Abstract
The inhibitory receptor programmed death-1 (PD-1) is present on CD8+ T cells in chronic hepatitis C virus (HCV), but expression patterns in spontaneously resolving infections are incompletely characterized. Here we report that PD-1 was usually absent on memory CD8+ T cells from chimpanzees with resolved infections, but sustained low-level expression was sometimes observed in the absence of apparent virus replication. PD-1-positive memory T cells expanded and displayed antiviral activity upon reinfection with HCV, indicating conserved function. This animal model should facilitate studies of whether PD-1 differentially influences effector and memory T-cell function in resolved versus persistent human infections.
The hepatitis C virus (HCV) is a major etiological agent of hepatic cirrhosis and hepatocellular carcinoma (3). Although the mechanism(s) facilitating chronic infection is not fully understood, HCV-specific cytotoxic T lymphocytes (CTLs) that persist during the chronic phase of infection have an immature phenotype (1), impaired proliferative capacity, and reduced cytotoxic function and cytokine secretion (9, 16, 19, 20). It is therefore probable that the dysfunction of HCV-specific CD8+ T cells plays a role in HCV persistence.
Much recent interest has centered upon the inhibitory T-cell receptor programmed death-1 (PD-1) as a marker and mediator of impaired CTL function in persistent viral infections. Studies in lymphocytic choriomeningitis virus (LCMV)-infected mice have demonstrated that in persistent infection virus-specific T cells express PD-1, while in resolved infection, LCMV-specific cells lose expression of this molecule (2). In addition, the in vivo blockade of PD-1 interaction with one of its ligands, programmed death-ligand 1 (PD-L1), improved proliferation and cytokine secretion by LCMV-specific CD8+ T cells and reduced viral titers in persistently infected mice (2). More recently, studies have shown that in human immunodeficiency virus (HIV)-infected individuals, HIV-specific T cells express PD-1 (4, 12, 17) and that the levels of expression of this molecule correlate with viremia and with CD4+ T-cell count (4, 17). HCV-specific CD8+ T cells from the blood and liver of humans with chronic hepatitis C also express PD-1, and the in vitro blockade of PD-1-PD-L1 interaction enhanced antigen-driven proliferation and cytokine secretion (8, 11, 13, 18). Furthermore, in one study of acute human infection, the loss of PD-1 expression by HCV-specific CD8+ T cells was shown to correlate with viral clearance (18). These data support the hypothesis that the PD-1-positive phenotype is associated with the impairment of virus-specific CD8+ T-cell function, and as such, PD-1 expression is a correlate of the outcome of HCV infection.
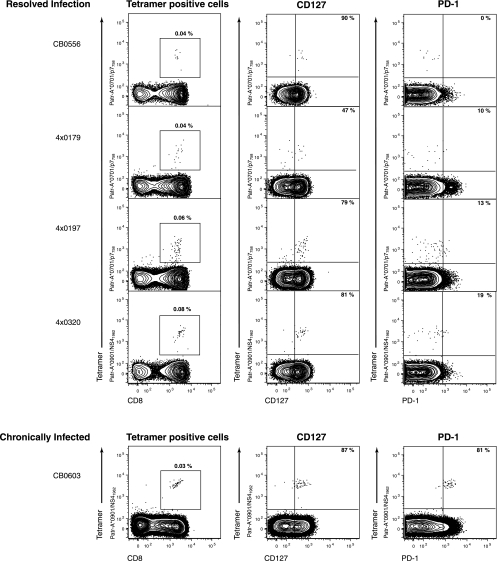
The goal of this study was to further evaluate the relationship between PD-1 expression and virus persistence, using the only animal model of human HCV infection. We analyzed the phenotype of HCV-specific CD8+ T cells in chimpanzees that had spontaneously resolved HCV infection 5 to 7 years prior. Flow cytometric analysis of peripheral blood mononuclear cells (PBMCs) by use of major histocompatibility complex (MHC) class I peptide tetrameric complexes (tetramers) revealed HCV-specific CD8+ memory T cells to be present at frequencies between 0.04% and 0.08% of CD8+ T cells (Fig. 1). The majority expressed CD127, the interleukin-7 receptor, which is a marker of memory T cells and their precursors (10, 21). Few of these HCV-specific CD8+ T cells were positive for PD-1 (Fig. 1). PD-1-negative memory CD8+ T cells were functional. For instance, reinfection of animal CBO556 with HCV resulted in vigorous expansion and gamma interferon (IFN-γ) production by the Patr-A*0701-restricted CD8+ T cells specific for epitope P7756 (reference 15 and data not shown). In contrast, the majority of HCV-specific CD8+ T cells circulating in the blood of an animal after 12 years of persistent infection were also CD127 positive but coexpressed PD-1 (Fig. 1). These findings in chimpanzees with acute resolving and persistent infections suggest that the broad patterns of PD-1 and CD127 expression on virus-specific CD8+ T cells generally mirror those in HCV-infected humans (8, 11, 13, 18).
FIG. 1.
Phenotype of HCV-specific CD8+ T cells in four chimpanzees resolving infection (CB0556, 4x0179, 4x0197, and 4x0320) versus a persistently infected animal (CB0603) several years postinfection. Plots show flow cytometric analysis of PBMCs. Percentages in far left panels indicate the proportion of peripheral blood CD8+ T cells comprised by the HCV-specific tetramer indicated (Patr-A*0701/p7758-SLAGTHGLVSFL-769 and Patr-A*0901/NS41962-QWISSECTTPC-1972). Percentages in the middle and far right panels indicate the proportion of tetramer-positive CD8+ T cells positive for CD127 (middle panels) or PD-1 (far right panels). The far left panels are gated on forward and side scatter appropriate for lymphocytes and live CD4− CD14− CD16− CD19− cells. The middle and far right panels are gated on forward and side scatter appropriate for lymphocytes and live CD4− CD14− CD16− CD19− CD8+ cells. Quadrant gates are derived from appropriate fluorescence minus one controls.
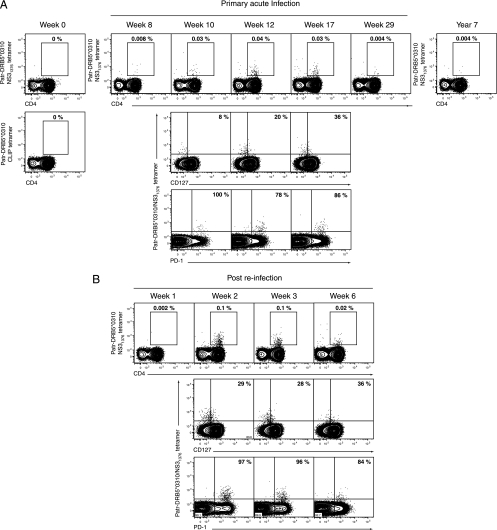
While differential PD-1 expression on CD8+ T cells during chronic versus resolved HCV infection in both species suggests its involvement in the functional silencing of the immune response, there may be unexplained exceptions to this pattern in some individuals. We previously described the evolution of T-cell immunity in an animal (CBO572) that spontaneously resolved two consecutive HCV infections separated by an interval of 7 years. Primary infection with the HCV-1/910 strain was cleared within 3 months of inoculation (15). Consistent with a previous analysis (15), tetramer staining of peripheral blood demonstrated CD8+ T cells specific for the Patr-B*2301-restricted epitope E2445-457 at 8 weeks postinfection, peaking at 17 weeks at 0.3% of CD8+ T cells (Fig. 2). At 7 years after resolution of primary HCV infection, 0.1% of circulating CD8+ T cells were still specific for this HCV epitope (Fig. 2). Further phenotypic analysis revealed that the majority of CD8+ T cells collected at the earliest time points of acute infection were CD127 negative but became positive by week 29 and remained so through 7 years of follow-up (Fig. 2). Most E2445-457-specific CD8+ T cells also expressed PD-1 throughout the acute phase of infection, even though the mean fluorescence intensity (MFI) of staining fell from 2,227 at week 8 to 626 at week 29 postinfection (Fig. 2), consistent with the notion that it can serve as a marker of activation. Surprisingly, 91% of memory E2445-457-specific CD8+ T cells remained positive for PD-1 at the 7-year time point (Fig. 2), with an MFI of 929, even though virus was never detected on multiple occasions during prolonged follow-up using a highly sensitive (6) HCV RNA transcription-mediated amplification assay (Versant HCV RNA qualitative assay; Bayer Diagnostics, Tarrytown, NY) that detects as few as 50 viral genomes per milliliter of plasma. This observation was unexpected in an animal that had spontaneously resolved infection, given that constitutive PD-1 expression has been associated with impaired CD8+ T-cell function and virus persistence in human infections.
FIG. 2.
Phenotype of E2445-HKFNSSGCPERL-456-specific CD8+ T cells through the course of acute HCV infection and 7 years post-HCV clearance in chimpanzee CB0572. Plots show flow cytometric analysis of PBMCs. Weeks or years indicated in plots are time-point post-primary inoculation with the 1/910 strain of HCV. Plots are gated as described in the legend for Fig. 1, and percentages indicated in plots are as per the legend for Fig. 1. Quadrant gates are derived from appropriate fluorescence minus one controls.
Reinfection of chimpanzee CB0572 at 7 years post-primary infection facilitated an analysis of PD-1 expression and memory CD8+ T-cell function after a reencounter with the antigen. Viremia was successfully terminated by 14 days post-secondary infection as described previously (15). Repeat HCV infection was associated with the loss of CD127 expression by E2445-457-specific memory CD8+ T cells (Fig. 3), consistent with observations for mice serially infected with LCMV (7). However, despite a predominant PD-1-positive phenotype prior to reinfection and the maintenance of this marker during resolution of viremia during viral rechallenge, E2445-457-specific CD8+ T cells were capable of expansion, reaching a peak frequency of 1.1% of CD8+ T cells in peripheral blood 2 weeks postinoculation (Fig. 3). This rapid, marked expansion of the E2445-457-specific CD8+ T-cell population makes it likely that it was derived from the majority PD-1-positive population, rather than from PD-1-negative cells, although derivation from this very minor subpopulation cannot be absolutely excluded. Three weeks after rechallenge, Patr-B*2301/E2445-457 tetramer-positive CD8+ T cells also displayed the activation marker CD69 and secreted IFN-γ in response to in vitro stimulation with cognate peptide (Fig. 3). Proliferation and cytokine secretion by these activated memory CD8+ T cells indicated that at least in this circumstance, a PD-1-positive phenotype was not associated with functional exhaustion.
FIG. 3.
Phenotype and function of E2445-HKFNSSGCPERL-456-specific CD8+ T cells through the course of a homologous HCV rechallenge in chimpanzee CB0572. (A) Flow cytometric analysis of PBMCs at various time points after repeat inoculation as indicated above plots. Plots are gated as described in the legend for Fig. 1, and percentages indicated in plots are as per the legend for Fig. 1. Quadrant gates are derived from appropriate fluorescence minus one controls. (B) Intracellular cytokine staining for IFN-γ production by E2445-specific CD8+ T cells 3 weeks post-homologous viral rechallenge. Plots show surface expression of CD69 versus intracellular staining for IFN-γ following 6 h in vitro incubation at 37°C in the presence of brefeldin A, without peptide (top panel) versus incubation with E2445-457 peptide (bottom panel). Plots are gated on forward and side scatter appropriate for lymphocytes and CD8+ Patr-B*2301/E2445-457-positive cells. Percentages indicate the proportion of CD8+ Patr-B*2301/E2445-457-positive cells in each quadrant.
HCV-specific CD4+ T helper cells were also directly visualized after primary and secondary infection of CBO572 using a Patr-DRB5*0310 class II tetramer that incorporated the previously described epitope NS31376 derived from the HCV nonstructural 3 (NS3) protein (14, 22). Chimp MHC class II tetramers were generated by peptide exchange from precursors with the low-affinity CLIP peptide, as previously described for human MHC class II molecules (5). The kinetics of NS31376-specific CD4+ T-cell expansion in blood was identical to that of the E2445-457 CD8+ T-cell population after both primary and secondary HCV challenge (Fig. 4). These CD4+ T cells also expressed PD-1 during the period of viremia and for several weeks after apparent clearance of the primary and secondary infections. PD-1 expression as measured by MFI was stable during primary viremia and after apparent resolution of the infection (Fig. 4), despite significant detectable proliferative responses against this epitope during that period (data not shown and reference 15) indicating that these cells were functional. By comparison, PD-1 expression dropped rapidly and sharply from the peak observed after reinfection, perhaps because viral antigens that could stimulate these CD4+ T cells were cleared more efficiently. The frequency of circulating NS31376-specific CD4+ T cells ultimately dropped below the limit of detection using the Patr class II tetramer, so it is unclear if long-lived memory populations retained PD-1 expression, as observed for E2445-457-specific CD8+ T cells.
FIG. 4.
Phenotype of NS31376-YGKAIPLEVI-1385-specific CD4+ T cells through the course of acute HCV-primary infection (A) and subsequent homologous viral rechallenge (B) in chimpanzee CB0572. Plots show flow cytometric analysis of PBMCs. Weeks or years indicated in plots are time-point post-primary (A) or -secondary (B) inoculation with the 1/910 strain of HCV. A control tetramer stain using Patr-DRB5*0310 complexed with the CLIP peptide is shown in panel A. Plots of CD4 versus tetramer are gated on forward and side scatter appropriate for lymphocytes and live CD8− CD14− CD16− CD19− cells. For CD4 versus CD127 and PD-1 plots, plots are gated on forward and side scatter appropriate for lymphocytes and live CD8− CD14− CD16− CD19− CD4+ cells. Quadrant gates are derived from appropriate fluorescence minus one controls.
Our findings suggest that at least in the chimpanzee model of HCV infection, functional memory CD8+ T cells can express PD-1 and that it is not always a marker of exhaustion as described in published studies of human infections (8, 11, 13, 18). While our experiments do support a model where PD-1 is usually lost from memory T cells as HCV infections resolve, they also suggest that this phenotype is not universal and predict that some human subjects will also harbor memory CD8+ T cells that constitutively express this coinhibitory molecule.
It is unlikely that PD-1 signaling acts in isolation on CD8+ T cells, and the balance between costimulation and coinhibition may regulate antiviral function during acute hepatitis C infection. Alternatively, differences in the levels of PD-1 expression on CD8+ T cells in resolved versus persistent infections might influence the quality of the antiviral response, perhaps by governing the threshold for the transduction of inhibitory signals. Indeed, although E2445-457-specific memory CD8+ T cells were PD-1 positive, levels of receptor expression fell from those observed early during acute primary infection. It is thus likely that relative expression levels of this receptor form a more useful marker of CD8+ T-cell function rather than the presence or absence of this molecule. The association of the expression levels of this molecule with viremia and CD4+ T-cell counts in HIV infection, as well as the observed decline in PD-1 expression during successful therapy for HIV infection (4, 17), would support this hypothesis. Our finding that a positive PD-1 phenotype does not invariably imply CD8+ T-cell dysfunction may also help to explain the apparent discrepancy between the recent finding of elevated PD-1 expression on cytomegalovirus-specific CD8+ T cells in chronically HCV-infected individuals (8) and the fact that generalized immune defects are not clinically observed with this population in the absence of advanced liver disease.
The expression of PD-1 by HCV-specific CD8+ T cells remains potentially important as a prognostic marker of infection outcome, as well as a possible therapeutic target. However, while the frequency of functional memory CD8+ T-cell populations with sustained PD-1 expression remains to be fully delineated, our findings indicate that the relationship between a successful outcome of infection and the loss of PD-1 expression is not absolute. Data presented here also suggest that the chimpanzee infection model is well suited to address these questions and to explore how PD-1 signaling might be interrupted to treat human chronic hepatitis C.
Acknowledgments
The authors thank the NIH tetramer core facility, Emory University, Atlanta, GA, and Russell Durbin and Priyani Fonseka for the synthesis of tetramers.
This work was supported by Public Health Service grants RO1 A147367 and U19 AI48231 to C. M. Walker and a grant from the Foundation for the National Institutes of Health through the Grand Challenges in Global Health Initiative (to C. M. Walker and G. J. Freeman). Work at New Iberia Research Center Animal was supported by grants 5U42RR15087, 1CO6RR1643-01, and 1C06RR014491-01 from the NCRR. D. G. Bowen was supported by a C. J. Martin Fellowship from the NHMRC, Australia, and a Postdoctoral Research Fellowship from the American Liver Foundation. N. H. Shoukry holds a New Investigator Award from the Canadian Institutes of Health Research (CIHR). A. G. Cawthon was the recipient of a fellowship from the U.S. Public Health Service (F32 AI055193). A. Grakoui was supported by a Cancer Research Institute Investigator Award, the Woodruff Health Sciences Fund, the Yerkes Research Center Base Grant RR-00165, and Public Health Service grant AI070101.
Footnotes
Published ahead of print on 12 March 2008.
REFERENCES
- 1.Appay, V., P. R. Dunbar, M. Callan, P. Klenerman, G. M. Gillespie, L. Papagno, G. S. Ogg, A. King, F. Lechner, C. A. Spina, S. Little, D. V. Havlir, D. D. Richman, N. Gruener, G. Pape, A. Waters, P. Easterbrook, M. Salio, V. Cerundolo, A. J. McMichael, and S. L. Rowland-Jones. 2002. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 8379-385. [DOI] [PubMed] [Google Scholar]
- 2.Barber, D. L., E. J. Wherry, D. Masopust, B. Zhu, J. P. Allison, A. H. Sharpe, G. J. Freeman, and R. Ahmed. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439682-687. [DOI] [PubMed] [Google Scholar]
- 3.Bowen, D. G., and C. M. Walker. 2005. Adaptive immune responses in acute and chronic hepatitis C virus infection. Nature 436946-952. [DOI] [PubMed] [Google Scholar]
- 4.Day, C. L., D. E. Kaufmann, P. Kiepiela, J. A. Brown, E. S. Moodley, S. Reddy, E. W. Mackey, J. D. Miller, A. J. Leslie, C. DePierres, Z. Mncube, J. Duraiswamy, B. Zhu, Q. Eichbaum, M. Altfeld, E. J. Wherry, H. M. Coovadia, P. J. Goulder, P. Klenerman, R. Ahmed, G. J. Freeman, and B. D. Walker. 2006. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature 443350-354. [DOI] [PubMed] [Google Scholar]
- 5.Day, C. L., N. P. Seth, M. Lucas, H. Appel, L. Gauthier, G. M. Lauer, G. K. Robbins, Z. M. Szczepiorkowski, D. R. Casson, R. T. Chung, S. Bell, G. Harcourt, B. D. Walker, P. Klenerman, and K. W. Wucherpfennig. 2003. Ex vivo analysis of human memory CD4 T cells specific for hepatitis C virus using MHC class II tetramers. J. Clin. Investig. 112831-842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Desombere, I., H. Van Vlierberghe, S. Couvent, F. Clinckspoor, and G. Leroux-Roels. 2005. Comparison of qualitative (COBAS AMPLICOR HCV 2.0 versus VERSANT HCV RNA) and quantitative (COBAS AMPLICOR HCV monitor 2.0 versus VERSANT HCV RNA 3.0) assays for hepatitis C virus (HCV) RNA detection and quantification: impact on diagnosis and treatment of HCV infections. J. Clin. Microbiol. 432590-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fuller, M. J., D. A. Hildeman, S. Sabbaj, D. E. Gaddis, A. E. Tebo, L. Shang, P. A. Goepfert, and A. J. Zajac. 2005. Cutting edge: emergence of CD127high functionally competent memory T cells is compromised by high viral loads and inadequate T cell help. J. Immunol. 1745926-5930. [DOI] [PubMed] [Google Scholar]
- 8.Golden-Mason, L., B. Palmer, J. Klarquist, J. A. Mengshol, N. Castelblanco, and H. R. Rosen. 2007. Upregulation of PD-1 expression on circulating and intrahepatic HCV-specific CD8+ T cells associated with reversible immune dysfunction. J. Virol. 819249-9258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gruener, N. H., F. Lechner, M. C. Jung, H. Diepolder, T. Gerlach, G. Lauer, B. Walker, J. Sullivan, R. Phillips, G. R. Pape, and P. Klenerman. 2001. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J. Virol. 755550-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaech, S. M., J. T. Tan, E. J. Wherry, B. T. Konieczny, C. D. Surh, and R. Ahmed. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 41191-1198. [DOI] [PubMed] [Google Scholar]
- 11.Penna, A., M. Pilli, A. Zerbini, A. Orlandini, S. Mezzadri, L. Sacchelli, G. Missale, and C. Ferrari. 2007. Dysfunction and functional restoration of HCV-specific CD8 responses in chronic hepatitis C virus infection. Hepatology 45588-601. [DOI] [PubMed] [Google Scholar]
- 12.Petrovas, C., J. P. Casazza, J. M. Brenchley, D. A. Price, E. Gostick, W. C. Adams, M. L. Precopio, T. Schacker, M. Roederer, D. C. Douek, and R. A. Koup. 2006. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 2032281-2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Radziewicz, H., C. C. Ibegbu, M. L. Fernandez, K. A. Workowski, K. Obideen, M. Wehbi, H. L. Hanson, J. P. Steinberg, D. Masopust, E. J. Wherry, J. D. Altman, B. T. Rouse, G. J. Freeman, R. Ahmed, and A. Grakoui. 2007. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J. Virol. 812545-2553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shoukry, N. H., D. G. Bowen, N. P. Seth, K. W. Wucherpfennig, and C. M. Walker. 2004. Abstr. 11th Int. Symp. Hepat. C Relat. Viruses, abstr. 170, Heidelberg, Germany, 3 to 7 October 2004.
- 15.Shoukry, N. H., A. Grakoui, M. Houghton, D. Y. Chien, J. Ghrayeb, K. A. Reimann, and C. M. Walker. 2003. Memory CD8+ T cells are required for protection from persistent hepatitis C virus infection. J. Exp. Med. 1971645-1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spangenberg, H. C., S. Viazov, N. Kersting, C. Neumann-Haefelin, D. McKinney, M. Roggendorf, F. von Weizsacker, H. E. Blum, and R. Thimme. 2005. Intrahepatic CD8+ T-cell failure during chronic hepatitis C virus infection. Hepatology 42828-837. [DOI] [PubMed] [Google Scholar]
- 17.Trautmann, L., L. Janbazian, N. Chomont, E. A. Said, S. Gimmig, B. Bessette, M. R. Boulassel, E. Delwart, H. Sepulveda, R. S. Balderas, J. P. Routy, E. K. Haddad, and R. P. Sekaly. 2006. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 121198-1202. [DOI] [PubMed] [Google Scholar]
- 18.Urbani, S., B. Amadei, D. Tola, M. Massari, S. Schivazappa, G. Missale, and C. Ferrari. 2006. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J. Virol. 8011398-11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urbani, S., C. Boni, G. Missale, G. Elia, C. Cavallo, M. Massari, G. Raimondo, and C. Ferrari. 2002. Virus-specific CD8+ lymphocytes share the same effector-memory phenotype but exhibit functional differences in acute hepatitis B and C. J. Virol. 7612423-12434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wedemeyer, H., X. S. He, M. Nascimbeni, A. R. Davis, H. B. Greenberg, J. H. Hoofnagle, T. J. Liang, H. Alter, and B. Rehermann. 2002. Impaired effector function of hepatitis C virus-specific CD8+ T cells in chronic hepatitis C virus infection. J. Immunol. 1693447-3458. [DOI] [PubMed] [Google Scholar]
- 21.Wherry, E. J., and R. Ahmed. 2004. Memory CD8 T-cell differentiation during viral infection. J. Virol. 785535-5545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Woollard, D. J., A. Grakoui, N. H. Shoukry, K. K. Murthy, K. J. Campbell, and C. M. Walker. 2003. Characterization of HCV-specific Patr class II restricted CD4+ T cell responses in an acutely infected chimpanzee. Hepatology 381297-1306. [DOI] [PubMed] [Google Scholar]