GFP. GFP expression and nuclear DNA fragmentation (detected using a TUNEL assay) 3 days after infection were visualized by fluorescence microscopy. Nuclei were stained with DAPI. Cells infected with MCMVΔm38.5 are also shown at a higher magnification (third row).
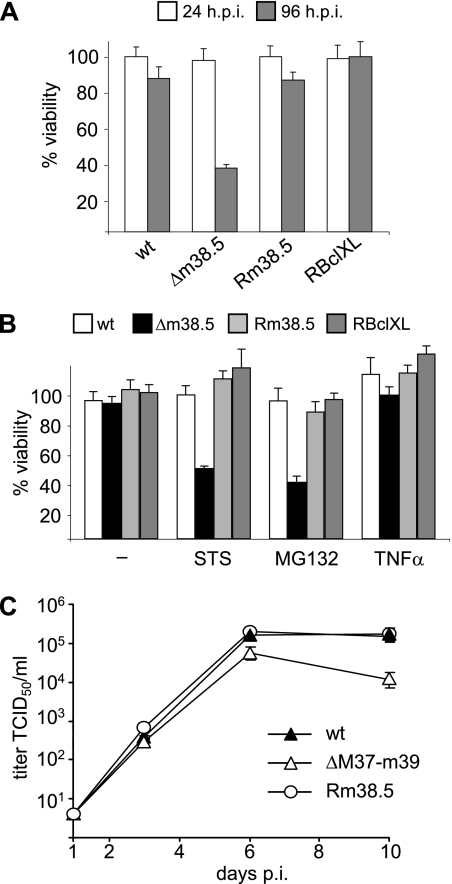
Single-step growth analyses of wt and mutant viruses (at an MOI of 5) showed that m38.5-deficient viruses had only modest growth defects (Fig. 4A). These growth defects were not detectable at 2 days postinfection (dpi) but became apparent at later time points (4 and 6 dpi). A decrease in virus release coincided with the appearance of massive cell death. Thus, we presumed that the clear differences in virus titers at the end of the experiment (>10-fold at 6 dpi) were a result of premature cell death that limited the production of viral progeny rather than replication defects per se (Fig. 4). Multistep growth analyses with a starting MOI of 0.01 50% tissue culture infective dose/ml showed even more pronounced growth defects (Fig. 4B). For these growth analyses, a 100-μl aliquot was collected at each time point and a corresponding amount of fresh medium was added. However, when the medium was exchanged completely at each time point in a parallel experiment, wt MCMV and the MCMVΔm38.5 mutant reached similar high titers (Fig. 4B). Fewer dead and disintegrated cells were observed in the MCMVΔm38.5-infected cell cultures under these conditions than under the conditions in which only portions of the medium were exchanged. This result suggested that cells infected with an m38.5-deficient virus were more sensitive to stress exerted by (partially) exhausted cell culture medium or toxic factors released by infected cells than cells infected with wt virus.
FIG. 4.
Replication kinetics of wt and mutant MCMVs. (A) Single-step growth curves for MCMVs in 10.1 fibroblasts infected at an MOI of 5. m38.5HA-kan, MCMV-m38.5HA-kan; m38.5HA, MCMV-m38.5HA; Δm38.5, MCMVΔm38.5; Δm38.5HA, MCMVΔm38.5HA; ΔM37-m39, MCMVΔM37-m39. (B) Multistep growth curves for MCMVs in 10.1 fibroblasts infected at an MOI of 0.01. Infected cells were incubated with (open symbols) or without (black symbols) the daily exchange of the cell culture medium. TCID50, 50% tissue culture infective dose.
m38.5-deficient viruses induce cell death and do not protect from STS- or MG132-induced apoptosis.
The results from the experiments shown in Fig. 3 and 4 indicated that m38.5 is required to inhibit premature cell death. To further characterize and quantify this requirement, cells were infected at a high MOI with different MCMV mutants, and cell viability levels at different times after infection were measured using an MTT assay. As shown in Fig. 5A, 10.1 fibroblasts infected with wt MCMV or the HA-tagged virus lost only a little viability between 24 and 96 h after infection. By contrast, cells infected with m38.5-deficient viruses showed severely reduced viability at 96 hpi, as determined by an MTT assay. At this time point, most cells were disintegrated and floating, whereas cells infected with the control viruses showed a cytopathic effect but were still attached to the cell culture dish and appeared to be viable (data not shown). The induction of premature cell death was seen not only in 10.1 fibroblasts but also in SVEC4-10 endothelial cells (Fig. 5B) and NIH 3T3 cells (Fig. 5C), suggesting that it is not a cell type- or cell line-specific phenomenon. The loss of viability was largely blocked by PAA, an inhibitor of viral DNA replication (Fig. 5D). This result indicated that viral DNA replication itself or a process occurring after DNA replication triggered PCD.
FIG. 5.
Cells infected with MCMVs lacking m38.5 show increased sensitivity to virus- and drug-induced cell death compared to cells infected with wt MCMV. (A to C) 10.1 fibroblasts (A), SVEC4-10 endothelial cells (B), and NIH 3T3 fibroblasts (C) were infected with the indicated viruses, and levels of cell viability at 24 and 96 hpi were measured by an MTT assay. No difference in viability among the cell types at 24 hpi was detected (data not shown in panels B and C). m38.5HA, MCMV-m38.5HA; Δm38.5HA, MCMVΔm38.5HA; ΔM37-m39, MCMVΔM37-m39. (D) The premature death of MCMVΔm38.5-infected cells was largely blocked by an inhibitor of viral DNA replication (PAA, 250 μg/ml) but not by the calpain inhibitor z-LLY-fmk (zLLY). −, control; Δm38.5, MCMVΔ38.5. (E) The addition of the pancaspase inhibitor z-VAD-fmk (zVAD), but not the solvent dimethyl sulfoxide (DM), also reduced premature cell death. (F) 10.1 cells infected with wt MCMV were resistant to cell death induced by STS, MG132, and TNF-α, but MCMVΔm38.5-infected cells were sensitive to STS- and MG132-induced cell death, and ΔM36-infected cells were sensitive to TNF-α-induced apoptosis. In all experiments, the 24-hpi values for untreated cells were normalized to 100%.
We also tested whether two families of proteases, caspases and calpains, play a role in the execution of cell death triggered by m38.5-deficient viruses. Cell death was partially inhibited by z-VAD-fmk, a broad-spectrum caspase inhibitor (Fig. 5E). This result may indicate that m38.5 is required for the inhibition of caspase-dependent as well as caspase-independent cell death. By contrast, the addition of a calpain inhibitor did not have a noticeable impact on the induction of premature cell death (Fig. 5D).
Next, we tested if cells infected with MCMVΔm38.5 would be more sensitive to exogenously induced apoptosis than those infected with wt MCMV. To this end, infected cells were treated with established inducers of apoptosis. Cells infected with MCMVΔm38.5 were sensitive to STS- and MG132-induced apoptosis but not to PCD induced by TNF-α, and cells infected with wt MCMV were resistant to all three apoptotic stimuli (Fig. 5F). By contrast, cells infected with an MCMV mutant lacking the viral inhibitor of caspase-8 activation (ΔM36) were highly sensitive to TNF-α but not to STS or MG132 (Fig. 5F). These results strongly suggest an interference of m38.5 with the intrinsic pathway of cell death.
The insertion of the m38.5 or Bcl-XL gene restores the wt phenotype in a deletion mutant lacking m38.5.
The deletion of the ATG start codon of the m38.5 gene by a bacterial zeocin resistance cassette may have an impact on the expression of the neighboring M38 gene due to the overlapping of the two ORFs (Fig. 1B). Hence, the observed phenotype of the MCMVΔm38.5 mutant virus may be the consequence of impaired expression of M38. However, this scenario seemed unlikely since the HA tagging of m38.5 resulted in the disruption of M38 and since the two HA-tagged viruses MCMV-m38.5HA and MCMV-m38.5HA-kan behaved like wt MCMV in replication and cell death assays (Fig. 4A and 5A to C). These findings argued against an important role for M38 in these settings. Yet the region surrounding the m38.5 gene has a very complex transcriptional pattern (29), and it cannot be ruled out that the phenotype of the MCMVΔm38.5 mutant was caused by a direct or indirect impact on other genes, distant from or in close proximity to the m38.5 gene. To resolve this question, we used a virus with a large deletion in the region surrounding m38.5 (MCMVΔM37-m39) and reinserted the HA sequence-tagged m38.5 gene under the control of an autonomous promoter at a distant site in the genome (between the m02 and m06 genes) known to be dispensable for virus replication in vitro (31). In the same way, a HA sequence-tagged version of the cellular antiapoptotic Bcl-XL gene was inserted. The resulting mutants were termed Rm38.5 and RBcl-XL (Fig. 6A and B). The expression of the HA-tagged proteins by the recombinant viruses was verified by Western blotting (Fig. 6C).
FIG. 6.
Construction of rescue mutants expressing m38.5 or Bcl-XL. (A) Sequences expressing HA-tagged versions of m38.5 and Bcl-XL driven by a phosphoglycerate kinase promoter (PGKp) were inserted into the nonessential region of the MCMVΔM37-m39 genome between the m02 and m06 genes. m38.5HA, gene expressing HA-tagged m38.5; HABclXL, gene expressing HA-tagged Bcl-XL. (B) This insertion results in the loss of a 2.16-kb HindIII fragment and the appearance of a new 1.25-kb fragment in the rescue mutants and an additional 0.96-kb fragment in RBcl-XL. Band patterns for two clones of each rescue mutant are shown. MW, molecular size standard; ΔM37-m39, MCMVΔM37-m39. (C) Detection of HA-tagged proteins (m38.5 and Bcl-XL) and the MCMV E1 protein in infected 10.1 cells 24 hpi. m38.5HA, MCMV-m38.5HA; Rm38.5(HA) and RBclXL(HA), Rm38.5 and RBcl-XL mutants expressing HA-tagged proteins.
The previous experiments had shown that an MCMV lacking the entire region from the M37 to the m39 genes had the same replication and cell death-inducing properties as MCMVΔm38.5 (Fig. 4A and 5A to C). The insertion of the m38.5 gene at an ectopic location was sufficient to reverse the phenotype of the MCMVΔM37-m39 mutant: cells infected with Rm38.5 retained a level of viability at 96 hpi similar to that of cells infected with wt MCMV (Fig. 7A), and Rm38.5-infected cells and wt MCMV-infected cells were equally resistant to STS- or MG132-induced apoptosis (Fig. 7B). The well-characterized cellular antiapoptotic protein Bcl-XL showed activity similar to that of m38.5 when the Bcl-XL gene was inserted into the viral genome, suggesting that these two mitochondrion-localized proteins have similar functions. The insertion of the m38.5 gene was also sufficient to reverse the growth defect of the MCMVΔM37-m39 mutant in a multistep growth kinetics experiment (Fig. 7C).
FIG. 7.
The insertion of the m38.5 or Bcl-XL gene eliminates the defects caused by the deletion of the m38.5 gene. (A and B) The levels of viability of infected 10.1 cells at 24 and 96 hpi (A) and of infected STS-, MG132-, or TNF-α-treated 10.1 cells at 24 hpi (B) were measured by an MTT assay. In all experiments, the 24-hpi values for untreated cells were normalized to 100%. Δm38.5, MCMVΔm38.5; −, no treatment. (C) Multistep growth kinetics of wt and mutant MCMVs in 10.1 fibroblasts (MOI, 0.01). TCID50, 50% tissue culture infective dose; ΔM37-m39, MCMVΔM37-m39.
m38.5 inhibits Bax-mediated cell death.
It has been shown previously that MCMV-infected cells are resistant to the induction of apoptosis via the mitochondrial pathway (2), but the molecular mechanism and the responsible viral gene products have remained unknown. Many cellular and viral antiapoptotic proteins operate at the mitochondrial membrane, inhibiting the oligomerization and/or activation of the proapoptotic Bcl-2 family members Bax and Bak (8, 47). The HCMV UL37x1 gene product (vMIA) differs from most Bcl-2-like proteins in that it inhibits only Bax- but not Bak-mediated cell death (4, 34). As the m38.5 gene is a positional homolog of the HCMV UL37x1 gene, we wondered if m38.5 would have similarly restricted activity. To test this, we infected fibroblasts lacking Bax, Bak, or both proteins with different MCMV mutants and stimulated the cells with STS. This drug is known to activate Bax- as well as Bak-mediated apoptosis (46). As shown in Fig. 8A, m38.5 was required to protect Bax+ but not Bax− cells from STS-induced cell death. The insertion of an m38.5, UL37x1, or Bcl-XL gene into the MCMVΔM37-m39 virus restored the ability to confer resistance to STS.
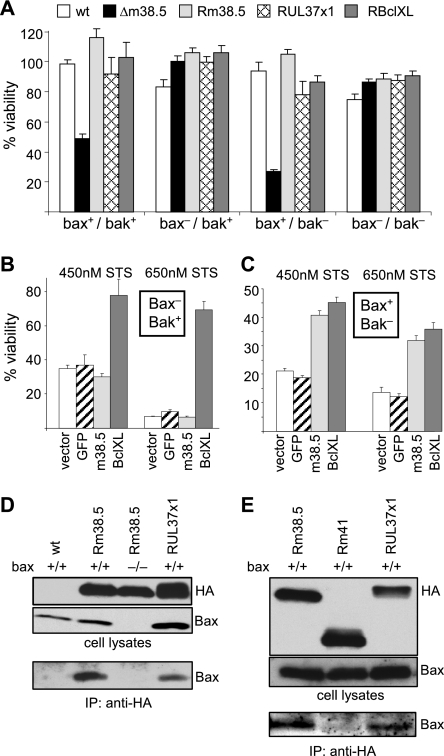
FIG. 8.
m38.5 inhibits Bax- but not Bak-mediated cell death. (A) Fibroblasts from wt, Bax knockout, Bak knockout, or double-knockout mice were infected with the indicated viruses and treated with STS. The percent viabilities of STS-treated cells versus dimethyl sulfoxide-treated control cells are shown. Δm38.5, MCMVΔm38.5. (B) Fibroblasts expressing only Bak were transduced with retroviral vectors encoding m38.5, Bcl-XL, or GFP as a negative control. Bcl-XL but not m38.5 protected cells against STS-induced cell death. Two different concentrations of STS were used. (C) In fibroblasts expressing only Bax, Bcl-XL and m38.5 both protected against STS-induced apoptosis. (D) wt and Bax−/− fibroblasts were infected with the indicated viruses. Lysates of infected cells were subjected to Western blotting and immunoprecipitation (IP) with an anti-HA antibody to precipitate HA-tagged m38.5 and UL37x1 proteins. Bax was detected as a coprecipitating protein, indicating an interaction between the viral mitochondrial proteins and Bax. (E) Results of an experiment similar to that described in the legend to panel D. A recombinant MCMV expressing HA-tagged m41 protein (Rm41) was used as a negative control.
We also wondered whether m38.5 by itself (i.e., outside of the context of viral infection) would be able to block apoptosis. In previous studies, m38.5 did not protect human HeLa, RPE-1, or fibroblast cells from Fas-mediated apoptosis but provided some protection against apoptosis induced by proteasome inhibitors such as MG132 (21, 28). HeLa and RPE-1 cells expressing m38.5 were also not protected from STS-induced cell death (our unpublished observations). To investigate if m38.5 possesses selective activity against Bax-mediated cell death, as suggested by the results of the previous experiment (Fig. 8A), we decided to use fibroblasts that expressed either Bax or Bak. These fibroblasts were transduced with retroviral vectors expressing m38.5, Bcl-XL, or GFP (as a negative control) and were then treated with different concentrations of STS. As expected, Bcl-XL inhibited apoptosis in both cell types. By contrast, m38.5 inhibited apoptosis only in Bax+ Bak− and not in Bax− Bak+ cells (Fig. 8B and C). To substantiate this finding, we examined if m38.5 interacts with Bax by immunoprecipitation. As shown in Fig. 8D and E, Bax could be coprecipitated with m38.5 from lysates of infected cells. These findings reveal a specific role for m38.5 in Bax-mediated apoptosis.
The results also show that MCMV-infected cells are protected from both Bax- and Bak-mediated cell death. As m38.5 is responsible for blocking only the Bax-mediated pathway, an additional, hitherto-unidentified mechanism of MCMV interference with the Bak-mediated pathway must exist.
DISCUSSION
In this study, we analyzed the role of the m38.5 protein during viral infection. Using mutant viruses, we demonstrated that the lack of m38.5 increased sensitivity to cell death induced by the virus itself, by STS, or by proteasome inhibitors compared to the sensitivity of cells infected with the wt virus. We further showed that m38.5 interacted with Bax and blocked Bax- but not Bak-mediated apoptosis, suggesting the existence of an additional, Bak-inhibiting mechanism.
By epitope tagging of the m38.5 ORF at its native position within the viral genome, we were able to determine the expression kinetics of the m38.5 protein and classify it as an early protein. This finding differs from that in a recent report, which mentioned that m38.5 was transcribed only at later times (41), but the data substantiating this conclusion were not given in the article. It seems likely that differences in the sensitivity of the assay systems caused this discrepancy.
In a previous study, m38.5 did not protect transfected HeLa cells from Fas-mediated apoptosis but did reduce PCD induced by proteasome inhibitors (28). This result suggested that the role of m38.5 is to inhibit cell death induced via the intrinsic pathway. The extrinsic, death receptor-induced pathway is already blocked during MCMV infection by means of the M36 gene product, which operates as a viral inhibitor of caspase-8 activation (30). When the M36 gene was deleted from the viral genome, infected cells became sensitive to TNF-α-induced cell death (Fig. 5F). This result indicated that the mitochondrial inhibitors of apoptosis encoded by MCMV were unable to protect from death receptor-derived signals, probably because caspase-8 can directly activate downstream effector caspases without the need to relay the signal through mitochondria.
Popkin and Virgin have shown previously that MCMV inhibits TNF-α-induced NF-κB activation in infected macrophages and have correlated this effect with the down-regulation of TNF receptor 1 (TNFR1) from the cell surface (35). Receptor down-regulation may render MCMV-infected cells unresponsive to TNF-α. However, the down-regulation was measured at 18 hpi and was not complete, suggesting that TNFR1 down-regulation may not be sufficient. We have recently shown that the MCMV M45 protein blocks TNFR1-dependent NF-κB activation by interacting with the adaptor molecule RIP1 (26). Fibroblasts infected with M45- and M36-deficient viruses are sensitive to TNF-α-induced NF-κB activation and apoptosis, respectively (reference 26 and this study). These results indicated that infected fibroblasts have enough TNFR1 molecules on the surface to respond to TNF-α stimulation, at least between 4 and 8 hpi, when TNF-α was added. Nevertheless, TNFR1 down-regulation may contribute to the unresponsiveness of MCMV-infected cells to TNF-α at later times.
Cells infected with m38.5-deficient MCMVs were more sensitive than wt virus-infected cells to stress induced by viral replication within the cells, as well as to stress exerted by exhausted growth medium, toxic secreted factors, or cytotoxic drugs such as STS and MG132. Premature cell death affected the growth kinetics of the MCMVΔm38.5 virus to various degrees, dependent on the infectious dose and the growth conditions. Surprisingly, the MCMVΔm38.5 mutant grew to almost the same titers as the wt virus when the cell culture medium was exchanged on a daily basis. This finding parallels results obtained previously with a UL37x1 deletion mutant of the HCMV strain Towne, which also grew almost to wt levels in human fibroblasts, despite the fact that increased apoptosis of infected cells was observed (28). However, UL37x1 deletion mutants of the HCMV strain AD169 were severely growth defective (12, 36, 40, 50), and a similar growth defect was observed with the HCMV strain FIX (our unpublished results). Since the UL37x1 vMIA proteins of AD169 and Towne are almost identical, it seems likely that the two HCMV strains differ in their propensities to induce apoptosis. The issue of which viral processes inside the cell induce apoptosis is not fully understood. However, the fact that the inhibition of viral DNA replication blocks the induction of apoptosis suggests that DNA replication itself or later processes such as virion assembly and egress are responsible. This possibility raises the question of why m38.5 is expressed at early times while its antiapoptotic function becomes important only at late times. The discrepancy is even more pronounced for HCMV, in which UL37x1 expression starts at immediate-early times (42). It is possible that enough vMIA protein needs to accumulate before DNA replication starts in order to block Bax-dependent cell death efficiently. Alternatively, m38.5 may have an additional function that is important already at early times, such as the induction of calcium release from endoplasmatic reticulum stores, a recently described function of the UL37x1 protein (40).
The complex structure of the M38-m38.5 locus made it difficult to construct a mutant virus in which only the m38.5 gene was inactivated. By deleting only 24 nt around the ATG start codon, a 391-nt stretch upstream of the M38 start codon was left intact. Whether this method was sufficient to preserve the normal expression of M38 could not be determined, because the boundaries of the M38 promoter have not been defined and an M38-specific antibody is not available. However, we provide two pieces of evidence showing that M38 does not play a significant role in the phenotype studied here. Firstly, the MCMV-m38.5HA virus carried the HA tag sequence at the C terminus of m38.5, which corresponds to the M38 ORF. The insertion of the HA tag coding sequence disrupted and terminated the M38 ORF (Fig. 1D). Nonetheless, the MCMV-m38.5HA mutant virus behaved like wt MCMV in replication and cell death assays. Secondly, the phenotype of the MCMVΔM37-m39 mutant was reversed by inserting only the m38.5 gene into the viral genome at a different location from the original gene position (Fig. 7). Hence, none of the M37, M38, and m39 ORFs seem to be required for the inhibition of apoptosis under the conditions tested here. This scenario is consistent with the results of a previous study, in which the authors found no mutant phenotype of an M37 mutant virus in cell culture (24). Interestingly, an antiapoptotic function was recently attributed to the HCMV UL38 protein (43), which shows sequence homology to M38 (27% identity and 46% similarity). Our results suggest that the experimental conditions in the present study may have been unsuitable to reveal a presumed antiapoptotic activity of M38. Alternatively, it is possible that M38 does not have the same antiapoptotic activity as UL38.
By contrast, m38.5 and UL37x1 proteins have little or no sequence homology but seem to be highly similar in their functions. Both localize to mitochondria, interact with Bax, and inhibit Bax-mediated PCD. Cells infected with m38.5 or UL37x1 deletion mutants show increased apoptosis compared to that of cells infected with wt virus and are more sensitive to stress and drugs activating the mitochondrial apoptosis pathway than wt virus-infected cells. The antiapoptotic function of m38.5 is probably conserved in rodent CMVs, as sequence homologs of the m38.5 gene can be found in the genomes of two different rat CMVs, i.e., the Maastricht and English isolates (reference 9 and S. Voigt, unpublished results). The m38.5 protein is slightly more homologous to e38.5 than to r38.5 but differs from both rat CMV proteins by a glutamine-rich sequence (Fig. 1A).
Mitochondrial inhibitors of apoptosis prevent the release of cytochrome c and the activation of caspase-9. However, there is also evidence that Bcl-2 and vMIA proteins can block certain forms of caspase-independent cell death (39). This possibility may explain why Bcl-XL can block the increased cell death caused by an m38.5-deficient virus while the pancaspase inhibitor z-VAD-fmk blocked cell death only partially, even at high concentrations.
The vMIAs of HCMV and MCMV differ from other viral mitochondrial apoptosis inhibitors in that they inhibit only Bax- and not Bak-mediated cell death (reference 4 and this study). This difference is surprising, because Bax and Bak can mediate apoptosis independently and many apoptosis-inducing stimuli activate both Bax and Bak (46). We have shown here that MCMV-infected cells are protected from PCD via Bax and via Bak but that m38.5 is responsible for the inhibition of the Bax-mediated pathway only. Consequently, MCMV must either encode a separate protein that inhibits Bak or up-regulate a cellular protein that interferes with the Bak-dependent pathway. The first possibility is supported by the recent identification of genes for four additional mitochondrion-localized proteins in the MCMV genome, the functions of which remain to be determined (41). By analogy, it seems likely that HCMV also encodes a protein with a Bak-inhibiting function.
Acknowledgments
We thank Kerstin Heyl for technical assistance and Georg Häcker and David Huang for making Bax/Bak knockout fibroblasts available to us.
This work was supported by the Deutsche Forschungsgemeinschaft grant SFB 421/TP B14 to W.B.
Footnotes
Published ahead of print on 5 March 2008.
REFERENCES
- 1.Altmann, M., and W. Hammerschmidt. 2005. Epstein-Barr virus provides a new paradigm: a requirement for the immediate inhibition of apoptosis. PLoS Biol. 3e404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andoniou, C. E., D. M. Andrews, M. Manzur, P. Ricciardi-Castagnoli, and M. A. Degli-Esposti. 2004. A novel checkpoint in the Bcl-2-regulated apoptotic pathway revealed by murine cytomegalovirus infection of dendritic cells. J. Cell Biol. 166827-837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andoniou, C. E., and M. A. Degli-Esposti. 2006. Insights into the mechanisms of CMV-mediated interference with cellular apoptosis. Immunol. Cell Biol. 8499-106. [DOI] [PubMed] [Google Scholar]
- 4.Arnoult, D., L. M. Bartle, A. Skaletskaya, D. Poncet, N. Zamzami, P. U. Park, J. Sharpe, R. J. Youle, and V. S. Goldmacher. 2004. Cytomegalovirus cell death suppressor vMIA blocks Bax- but not Bak-mediated apoptosis by binding and sequestering Bax at mitochondria. Proc. Natl. Acad. Sci. USA 1017988-7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aubert, M., L. E. Pomeranz, and J. A. Blaho. 2007. Herpes simplex virus blocks apoptosis by precluding mitochondrial cytochrome c release independent of caspase activation in infected human epithelial cells. Apoptosis 1219-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Benedict, C. A., T. A. Banks, and C. F. Ware. 2003. Death and survival: viral regulation of TNF signaling pathways. Curr. Opin. Immunol. 1559-65. [DOI] [PubMed] [Google Scholar]
- 7.Benedict, C. A., P. S. Norris, and C. F. Ware. 2002. To kill or be killed: viral evasion of apoptosis. Nat. Immunol. 31013-1018. [DOI] [PubMed] [Google Scholar]
- 8.Boya, P., A. L. Pauleau, D. Poncet, R. A. Gonzalez-Polo, N. Zamzami, and G. Kroemer. 2004. Viral proteins targeting mitochondria: controlling cell death. Biochim. Biophys. Acta 1659178-189. [DOI] [PubMed] [Google Scholar]
- 9.Brocchieri, L., T. N. Kledal, S. Karlin, and E. S. Mocarski. 2005. Predicting coding potential from genome sequence: application to betaherpesviruses infecting rats and mice. J. Virol. 797570-7596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brune, W., H. Hengel, and U. H. Koszinowski. 1999. A mouse model for cytomegalovirus infection, unit 19.7, p. 19.7.1-19.7.13. In J. E. Coligan, A. M. Kruisbeek, D. H. Margulies, E. M. Shevach, and W. Strober (ed.), Current protocols in immunology. John Wiley & Sons, New York, NY. [DOI] [PubMed]
- 11.Brune, W., C. Ménard, J. Heesemann, and U. H. Koszinowski. 2001. A ribonucleotide reductase homolog of cytomegalovirus and endothelial cell tropism. Science 291303-305. [DOI] [PubMed] [Google Scholar]
- 12.Brune, W., M. Nevels, and T. Shenk. 2003. Murine cytomegalovirus m41 open reading frame encodes a Golgi-localized antiapoptotic protein. J. Virol. 7711633-11643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brune, W., M. Wagner, and M. Messerle. 2006. Manipulating cytomegalovirus genomes by BAC mutagenesis: strategies and applications, p. 61-89. In M. J. Reddehase (ed.), Cytomegaloviruses: molecular biology and immunology. Caister Academic Press, Norfolk, United Kingdom.
- 14.Everett, H., M. Barry, S. F. Lee, X. Sun, K. Graham, J. Stone, R. C. Bleackley, and G. McFadden. 2000. M11L: a novel mitochondria-localized protein of myxoma virus that blocks apoptosis of infected leukocytes. J. Exp. Med. 1911487-1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldmacher, V. S. 2005. Cell death suppression by cytomegaloviruses. Apoptosis 10251-265. [DOI] [PubMed] [Google Scholar]
- 16.Goldmacher, V. S., L. M. Bartle, A. Skaletskaya, C. A. Dionne, N. L. Kedersha, C. A. Vater, J. Han, R. J. Lutz, S. Watanabe, E. D. McFarland, E. D. Kieff, E. S. Mocarski, and T. Chittenden. 1999. A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc. Natl. Acad. Sci. USA 9612536-12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Häcker, G., and A. Weber. 2007. BH3-only proteins trigger cytochrome c release, but how? Arch. Biochem. Biophys. 462150-155. [DOI] [PubMed] [Google Scholar]
- 18.Harvey, D. M., and A. J. Levine. 1991. p53 alteration is a common event in the spontaneous immortalization of primary BALB/c murine embryo fibroblasts. Genes Dev. 52375-2385. [DOI] [PubMed] [Google Scholar]
- 19.Hay, S., and G. Kannourakis. 2002. A time to kill: viral manipulation of the cell death program. J. Gen. Virol. 831547-1564. [DOI] [PubMed] [Google Scholar]
- 20.Hayajneh, W. A., A. M. Colberg-Poley, A. Skaletskaya, L. M. Bartle, M. M. Lesperance, D. G. Contopoulos-Ioannidis, N. L. Kedersha, and V. S. Goldmacher. 2001. The sequence and antiapoptotic functional domains of the human cytomegalovirus UL37 exon 1 immediate early protein are conserved in multiple primary strains. Virology 279233-240. [DOI] [PubMed] [Google Scholar]
- 21.Jurak, I., and W. Brune. 2006. Induction of apoptosis limits cytomegalovirus cross-species infection. EMBO J. 252634-2642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuwana, T., and D. D. Newmeyer. 2003. Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr. Opin. Cell Biol. 15691-699. [DOI] [PubMed] [Google Scholar]
- 23.Kvansakul, M., M. F. van Delft, E. F. Lee, J. M. Gulbis, W. D. Fairlie, D. C. Huang, and P. M. Colman. 2007. A structural viral mimic of prosurvival Bcl-2: a pivotal role for sequestering proapoptotic Bax and Bak. Mol. Cell 25933-942. [DOI] [PubMed] [Google Scholar]
- 24.Lee, M., J. Xiao, E. Haghjoo, X. Zhan, G. Abenes, T. Tuong, W. Dunn, and F. Liu. 2000. Murine cytomegalovirus containing a mutation at open reading frame M37 is severely attenuated in growth and virulence in vivo. J. Virol. 7411099-11107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loh, L. C., N. Balachandran, and L. F. Qualtiere. 1988. Characterization of a major virion envelope glycoprotein complex of murine cytomegalovirus and its immunological cross-reactivity with human cytomegalovirus. Virology 166206-216. [DOI] [PubMed] [Google Scholar]
- 26.Mack, C., A. Sickmann, D. Lembo, and W. Brune. 2008. Inhibition of proinflammatory and innate immune signaling pathways by a cytomegalovirus RIP1-interacting protein. Proc. Natl. Acad. Sci. USA 1053094-3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahy, B. W. J., and H. O. Kangro. 1996. Virology methods manual. Academic Press, San Diego, CA.
- 28.McCormick, A. L., C. D. Meiering, G. B. Smith, and E. S. Mocarski. 2005. Mitochondrial cell death suppressors carried by human and murine cytomegalovirus confer resistance to proteasome inhibitor-induced apoptosis. J. Virol. 7912205-12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCormick, A. L., A. Skaletskaya, P. A. Barry, E. S. Mocarski, and V. S. Goldmacher. 2003. Differential function and expression of the viral inhibitor of caspase 8-induced apoptosis (vICA) and the viral mitochondria-localized inhibitor of apoptosis (vMIA) cell death suppressors conserved in primate and rodent cytomegaloviruses. Virology 316221-233. [DOI] [PubMed] [Google Scholar]
- 30.Ménard, C., M. Wagner, Z. Ruzsics, K. Holak, W. Brune, A. Campbell, and U. Koszinowski. 2003. Role of murine cytomegalovirus US22 gene family members for replication in macrophages. J. Virol. 775557-5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Oliveira, S. A., S. H. Park, P. Lee, A. Bendelac, and T. E. Shenk. 2002. Murine cytomegalovirus m02 gene family protects against natural killer cell-mediated immune surveillance. J. Virol. 76885-894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pauleau, A. L., N. Larochette, F. Giordanetto, S. R. Scholz, D. Poncet, N. Zamzami, V. S. Goldmacher, and G. Kroemer. 2007. Structure-function analysis of the interaction between Bax and the cytomegalovirus-encoded protein vMIA. Oncogene 267067-7080. [DOI] [PubMed] [Google Scholar]
- 33.Pilder, S., J. Logan, and T. Shenk. 1984. Deletion of the gene encoding the adenovirus 5 early region 1b 21,000-molecular-weight polypeptide leads to degradation of viral and host cell DNA. J. Virol. 52664-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Poncet, D., N. Larochette, A. L. Pauleau, P. Boya, A. A. Jalil, P. F. Cartron, F. Vallette, C. Schnebelen, L. M. Bartle, A. Skaletskaya, D. Boutolleau, J. C. Martinou, V. S. Goldmacher, G. Kroemer, and N. Zamzami. 2004. An anti-apoptotic viral protein that recruits Bax to mitochondria. J. Biol. Chem. 27922605-22614. [DOI] [PubMed] [Google Scholar]
- 35.Popkin, D. L., and H. W. Virgin. 2003. Murine cytomegalovirus infection inhibits tumor necrosis factor alpha responses in primary macrophages. J. Virol. 7710125-10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reboredo, M., R. F. Greaves, and G. Hahn. 2004. Human cytomegalovirus proteins encoded by UL37 exon 1 protect infected fibroblasts against virus-induced apoptosis and are required for efficient virus replication. J. Gen. Virol. 853555-3567. [DOI] [PubMed] [Google Scholar]
- 37.Reeves, M. B., A. A. Davies, B. P. McSharry, G. W. Wilkinson, and J. H. Sinclair. 2007. Complex I binding by a virally encoded RNA regulates mitochondria-induced cell death. Science 3161345-1348. [DOI] [PubMed] [Google Scholar]
- 38.Roulston, A., R. C. Marcellus, and P. E. Branton. 1999. Viruses and apoptosis. Annu. Rev. Microbiol. 53577-628. [DOI] [PubMed] [Google Scholar]
- 39.Roumier, T., H. L. Vieira, M. Castedo, K. F. Ferri, P. Boya, K. Andreau, S. Druillennec, N. Joza, J. M. Penninger, B. Roques, and G. Kroemer. 2002. The C-terminal moiety of HIV-1 Vpr induces cell death via a caspase-independent mitochondrial pathway. Cell Death Differ. 91212-1219. [DOI] [PubMed] [Google Scholar]
- 40.Sharon-Friling, R., J. Goodhouse, A. M. Colberg-Poley, and T. Shenk. 2006. Human cytomegalovirus pUL37x1 induces the release of endoplasmic reticulum calcium stores. Proc. Natl. Acad. Sci. USA 10319117-19122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tang, Q., E. A. Murphy, and G. G. Maul. 2006. Experimental confirmation of global murine cytomegalovirus open reading frames by transcriptional detection and partial characterization of newly described gene products. J. Virol. 806873-6882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tenney, D. J., and A. M. Colberg-Poley. 1991. Expression of the human cytomegalovirus UL36-38 immediate early region during permissive infection. Virology 182199-210. [DOI] [PubMed] [Google Scholar]
- 43.Terhune, S., E. Torigoi, N. Moorman, M. Silva, Z. Qian, T. Shenk, and D. Yu. 2007. Human cytomegalovirus UL38 protein blocks apoptosis. J. Virol. 813109-3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tschopp, J., M. Thome, K. Hofmann, and E. Meinl. 1998. The fight of viruses against apoptosis. Curr. Opin. Genet. Dev. 882-87. [DOI] [PubMed] [Google Scholar]
- 45.Wasilenko, S. T., T. L. Stewart, A. F. Meyers, and M. Barry. 2003. Vaccinia virus encodes a previously uncharacterized mitochondrial-associated inhibitor of apoptosis. Proc. Natl. Acad. Sci. USA 10014345-14350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wei, M. C., W. X. Zong, E. H. Cheng, T. Lindsten, V. Panoutsakopoulou, A. J. Ross, K. A. Roth, G. R. MacGregor, C. B. Thompson, and S. J. Korsmeyer. 2001. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292727-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.White, E. 2006. Mechanisms of apoptosis regulation by viral oncogenes in infection and tumorigenesis. Cell Death Differ. 131371-1377. [DOI] [PubMed] [Google Scholar]
- 48.Willis, S. N., L. Chen, G. Dewson, A. Wei, E. Naik, J. I. Fletcher, J. M. Adams, and D. C. Huang. 2005. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 191294-1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yu, D., H. M. Ellis, E. C. Lee, N. A. Jenkins, N. G. Copeland, and D. L. Court. 2000. An efficient recombination system for chromosome engineering in Escherichia coli. Proc. Natl. Acad. Sci. USA 975978-5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu, D., M. C. Silva, and T. Shenk. 2003. Functional map of human cytomegalovirus AD169 defined by global mutational analysis. Proc. Natl. Acad. Sci. USA 10012396-12401. [DOI] [PMC free article] [PubMed] [Google Scholar]