Abstract
A flurry of recent reports on the role of activating and inhibitory forms of the killer cell immunoglobulin-like receptors (KIR) in natural killer (NK) cell activity against human immunodeficiency virus type 1 (HIV-1) have yielded widely divergent results. The role of the activating NK receptor encoded by the KIR3DS1 allele and its putative ligands, members of the HLA class I Bw4Ile80 cluster, in early HIV-1 disease is controversial. We selected 60 treatment-naïve adults for study from the OPTIONS cohort of individuals with early HIV-1 infection in San Francisco. We performed NK cell functional assays measuring gamma interferon (IFN-γ) and CD107a expression by NK cells in the unstimulated state and after stimulation by the major histocompatibility complex class I-deficient 721.221 B-lymphoblastoid cell line. In addition, we measured CD38 expression (a T-cell activation marker) on T and NK cells. Persons who have at least one copy of the KIR3DS1 gene had higher IFN-γ and CD107a expression in the unstimulated state compared to those who do not possess this gene. After stimulation, both groups experienced a large induction of IFN-γ and CD107a, with KIR3DS1 carriers achieving a greater amount of IFN-γ expression. Differences in effector activity correlating with KIR3DS1 were not attributable to joint carriage of HLA Bw4Ile80 and KIR3DS1. We detected a partial but not complete dependence of KIR3DS1 on the members of B*58 supertype (B*57 and B*58) leading to higher NK cell function. Possessing KIR3DS1 was associated with lower expression of CD38 on both CD8+ T and NK cells and with a loss or weakening of the known strong associations between CD8+ T-cell expression of CD38 mean fluorescence intensity and the HIV-1 viral load. We observed that possessing KIR3DS1 was associated with higher NK cell effector functions in early HIV-1 disease, despite the absence of HLA Bw4Ile80, a putative ligand of KIR3DS1. Carriage of KIR3DS1 was associated with diminished CD8+ T-cell activation, as determined by expression of CD38, and a disruption of the traditional relationship between viral load and activation in HIV-1 disease, which may lead to better clinical outcomes for these individuals.
NK cell function is regulated by a family of receptors encoded by the killer cell immunoglobulin-like receptor (KIR) genes (18, 33). Within the KIR family, certain genes encode inhibitory receptors that recognize HLA class I ligands (i.e., HLA-Bw4 or HLA-C), whereas other KIR genes encode activating receptors which are not completely known. Studies on the role of KIRs in human immunodeficiency virus (HIV) disease have focused on the activating receptor encoded by the KIR3DS1 allele. However, recent genetic association and functional studies of KIR and HIV disease have yielded widely disparate results on the role of KIR3DS1 and its putative ligands, a subset of HLA class I-B alleles referred to as Bw4Ile80. The Bw4Ile80 cluster is a subset of HLA-B alleles that bear an isoleucine at position 80 in the α-1 helix, on the rim of the peptide-binding cleft. The inhibitory receptors encoded by KIR3DL1 alleles, which are highly related in the extracellular domains to the activating receptor encoded by KIR3DS1, specifically recognize HLA-Bw4 ligands (5). Because of this similarity, KIR3DS1 has been assumed to also recognize Bw4Ile80 ligands. In 2002, Martin et al. reported that HIV-infected individuals in the Multicenter AIDS Cohort Study possessing the KIR3DS1 allele demonstrated significantly delayed progression to AIDS, provided that the individuals also expressed a Bw4Ile80 allele (20).
In 2005, Gaudieri et al. reported on the association of the entire KIR gene cluster and HLA class I in HIV disease progression in an Australian HIV cohort (8, 9). These authors observed a trend toward slowed CD4+ T-cell percent loss among those who carried both Bw4Ile80 and KIR3DS1 (8). However, this trend was not statistically significant, and Gaudieri et al. simultaneously observed an acceleration of time to AIDS (1987 definition) among joint KIR3DS1 and Bw4Ile80 carriers. In 2006, Qi et al. published a follow-up report from the Multicenter AIDS Cohort Study cohort documenting an association between the coexpression of KIR3DS1 and Bw4Ile80 and enhanced protection against certain opportunistic infections in HIV-infected individuals (26), an effect partially attributed to very modest differences in viral load. In 2007, our group observed that KIR3DS1 gene carriage was associated with higher CD4+ T-cell counts and hence protection against HIV type 1 (HIV-1) progression in early disease (4); however, we observed that this effect was not attributable to differences in the viral load and further was independent of Bw4Ile80. In other words, our analyses suggested that the KIR3DS1 and Bw4Ile80 genes were each associated with protection against HIV disease but via different mechanisms.
Until recently, it was not clear if KIR3DS1 was expressed on the surface of NK cells; however, two recent reports have conclusively established that KIR3DS1 is expressed on NK cells (6, 24) and that expression is dose dependent, with higher expression for homozygotes. These studies also demonstrated that KIR3DS1 recognizes neither HLA-Bw4 nor HLA-Bw6 ligands, at least when these major histocompatibility complex (MHC) class I molecules are expressed on Epstein-Barr virus-transformed B-lymphoblastoid cell lines. Similarly, an independent study by another group reported that KIR3DS1 fails to bind to soluble Bw4Ile80 tetrameric complexes (10). In contrast, Alter et al. have presented results from in vitro cytotoxicity assays suggesting that target cells possessing HLA-Bw4Ile80 are better targets for NK cells possessing KIR3DS1 (1); however, no evidence was provided to confirm a physical interaction between the KIR3DS1 and HLA-Bw4 proteins.
Here, we present a study of the NK cell phenotype and function in 60 treatment-naïve, recently HIV-1-infected persons with defined HLA-B and KIR3DS1/KIR3DL1 allotypes. We also measured the expression of CD38 on NK cells and CD8+ T cells, a widely used marker of disease progression and virulence in HIV research and a marker of immune activation. The expression of CD38, as measured by flow cytometry, is known to be elevated on CD8+ T cells in HIV disease, reaching steady-state levels in early HIV-1 infection (7), and predicts disease progression independently of the viral load (19). The individuals studied were selected from our recent genetic association study of KIR and HLA among 255 recently HIV-1-infected persons (4), in which KIR3DS1 carriage alone was associated with higher CD4+ T-cell counts, despite the absence of a difference in the viral loads. On the basis of these clinical findings, we performed this study to determine whether persons who carried the KIR3DS1 gene had enhanced NK cell phenotypic and functional profiles and if these profiles were further enhanced by carriage of the putative KIR3DS1 ligands encoded by HLA-Bw4Ile80 alleles. Flow cytometry-based detection of KIR3DS1 has been hampered by the absence of a monoclonal antibody that can bind to KIR3DS1 specifically and not cross-react with the related KIR3DL1 proteins (25). Hence, we used genotypic KIR assignments for our analyses rather than flow cytometry-based methods.
MATERIALS AND METHODS
Study overview.
We selected 60 adults from the OPTIONS cohort study of early HIV-1 infection in San Francisco (3). All persons gave informed consent to participate in this study, and this study was approved by the University of California, San Francisco (UCSF), Committee on Human Research. Individuals were selected on the basis of having known HLA-A and -B types, KIR3DS1 and KIR3DL1 genotype assignments, a record of clinical parameters of HIV-1 infection prior to treatment, and banked, viably frozen peripheral blood mononuclear cells (PBMCs). The date of estimated HIV-1 infection was derived through an algorithm developed by F. M. Hecht and is based on the time of detection of HIV-1 RNA, the level and timing of the results of the detuned HIV-1 enzyme immunosorbent assay (14, 15), the timing of prior HIV-1 test results, self-reported HIV-1 exposures, and symptomatology consistent with acute HIV-1 infection.
Determination of KIR3DS1 and KIR3DL1 genotypes.
We determined the KIR3DS1 and KIR3DL1 genotypes as previously described (4).
Cell staining and flow cytometric analysis.
Cryopreserved PBMCs were thawed and washed with phosphate-buffered saline supplemented with 1% bovine serum albumin and 2 mM EDTA (FACS buffer). For staining, 5 × 105 cells were incubated with purified human immunoglobulin G (100 μg/ml) to block nonspecific binding. To define NK cells, we stained the cells with phycoerythrin-Texas Red-conjugated anti-CD3, Alexa700-conjugated anti-CD4, phycoerythrin-Cy7-conjugated anti-CD56, and Pacific Blue-conjugated anti-CD16. Anti-CD14 and anti-CD19 (both conjugated to allophycocyanin-Cy7) were used to collectively exclude monocytes and B cells. Freshly thawed or stimulated PBMCs were stained for cell surface antigens (CD3, CD4, CD8, CD14, CD19, CD56, and CD16; all antibodies were from Becton Dickinson, San Jose, CA) and stained with Amine Aqua (Invitrogen) to exclude dead cells. Cells were fixed in 2% paraformaldehyde and permeabilized with FACS-perm (Becton Dickinson, San Jose, CA), with the exception of CD3-phycoerythrin-Texas Red (Beckman Coulter, Miami, FL). Permeabilized cells were stained for intracellular gamma interferon (IFN-γ). Fluorescence minus one samples were prepared for each fluorochrome to facilitate gating (28). All cells were fixed with 2% paraformaldehyde and analyzed by flow cytometry with a four-laser LSR-II instrument (Becton Dickinson, San Jose, CA). Anti-mouse immunoglobulin G-coated beads were stained with each fluorochrome-conjugated mouse antibody separately and used for software-based compensation.
Cell culture and antigenic stimulation.
Cryopreserved PBMCs stored by the UCSF/ARI AIDS Specimen Bank were thawed and used for measurements of NK cell frequency, number, and receptor expression. The thawed cells were washed with RPMI 1640 medium supplemented with 15% fetal bovine serum before staining or stimulation. NK cells and T-cell functions were assessed by cytokine flow cytometry. To measure NK cell function, PBMCs were cultured in medium alone or with MHC class I-deficient 721.221 cells (1:5 ratio). PBMCs cultured in medium alone were used for the measurement of endogenous NK cell function. Briefly, a 100-μl volume of thawed PBMCs was cultured at 5 × 105/ml in 96-well plates with the respective stimulant and 10 μg/ml fluorescein isothiocyanate-conjugated anti-CD107a antibody (Becton Dickinson, San Jose, CA) for 24 h; during the last 6 h of culture, monensin and brefeldin A were added to allow intracellular accumulation of cytokines. Data analysis was performed by using FlowJo flow cytometric analysis software (Tree Star, Ashland, OR). Statistical analysis was performed by using SAS System 9 for Windows XP (SAS Institute, Cary, NC) or GraphPad Prism statistical software (GraphPad Software, San Diego, CA). The Mann-Whitney U or unpaired t test with Welch's correction (which does not assume equal variances) was used for comparisons based on the results of Kolmogorov Smirnov normality tests. The Spearman rank test was used for correlation analyses.
RESULTS
Study sample description.
Demographic and baseline characteristics of the patient population are shown in Table 1. The persons selected for this study did not differ from other members of the OPTIONS early-infection cohort with respect to age, gender, ethnicity, or KIR or HLA type (4). Forty-three percent of the cohort (26 persons) had at least one copy of KIR3DS1. Four patients were homozygous for KIR3DS1. Fifty percent, or 30 persons, possessed at least one copy of a HLA-Bw4Ile80 gene, the putative ligand for KIR3DS1. Twenty percent of the cohort, or 12 persons, carried at least one copy of both KIR3DS1 and Bw4Ile80. We detected no difference in the estimated length of infection at the time of this study by KIR3DS1 carriage (P = 0.25), joint KIR3DS1 and Bw4Ile80 carriage (P = 0.8), Bw4Ile80 carriage (P = 0.4), B*58 supertype carriage (P = 0.6), or joint KIR3DS1 and B*58 supertype carriage (P = 0.2) (Mann-Whitney U test).
TABLE 1.
KIR and HLA genetics and characteristics of treatment-naïve recently HIV-1-infected adults
| Variable | Median (IQRa) or % | No. of patients |
|---|---|---|
| Age (yr) | 35 (32, 39) | 60 |
| HIV-1 RNA (log10 copies/ml) | 4.7 (3.8, 5.1) | 60 |
| CD4+ T-cell counts/μl | 552 (462, 748) | 60 |
| Estimated time (wk) infectedb | 13.1 (10.1, 19.1) | 60 |
| Male gender | 97 | 58 |
| Ethnicity | ||
| Asian | 8 | 5 |
| Caucasian | 68 | 41 |
| African-American | 8 | 5 |
| Latino | 15 | 9 |
| Gene carriage | ||
| KIR3DS1 | 43 | 26 |
| Homozygous | 7 | 4 |
| KIR3DL1 | 93 | 56 |
| Homozygous | 57 | 34 |
| HLA class I Bw4 | 67 | 40 |
| Bw4Ile80 | 50 | 30 |
| KIR3DS1 and Bw4Ile80 | 20 | 12 |
| HLA-B*57 | 12 | 7 |
| HLA-B*58 | 7 | 4 |
| B*58 supertype | 18.3 | 11 |
| KIR3DS1 and B*58 supertype | 5 | 3 |
IQR, interquartile range (25th, 75th percentiles).
Estimated time since HIV-1 infection (see Materials and Methods for reference).
NK cell effector measurements.
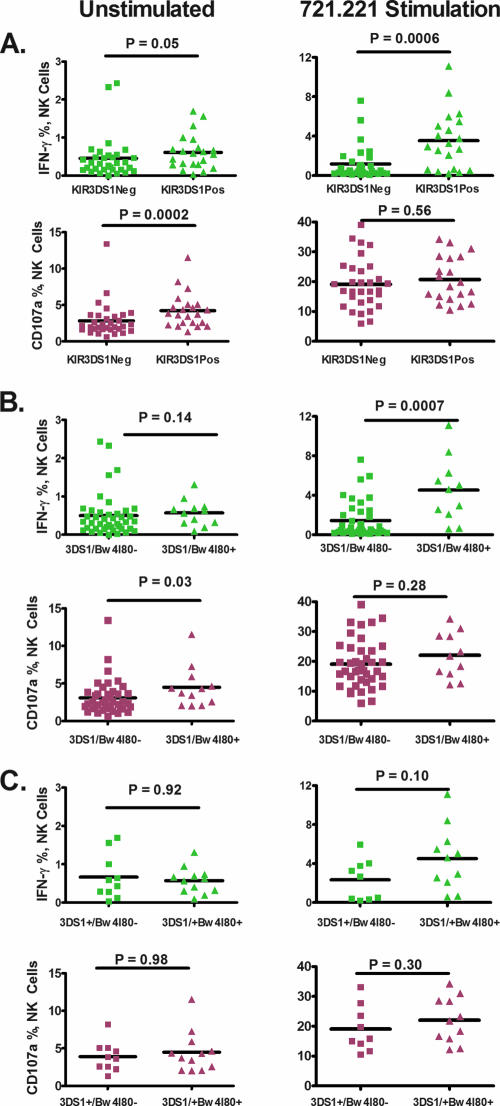
Staining and flow cytometry gating strategies for NK cell assignment and measurement of function are shown in Fig. 1. We observed that persons with at least one copy of the KIR3DS1 gene had higher IFN-γ and CD107a expression on NK cells in the basal, unstimulated state than those who did not bear at least one copy (Fig. 2A, column 1). We stimulated PBMCs from these individuals by coculture with the MHC class I-deficient 721.221 B-lymphoblastoid cell line (column 2, all panels, Fig. 2). 721.221 cells do not express any MHC class I proteins (HLA-A, HLA-B, HLA-C, HLA-E, or HLA-G) on their surface; therefore, they fail to express any known ligands for the inhibitory and activating KIR. Although these 721.221 stimulator cells lack all MHC class I proteins, we observed that NK cells from individuals with at least one copy of KIR3DS1 had higher IFN-γ expression, and equivalent CD107a expression, compared with those who did not bear KIR3DS1 (and are therefore KIR3DL1 carriers) (Fig. 2A, column 2). KIR3DS1 homozygotes did not differ from KIR3DS1 heterozygotes by IFN-γ or CD107a measurements in the stimulated or unstimulated state (data not shown). As we have previously observed in the larger cohort of 255 patients from which these patients were drawn (4), we did not detect a difference in viral loads between the KIR3DS1 carriers and those negative for this gene (P = 0.62). In the subsample, we observed no difference between the viral loads of those with joint carriage of KIR3DS1 and Bw4Ile80 (P = 0.55) and those who carried either one of the genes or neither, nor did we detect a difference in the viral loads of KIR3DS1 carriers who did or did not possess Bw4Ile80 (P = 0.2).
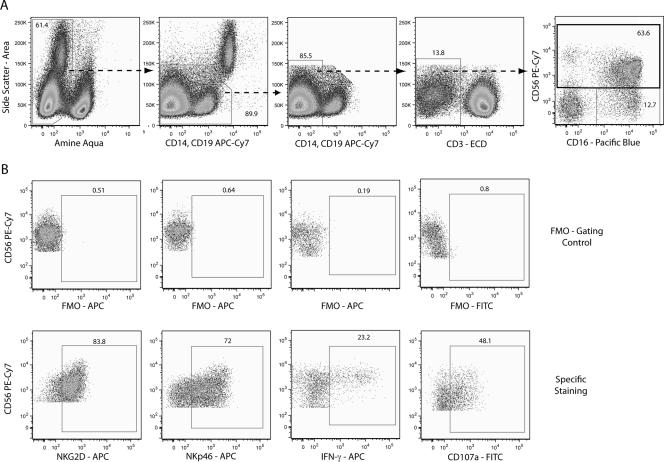
FIG. 1.
Gating strategy to identify NK cell populations and functional measurements. (A) Dead cells were excluded by using amine-reactive dye, and monocytes and B cells were excluded on the basis of CD14 and CD19 gating, respectively. CD4+ and CD8+ T cells and CD3− cells were gated from the CD14- and CD19-negative lymphocyte population, and NK cells were derived from the CD3− gate on the basis of the expression of CD16 and CD56. NK cells were then subdivided into CD56bright and CD56dim. (B) Cells positive for CD16 and CD56 were analyzed for surface expression of NKG2D, NKp46, and CD107a and intracellular expression of IFN-γ. FMO, fluorescence minus one; APC, allophycocyanin; ECD, phycoerythrin-Texas Red; FITC, fluorescein isothiocyanate.
FIG. 2.
NK cell functional activity by KIR and Bw4 gene combinations. Columns 1 and 2 refer to IFN-γ (green) or CD107a (purple) measurements in unstimulated cells and cells stimulated by coculture with the MHC class I-deficient 721.221 cell line, respectively. (A) KIR3DS1Pos refers to those who carry at least one copy of KIR3DS1. Those who are KIR3DS1 negative (KIR3DS1Neg) are homozygous for KIR3DL1. (B) 3DS1/Bw4I80+ refers to those who have the compound genotype, possessing both KIR3DS1 and Bw4Ile80, whereas those who are 3DS1/Bw4I80 negative are those who have one or the other gene (but not both) or have neither. This is the comparison used in the earliest genetic association studies, and its results were interpreted to signify synergy between the two gene products. (C) Persons who are KIR3DS1 carriers. The 3DS1+/Bw4I80− persons are those who carry KIR3DS1 but not the Bw4Ile80 gene, whereas the 3DS1/Bw4I80+ persons are those who have KIR3DS1 and also carry Bw4Ile80. This third comparison allows one to directly test if the presence or absence of Bw4Ile80 among KIR3DS1 carriers is associated with differences in NK cell functional measurements. The Mann-Whitney U test was used for comparisons because of the nonnormal distribution of the underlying data.
Discerning the role of Bw4Ile80 in KIR3DS1-associated NK cell function.
We then compared IFN-γ and CD107a expression in NK cells from individuals with defined KIR and Bw4Ile80 haplotypes. As shown in Fig. 2B, we compared those who carried both KIR3DS1 and Bw4Ile80 to those carrying only one of these genes or neither gene. This was the same category definition used for comparisons in the genetic association studies reported by Martin et al. (20). We observed that carriers of this compound genotype, that is, those who carried both KIR3DS1 and Bw4Ile80, had higher IFN-γ and CD107a expression levels than those who were not carriers of both genes (Fig. 2B).
Does possession of Bw4Ile80 augment KIR3DS1-associated NK cell effector function?
As shown in Fig. 2C, we performed one further comparison, in which we restricted the analysis to individuals carrying KIR3DS1. We sought to determine if those who bear both KIR3DS1 and a Bw4Ile80 allele had higher levels of IFN-γ or CD107a expression, in either the unstimulated or the stimulated state, than those who possess KIR3DS1 but not Bw4Ile80. By making this comparison, we were testing to see if the presence of a putative activating ligand for KIR3DS1 is associated with greater effector activity in the unstimulated or stimulated state. This is a more direct test of KIR3DS1 dependence on the Bw4Ile80 ligand than that shown in Fig. 2B. When this comparison was made, we observed no difference in the levels of IFN-γ or CD107a expression by NK cells among KIR3DS1 carriers who did or did not carry a Bw4Ile80 allele (Fig. 2C). Taken together, our data suggest that Bw4Ile80 carriage does not influence the functional profile of NK cells among persons who bear KIR3DS1.
Role of HLA-B*57 and -B*58 in KIR3DS1- or KIR3DL1-associated NK cell activity.
In our study population, 11 persons carried either the HLA-B*57 or the HLA-B*58 allele (Table 1), which form the B*58 supertype (31). We used the B*58 supertype, rather than either allele alone, to increase the power of our statistical analysis and hence our ability to detect differences in NK cell activity, should they exist. B*58 supertype carriers are distinct from the individuals possessing other alleles within the Bw4Ile80 cluster by virtue of their independent association with greatly slowed HIV disease progression. A recent report suggested that B*57 interacts differentially with specific KIR allotypes to confer added protection (22). We sought to determine if there was evidence of enhancement of joint carriage among B*58 supertype and KIR3DS1 carriers. We compared joint KIR3DS1 and B*58 supertype carriers to those who carried KIR3DS1 but not a B*58 supertype allele. In an analysis limited by sample size (only three persons carried both KIR3DS1 and B*58 supertype), we observed that joint carriers had higher NK CD107a levels in the unstimulated state (5.9% versus 3.5% [P = 0.04]) and higher CD107a and IFN-γ levels when cocultured with MHC class I-deficient 721.221 target cells (28.4% versus 16.2% [P = 0.04] and 8.4% versus 2.6% [P = 0.007], respectively). We observed no difference in IFN-γ levels in NK cells in the unstimulated state (0.56% versus 0.51% [P = 0.9]). B*58 supertype carriage did not account for the generally higher NK cell functional measurements among the KIR3DS1 carriers.
CD38 expression on NK cells and CD8+ T cells in early HIV-1 infection and the role of KIR3DS1.
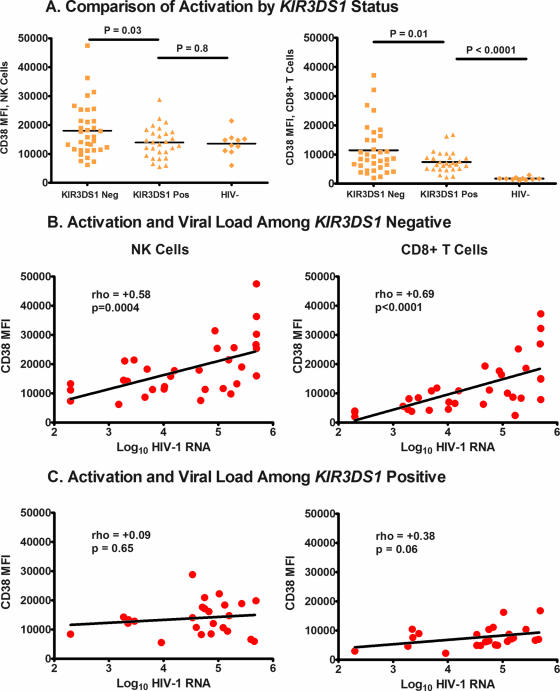
We examined the expression of CD38 on NK cells and CD8+ T cells by measuring the median fluorescence intensity (MFI) by flow cytometry. T-cell levels of CD38 achieved in early infection are an effective proxy for the risk of disease progression (7). We evaluated whether the KIR type might influence CD38 expression levels on CD8+ T cells and NK cells. The significance of CD38 up-regulation on NK cells in HIV disease is not clear. Between those who carried and those who did not carry KIR3DS1, the MFI of CD38 on NK and T cells was strongly associated (rho = 0.61 [P = 0.001] and rho = 0.59 [P = 0.0003], respectively [Spearman test]; data not shown). However, we observed that the level of CD38 expression (as reflected by the MFI) was lower on both NK and CD8+ T cells among those who possessed KIR3DS1 than among those who did not (Fig. 3A). There was no difference in the CD38 MFI on NK cells between those who were HIV-1 infected and carried KIR3DS1 and those were not infected (and were of unknown KIR type). Among those who carried KIR3DS1, we observed (i) no relationship between CD38 MFI on NK cells and the HIV-1 load (Fig. 3C) and (ii) a weakening of the relationship between CD38 MFI expression on CD8+ T cells and the HIV-1 load (Fig. 3C). Among those who do not carry KIR3DS1, there was a strong and direct correlation between the NK and CD8+ T-cell CD38 MFI and the HIV-1 load (Fig. 3B).
FIG. 3.
(A) MFI for CD38 on NK and CD8+ T cells, respectively, by KIR3DS1 carriage. We used an unpaired t test with the Welch correction for these comparisons. (B) Relationship of CD38 expression on NK and CD8+ T cells to the HIV-1 load among those who are not KIR3DS1 carriers. The Spearman rank correlation test was used for the correlation analysis. (C) Relationship of CD38 expression on NK and CD8+ T cells to the HIV-1 load among those who are KIR3DS1 carriers. The Spearman rank correlation test was used for the correlation analysis.
We considered the possibility that the association of KIR3DS1 and the CD38 MFI was due to a direct or indirect interaction with MHC class I ligands. We found that joint carriage of KIR3DS1 and Bw4Ile80 did not associate with differences in NK or CD8+ T-cell CD38 MFI levels (P = 0.69 and P = 0.32, respectively). We did find carriage of B*58 supertype to be marginally associated with a lower CD8+ T-cell CD38 MFI (P = 0.09) but not with the NK cell CD38 MFI (P = 0.31). We found carriage of Bw4Ile80 cluster alleles not to be associated with differences in either the CD8+ T-cell or the NK cell CD38 MFI (P = 0.66 and P = 0.88, respectively). Those who carried both B*58 supertype and KIR3DS1 did not show a lower CD8+ T-cell or NK cell CD38 MFI (P = 0.39 and P = 0.86, respectively [Mann-Whitney U test]).
DISCUSSION
In our study, we observed that recently HIV-1-infected persons who carried at least one copy of the KIR3DS1 gene had higher NK cell effector activity in the unstimulated and stimulated state and had lower expression of CD38 (a marker previously associated with T-cell activation) on their NK and T cells compared with those who did not have KIR3DS1. We observed that the effect of KIR3DS1 on NK cell function was independent of carriage of HLA-Bw4Ile80, the putative ligand for KIR3DS1.
KIR3DS1 carriage was associated with higher expression of IFN-γ and CD107a on NK cells in the unstimulated state. After stimulation by MHC class I-deficient 721.221 target cells and despite the lack of presentation of any ligand to the activating KIR3DS1 receptor, we observed that KIR3DS1 carriers expressed more IFN-γ and equivalent levels of CD107a (Fig. 2A). The increase in functional activity among KIR3DS1 carriers was not attributable to carriage of an HLA-Bw4Ile80 allele by other cells in the culture. That is, among persons who carried KIR3DS1, the carriage of an HLA-Bw4Ile80 allele was not associated with an additional increases in either basal or stimulated levels of IFN-γ or CD107a expression on NK cells (Fig. 2C). Hence, we have presented evidence that KIR3DS1 correlates with greater NK cell effector function among persons in early HIV-1 infection. We cannot exclude the possibility that KIR3DS1 has ligands other than HLA-Bw4 that might be present in these cultures (2).
Although widely expressed on NK cells, KIRs are also expressed on T cells (32), which we observed at low frequencies (in the range of 2 to 4% of the T cells in these samples; data not shown) (6, 32). KIR expression on T cells may be up-regulated in certain chronic inflammatory conditions, such as arthritis (12). In HIV-1 disease, itself a chronic inflammatory condition, KIR might be acting to modulate T-cell signaling, which may have broad effects on these T cells (13), thereby influencing their activation state.
We observed that KIR3DS1 carriage was associated with lower expression of CD38 on CD8+ T cells and NK cells. This effect was not attributable to carriage of the HLA-B*57 or HLA-B*58 protective allele. The significance of CD38 expression on NK cells in HIV-1 disease is not known. In prior studies, CD38 expression on NK cells has been shown to be up-regulated after interleukin-2 exposure, and cross-linking of CD38 causes NK cells to release granzyme and cytokines (29). Therefore, it is not clear if higher CD38 expression on NK cells is predictive of poorer HIV disease outcomes but in our analyses does appear to correlate with a higher viral load. In HIV-1 disease, it is commonly observed that CD38 levels on CD8+ T cells strongly correlate with HIV-1 viral loads (7). In our study, among those who carried KIR3DS1, we observed a weakening or loss of the expected strong associations between CD8+ T-cell CD38 expression and the viral load. This association was preserved in those who did not carry KIR3DS1 (Fig. 3B.). The viral load and expression of CD38 on T cells may be partially uncoupled among KIR3DS1 carriers.
Elevated CD8+ T-cell activation, as measured by the CD38 MFI, is known to be an independent predictor of poor disease outcomes in HIV disease (11, 19). Elevated T-cell activation levels, but not high-level viral replication, distinguish pathogenic from nonpathogenic primate lentiviral infections (17). In other words, a high viral load without accompanying elevated CD8+ T-cell activation is not associated with progressive immunodeficiency. Hence, the difference in activation state and NK cell function we observed here are consistent with a prior clinical genetic epidemiology report by our group that KIR3DS1 carriage is associated with elevated CD4+ T-cell counts in early HIV-1 infection, despite little or no difference in the viral load (4). It has recently been observed that the HIV-1 load only partially explains the variation in CD4+ T-cell decline among individuals, suggesting that other factors—viral or host derived—might modulate the relationship between the viral load and the virulence of the infection (27). Our findings suggest that KIR3DS1 carriage may be one of these factors.
Most of the persons in our study who carried KIR3DS1 (Table 1) also carried a copy of KIR3DL1. Individual NK cells typically only express a single KIR allele from a given locus. However, because of a lack of specific serological reagents, we were unable to directly address the frequency of NK cells within the population that expressed KIR3DS1, KIR3DL1, or both receptors in these heterozygous individuals (25). In our studies, we measured the behavior of the entire NK cell pool from these persons; hence, the effects of KIR3DS1 on NK cell function and disease outcome seen here might be diluted. In this study, we examined only four persons homozygous for KIR3DS1 and thus were underpowered to characterize them in detail. The low frequency of KIR3DS1 homozygosity in our study population of HIV-1-infected persons might indicate protection against HIV-1 transmission (23, 30) among exposed carriers. Absence of KIR inhibitory signals, and therefore the potential for higher NK cell activation, has been associated with protection against HIV-1 acquisition among highly exposed individuals in other studies (16).
In an analysis of limited statistical power (n = 3 versus n = 18), we observed some evidence that those carrying B*58 supertype (a B*57 or B*58 allele) and KIR3DS1 had higher NK cell functional activity in both the unstimulated and stimulated states. This result may suggest that KIR3DS1 is preferentially ligated by these select members of the Bw4Ile80 cluster. Alternatively, these MHC class I alleles might have effects on NK cell activity through another mechanism, perhaps affecting another NK-activating receptor family or via an effect on dendritic cells. Of note, KIR3DS1 carriage was associated with elevated NK cell functional measurements even among those who do not carry B*58 supertype, suggesting only a partial dependence on this allele. Persons who carry KIR3DS1 and B*58 supertype likely represent around 3 to 5% of the general population of persons with HIV-1 infection (based on our data), making them a rare population. Although this analysis is intriguing, it must be regarded as preliminary, bearing examination in a larger cohort.
A recent KIR genetic association study addressing HIV outcomes has examined KIR3DL1*004, an inhibitory KIR variant that is not expressed on the cell surface (22). When present along with HLA-B*57, KIR3DL1*004 carriage was associated with strong protection against HIV disease progression. That a KIR variant that does not reach the cell surface would be associated with dramatic protection is intriguing. The simplest explanation of these recent data is that inhibitory KIR3DL1*004 is rarely or never ligated and therefore fails to restrain NK cell activity, leading to more NK effector activity in vivo. The emerging story for KIR3DL1*004 may have characteristics in common with that for KIR3DS1, which, by our studies, does not appear to require its putative ligand (Bw4Ile80 cluster) to confer protection against HIV-1. Functional studies of KIR ligands that do not reach the NK cell surface may prove challenging (21) but are required to define the role of these intriguing variants.
In summary, we observed that recently HIV-1-infected adults who carried KIR3DS1 had higher NK cell effector functions in both the unstimulated and stimulated states than those who did not carry KIR3DS1. This effect was not dependent on the carriage of a Bw4Ile80 allele, the putative ligand for KIR3DS1. We further observed that persons who carried KIR3DS1 had lower CD38 expression on NK and CD8+ T cells, the latter of which is an important marker of disease progression in HIV disease. These effects were not attributable to joint carriage of Bw4Ile80. We previously observed that recently HIV-1-infected persons who carried KIR3DS1 had higher CD4+ T-cell counts despite the absence of a difference in the viral load. KIR3DS1 may be mediating that effect on CD4+ T-cell counts via partial uncoupling of T-cell activation and the viral load. How KIR3DS1 carriage or expression might mediate this effect is not known but will be an important area of research.
Acknowledgments
This work was supported by grants from the National Institutes of Health (NIH 1UO1 AI41531 to J. A. Levy and F. M. Hecht, P01-AI064520 to D. F. Nixon and L. L. Lanier, and AI 066917 and AI076014 to J. D. Barbour) and the Irvington Institute for Immunological Research (to L. C. Ndhlovu). L.L.L. is an American Cancer Society Research Professor.
We thank Uma Srirams and Stacy Caillier for assistance with KIR genotyping. We thank Gerald Spotts for assistance with data management and the OPTIONS study staff for assistance with patient recruitment and follow-up. We acknowledge the UCSF/AIDS Research Institute AIDS Specimen Bank (J. S. Greenspan), which prepared and stored the viably frozen PBMC aliquots used here. We thank the OPTIONS study participants for their time and effort.
Footnotes
Published ahead of print on 27 February 2008.
REFERENCES
- 1.Alter, G., M. P. Martin, N. Teigen, W. H. Carr, T. J. Suscovich, A. Schneidewind, H. Streeck, M. Waring, A. Meier, C. Brander, J. D. Lifson, T. M. Allen, M. Carrington, and M. Altfeld. 2007. Differential natural killer cell-mediated inhibition of HIV-1 replication based on distinct KIR/HLA subtypes. J. Exp. Med. 2043027-3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altfeld, M., and P. Goulder. 2007. ‘Unleashed’ natural killers hinder HIV. Nat. Genet. 39708-710. [DOI] [PubMed] [Google Scholar]
- 3.Barbour, J. D., F. M. Hecht, T. Wrin, M. R. Segal, C. A. Ramstead, T. J. Liegler, M. P. Busch, C. J. Petropoulos, N. S. Hellmann, J. O. Kahn, and R. M. Grant. 2004. Higher CD4+ T cell counts associated with low viral pol replication capacity among treatment-naive adults in early HIV-1 infection. J. Infect. Dis. 190251-256. [DOI] [PubMed] [Google Scholar]
- 4.Barbour, J. D., U. Sriram, S. J. Caillier, J. A. Levy, F. M. Hecht, and J. R. Oksenberg. 2007. Synergy or independence? Deciphering the interaction of HLA class I and NK cell KIR alleles in early HIV-1 disease progression. PLoS Pathog. 3e43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carr, W. H., M. J. Pando, and P. Parham. 2005. KIR3DL1 polymorphisms that affect NK cell inhibition by HLA-Bw4 ligand. J. Immunol. 1755222-5229. [DOI] [PubMed] [Google Scholar]
- 6.Carr, W. H., D. B. Rosen, H. Arase, D. F. Nixon, J. Michaelsson, and L. L. Lanier. 2007. Cutting edge: KIR3DS1, a gene implicated in resistance to progression to AIDS, encodes a DAP12-associated receptor expressed on NK cells that triggers NK cell activation. J. Immunol. 178647-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deeks, S. G., C. M. Kitchen, L. Liu, H. Guo, R. Gascon, A. B. Narvaez, P. Hunt, J. N. Martin, J. O. Kahn, J. Levy, M. S. McGrath, and F. M. Hecht. 2004. Immune activation set point during early HIV infection predicts subsequent CD4+ T-cell changes independent of viral load. Blood 104942-947. [DOI] [PubMed] [Google Scholar]
- 8.Gaudieri, S., D. DeSantis, E. McKinnon, C. Moore, D. Nolan, C. S. Witt, S. A. Mallal, and F. T. Christiansen. 2005. Killer immunoglobulin-like receptors and HLA act both independently and synergistically to modify HIV disease progression. Genes Immun. 6683-690. [DOI] [PubMed] [Google Scholar]
- 9.Gaudieri, S., D. Nolan, E. McKinnon, C. S. Witt, S. Mallal, and F. T. Christiansen. 2005. Associations between KIR epitope combinations expressed by HLA-B/-C haplotypes found in an HIV-1 infected study population may influence NK mediated immune responses. Mol. Immunol. 42557-560. [DOI] [PubMed] [Google Scholar]
- 10.Gillespie, G. M., A. Bashirova, T. Dong, D. W. McVicar, S. L. Rowland-Jones, and M. Carrington. 2007. Lack of KIR3DS1 binding to MHC class I Bw4 tetramers in complex with CD8+ T cell epitopes. AIDS Res. Hum. Retrovir. 23451-455. [DOI] [PubMed] [Google Scholar]
- 11.Giorgi, J. V., L. E. Hultin, J. A. McKeating, T. D. Johnson, B. Owens, L. P. Jacobson, R. Shih, J. Lewis, D. J. Wiley, J. P. Phair, S. M. Wolinsky, and R. Detels. 1999. Shorter survival in advanced human immunodeficiency virus type 1 infection is more closely associated with T lymphocyte activation than with plasma virus burden or virus chemokine coreceptor usage. J. Infect. Dis. 179859-870. [DOI] [PubMed] [Google Scholar]
- 12.Goronzy, J. J., and C. M. Weyand. 2005. Rheumatoid arthritis. Immunol. Rev. 20455-73. [DOI] [PubMed] [Google Scholar]
- 13.Henel, G., K. Singh, D. Cui, S. Pryshchep, W. W. Lee, C. M. Weyand, and J. J. Goronzy. 2006. Uncoupling of T-cell effector functions by inhibitory killer immunoglobulin-like receptors. Blood 1074449-4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janssen, R. S., G. A. Satten, S. L. Stramer, B. D. Rawal, T. R. O'Brien, B. J. Weiblen, F. M. Hecht, N. Jack, F. R. Cleghorn, J. O. Kahn, M. A. Chesney, and M. P. Busch. 1998. New testing strategy to detect early HIV-1 infection for use in incidence estimates and for clinical and prevention purposes. JAMA 28042-48. [DOI] [PubMed] [Google Scholar]
- 15.Janssens, W., L. Heyndrickx, G. Van der Auwera, J. Nkengasong, E. Beirnaert, K. Vereecken, S. Coppens, B. Willems, K. Fransen, M. Peeters, P. Ndumbe, E. Delaporte, and G. van der Groen. 1999. Interpatient genetic variability of HIV-1 group O. AIDS 1341-48. [PubMed] [Google Scholar]
- 16.Jennes, W., S. Verheyden, C. Demanet, C. A. Adje-Toure, B. Vuylsteke, J. N. Nkengasong, and L. Kestens. 2006. Cutting edge: resistance to HIV-1 infection among African female sex workers is associated with inhibitory KIR in the absence of their HLA ligands. J. Immunol. 1776588-6592. [DOI] [PubMed] [Google Scholar]
- 17.Kaur, A., R. M. Grant, R. E. Means, H. McClure, M. Feinberg, and R. P. Johnson. 1998. Diverse host responses and outcomes following simian immunodeficiency virus SIVmac239 infection in sooty mangabeys and rhesus macaques. J. Virol. 729597-9611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lanier, L. L. 2005. NK cell recognition. Annu. Rev. Immunol. 23225-274. [DOI] [PubMed] [Google Scholar]
- 19.Liu, Z., W. G. Cumberland, L. E. Hultin, A. H. Kaplan, R. Detels, and J. V. Giorgi. 1998. CD8+ T-lymphocyte activation in HIV-1 disease reflects an aspect of pathogenesis distinct from viral burden and immunodeficiency. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 18332-340. [DOI] [PubMed] [Google Scholar]
- 20.Martin, M. P., X. Gao, J. H. Lee, G. W. Nelson, R. Detels, J. J. Goedert, S. Buchbinder, K. Hoots, D. Vlahov, J. Trowsdale, M. Wilson, S. J. O'Brien, and M. Carrington. 2002. Epistatic interaction between KIR3DS1 and HLA-B delays the progression to AIDS. Nat. Genet. 31429-434. [DOI] [PubMed] [Google Scholar]
- 21.Martin, M. P., V. Pascal, M. Yeager, J. Phair, G. D. Kirk, K. Hoots, J. O'Brien, S., S. K. Anderson, and M. Carrington. 2007. A mutation in KIR3DS1 that results in truncation and lack of cell surface expression. Immunogenetics 59823-829. [DOI] [PubMed] [Google Scholar]
- 22.Martin, M. P., Y. Qi, X. Gao, E. Yamada, J. N. Martin, F. Pereyra, S. Colombo, E. E. Brown, W. L. Shupert, J. Phair, J. J. Goedert, S. Buchbinder, G. D. Kirk, A. Telenti, M. Connors, S. J. O'Brien, B. D. Walker, P. Parham, S. G. Deeks, D. W. McVicar, and M. Carrington. 2007. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat. Genet. 39733-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Montoya, C. J., P. A. Velilla, C. Chougnet, A. L. Landay, and M. T. Rugeles. 2006. Increased IFN-γ production by NK and CD3+/CD56+ cells in sexually HIV-1-exposed but uninfected individuals. Clin. Immunol. 120138-146. [DOI] [PubMed] [Google Scholar]
- 24.O'Connor, G. M., K. J. Guinan, R. T. Cunningham, D. Middleton, P. Parham, and C. M. Gardiner. 2007. Functional polymorphism of the KIR3DL1/S1 receptor on human NK cells. J. Immunol. 178235-241. [DOI] [PubMed] [Google Scholar]
- 25.Pascal, V., E. Yamada, M. P. Martin, G. Alter, M. Altfeld, J. A. Metcalf, M. W. Baseler, J. W. Adelsberger, M. Carrington, S. K. Anderson, and D. W. McVicar. 2007. Detection of KIR3DS1 on the cell surface of peripheral blood NK cells facilitates identification of a novel null allele and assessment of KIR3DS1 expression during HIV-1 infection. J. Immunol. 1791625-1633. [DOI] [PubMed] [Google Scholar]
- 26.Qi, Y., M. P. Martin, X. Gao, L. Jacobson, J. J. Goedert, S. Buchbinder, G. D. Kirk, J. O'Brien, S. J. Trowsdale, and M. Carrington. 2006. KIR/HLA pleiotropism: protection against both HIV and opportunistic infections. PLoS Pathog. 2e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodríguez, B., A. K. Sethi, V. K. Cheruvu, W. Mackay, R. J. Bosch, M. Kitahata, S. L. Boswell, W. C. Mathews, D. R. Bangsberg, J. Martin, C. C. Whalen, S. Sieg, S. Yadavalli, S. G. Deeks, and M. M. Lederman. 2006. Predictive value of plasma HIV RNA level on rate of CD4 T-cell decline in untreated HIV infection. JAMA 2961498-1506. [DOI] [PubMed] [Google Scholar]
- 28.Roederer, M. 2001. Spectral compensation for flow cytometry: visualization artifacts, limitations, and caveats. Cytometry 45194-205. [DOI] [PubMed] [Google Scholar]
- 29.Sconocchia, G., J. A. Titus, A. Mazzoni, A. Visintin, F. Pericle, S. W. Hicks, F. Malavasi, and D. M. Segal. 1999. CD38 triggers cytotoxic responses in activated human natural killer cells. Blood 943864-3871. [PubMed] [Google Scholar]
- 30.Scott-Algara, D., L. X. Truong, P. Versmisse, A. David, T. T. Luong, N. V. Nguyen, I. Theodorou, F. Barre-Sinoussi, and G. Pancino. 2003. Cutting edge: increased NK cell activity in HIV-1-exposed but uninfected Vietnamese intravascular drug users. J. Immunol. 1715663-5667. [DOI] [PubMed] [Google Scholar]
- 31.Sette, A., and J. Sidney. 1999. Nine major HLA class I supertypes account for the vast preponderance of HLA-A and -B polymorphism. Immunogenetics 50201-212. [DOI] [PubMed] [Google Scholar]
- 32.Snyder, M. R., T. Nakajima, P. J. Leibson, C. M. Weyand, and J. J. Goronzy. 2004. Stimulatory killer Ig-like receptors modulate T cell activation through DAP12-dependent and DAP12-independent mechanisms. J. Immunol. 1733725-3731. [DOI] [PubMed] [Google Scholar]
- 33.Vilches, C., and P. Parham. 2002. KIR: diverse, rapidly evolving receptors of innate and adaptive immunity. Annu. Rev. Immunol. 20217-251. [DOI] [PubMed] [Google Scholar]