Abstract
The E7 proteins of human papillomaviruses (HPVs) promote S-phase reentry in differentiated keratinocytes of the squamous epithelia to support viral DNA amplification. In this study, we showed that nuclear p130 was present in the differentiated strata of several native squamous epithelia susceptible to HPV infection. In contrast, p130 was below the level of detection in HPV-infected patient specimens. In submerged and organotypic cultures of primary human keratinocytes, the E7 proteins of the high-risk mucosotrophic HPV-18, the benign cutaneous HPV-1, and, to a lesser extent, the low-risk mucosotropic HPV-11 destabilized p130. This E7 activity depends on an intact pocket protein binding domain and a casein kinase II (CKII) phosphorylation motif. Coimmunoprecipitation experiments showed that both E7 domains were important for binding to p130 in extracts of organotypic cultures. Metabolic labeling in vivo demonstrated that E7 proteins were indeed phosphorylated in a CKII motif-dependent manner. Moreover, the efficiencies of the E7 proteins of various HPV types or mutations to induce S-phase reentry in spinous cells correlated with their relative abilities to bind and to destabilize p130. Collectively, these data support the notion that p130 controls the homeostasis of the differentiated keratinocytes and is therefore targeted by E7 for degradation to establish conditions permissive for viral DNA amplification.
Human papillomaviruses (HPVs) are ubiquitous, medically important pathogens. Infections can cause hyperproliferative lesions in mucosal or cutaneous epithelia. HPV types are grouped on the basis of their sequence homologies. Closely related types have similar tissue tropism and pathogenicity. The mucosotropic HPVs are further categorized as low-risk (LR) or high-risk (HR) genotypes according to their oncogenic potentials. The LR HPV-6 and HPV-11 are the most common types that induce exophytic, benign anogenital warts and laryngeal papillomas but seldom cause cancers. In contrast, a small fraction of the infections by HR HPV-16, HPV-18, and closely related types can progress to high-grade dysplasias and cancers (for reviews, see references 21 and 68). A direct causal correlation has been established between virtually all cervical cancers and HR HPV infections (64).
The molecular basis for the diverse pathological outcomes lies in the virus-host interactions necessary to support productive infections. In normal squamous epithelium, ability to proliferate is restricted to the basal and parabasal cells, whereas all other suprabasal cells have withdrawn from the cell cycle and undergo successive stages of differentiation (38). Papillomavirus gains entry into the cycling cells through wounds and can establish persistent infection, where the double-stranded, circular viral DNA genome is maintained as low-copy extrachromosomal nuclear plasmids. Productive viral DNA amplification takes place only in the differentiated strata (60; for a review, see reference 14). Since viral DNA replication requires host DNA replication proteins and substrates (for a review, see reference 13), HPVs must recondition the postmitotic, differentiated cells to support viral DNA amplification and progeny production. In brief, E7 proteins of the HR HPV types bind the retinoblastoma-susceptibility protein (pRB) and related pocket proteins, accelerating their degradation (22, 49, 55). Binding is mediated by the LxCxE motif conserved among most HPV E7 proteins (49). In contrast, the E7 proteins of LR HPV types bind to pRb with much reduced affinity and destabilize it inefficiently (33, 54, 55, 66). However, in organotypic cultures of primary human keratinocytes (PHKs), expression of HR or LR HPV E7 alone induces S-phase reentry in a subset of differentiated cells, albeit at different efficiencies (4, 11), thereby establishing a cellular milieu necessary to support viral DNA amplification (26, 45). This E7 activity is dependent upon an intact LxCxE motif (4, 12). These results suggest that, in the context of the viral productive program in the differentiating squamous epithelium, a pRB-related pocket protein is likely to be the primary target of E7.
pRB and related proteins p107 and p130 control cell proliferation, differentiation, senescence, and apoptosis (for a review, see reference 30). These proteins share a distinct pocket domain necessary for binding E2F transcription factors and LxCxE motif-containing cellular proteins, including the D-type cyclins and histone deacetylases (HDACs). In their hypo- or unphosphorylated forms, the pocket proteins negatively regulate cell cycle progression through interaction with E2F/DP heterodimers and the recruitment of HDACs that promote chromatin condensation and repress transcription (3; for reviews, see references 18, 20, 27, and 37). The suppressive activity of pRB bound to activating E2F1, E2F2, or E2F3a is abrogated to allow progression from G1 to S phase. pRB also forms repressive complexes with E2F3b in quiescent cells (41). In contrast, p107 and p130 associate with repressive E2F4 and E2F5 to ensure G0 and G1 states (17). The hypophosphorylated pRB is inactivated through phosphorylation mediated by cyclin D/cdk4 or cdk6 and cyclin E/cdk2. During late G1 and S phase, p107 is phosphorylated and inactivated by Cdk4 (40) while p130 is phosphorylated by cdk4/6 or cdk2 followed by SCF/Skp2 ubiquitination complex-mediated destabilization (8-10, 61). Repressive E2F4- or E2F5-p107/p130 complexes are then replaced with E2F1 to -3a, concomitant with dissociation of HDACs and recruitment of transcription factors with acetyltransferase activities (CBP, p300, p/CAF, or Tip 60) necessary for chromatin decondensation and transcriptional transactivation (27).
The pocket proteins also control the differentiation and homeostasis of the squamous epithelia. In humans, pRb is detected in basal and parabasal transit-amplifying cells, whereas p130 is detected from the basal through the suprabasal strata (51). Epithelial differentiation requires pRB as its conditional depletion is associated with hyperproliferation and hyperkeratosis in mouse skin (53). However, homeostasis of the differentiated epithelium appears to require p130 expression (32, 52). For instance, in the esophagus, p130 is abundantly expressed but is lost during metaplastic conversion to Barrett's esophagus (47). At present, the role of pRB in the maintenance of the differentiated state is not clear (37).
HPV-16 E7 preferentially binds to p130 in serum-starved quiescent human fibroblasts (58). Western blot analysis revealed that p130 is destabilized in organotypic raft culture of an epithelial cell line which harbors HPV-16 plasmids (19). The E7 proteins of HPV-16 and HPV-6 destabilize p130 in submerged PHKs cultured under differentiating conditions (66). Thus, an investigation of E7 activity in the squamous epithelium would provide further insights into the roles of the pocket proteins. In this study, we conducted a detailed examination of p130 and, to a lesser extent, pRB in native squamous epithelia, HPV-infected lesions, and organotypic cultures of PHKs acutely transduced with retroviruses expressing E7 of different genotypes or their mutations. Our results show that p130 is present in differentiated keratinocytes, whereas pRB is detected in cycling cells. Our results also demonstrate that p130 destabilization by E7 leads to S-phase reentry by differentiated keratinocytes, supporting the notion that p130 is necessary to maintain the homeostasis of differentiated cells, preventing them from S-phase reentry.
We also investigated the molecular mechanism by which phosphorylation regulates E7 activity. E7 proteins of the great majority of HPV genotypes contain a casein kinase II (CKII) motif which follows closely downstream of the LxCxE motif (Fig. 1). Indeed, HPV-6, HPV-16, and HPV-18 E7 can be phosphorylated by CKII in vitro or in vivo (6, 12, 24, 54). Maximal phosphorylation of HPV-16 E7 by CKII occurs during mid to late G1 (43). The conserved proximal arrangement of the LxCxE and CKII phosphorylation motifs in small DNA tumor virus oncoproteins as well as in a number of cellular proteins implicates a possible link between CKII phosphorylation and their functional interactions with pocket proteins. Experimentally, the significance of E7 phosphorylation by CKII on binding to and destabilization of pRB has been a matter of debate (6, 7, 12, 25, 28, 33, 35, 49), while the consequence of phosphorylation by CKII on the E7-p130 interaction has not been investigated. We have previously reported that HPV-18 E7 mutations at the CKII consensus docking motif or at the two serine substrates are not phosphorylated in vivo and these mutant forms of E7 cannot induce S-phase reentry in differentiated keratinocytes (12). But the mechanism by which CKII phosphorylation regulates this E7 activity has not been explored. We now show that the ability of E7 to bind and destabilize p130 is dependent on an intact pocket protein binding motif. Importantly, binding is significantly enhanced by CKII-mediated phosphorylation.
FIG. 1.
Amino acid sequence alignment of E7 pocket protein binding regions of HPV-1, HPV-11, and HPV-18. Numbers represent the position of the first residue displayed. The solid underlined sequence indicates the conserved LxCxE pocket protein binding domain. The dashed underlined sequence signifies the consensus SxxD/E CKII motif. Like symbols above or below the residues denote mutated residues in mutant forms of E7 characterized in this study.
MATERIALS AND METHODS
Plasmids.
The wild-type HPV-18 E7 and mutations H18 E7ΔDLLC, H18 E7S32,S34D, and H18 E7E35,36,37Q were each PCR amplified from previously described clones (12) and inserted into the retroviral vector pBabe puro (48) under the control of the retroviral long terminal repeat (LTR) promoter. These clones were used to transduce PHKs for studies in submerged cultures. A different set of retroviruses containing wild-type or mutated HPV-18 E7 was constructed for raft cultures as follows. The vector pLC contains the bacterial neomycin resistance gene, while the E7 gene is under the control of the 1-kb long upstream regulatory region (URR) spanning the HPV-18 enhancer and E6 promoter. One modification was introduced in that the E7 has a long untranslated leader sequence derived from the E6 gene (nucleotides 321 to 589). This new clone, H18 LL-E7, induced S-phase reentry more efficiently than the original H18 URR-E7 (11, 12). This long leader (LL) sequence was then introduced into the previously described H18 E7 mutations in the pocket protein binding site (H18 LL-E7C27S), CKII recognition sequence (H18 LL-E7E35,36,37Q), and CKII phosphorylation substrates (H18 LL-E7S32,34Q) (12). HPV-11 E7 wild type, H11 E7S32N,S33Q, HPV-1 E7 wild type, and H1 E7P31S,P32S,I35E were prepared by PCR from cloned genomic templates and then inserted downstream of the retroviral LTR in pBabe puro. pBabe puro vectors expressing the ligand binding domain (nucleotides 844 to 1788) of the human estrogen receptor α (ER) or ER fused to the carboxyl terminus of HPV-11 E7 via a linker, H11 E7-ER and H11 E7ΔGLHC-ER, have been described previously (4). pBabe puro-H18 E7-ER (H18 E7-ER), -H18 E7ΔDLLC-ER, -H18E7 E35,36,37Q-ER, -H18 E7S32,S34D-ER, -H11 E7S32N,S33Q-ER, -H1 E7-ER, and -H1 E7P31,32S,I35E-ER fusion constructs were each created by three-piece ligation of pBabe puro, ER, and the E7 open reading frame with a linker oligonucleotide. ER, H18 E7-ER, H18 E7S32,34D-ER, H11 E7-ER, H11 E7S32N,S33Q-ER, H1 E7-ER, and H1 E7P31S,32S,I35E-ER were additionally cloned into the pMTX expression vector (67), a derivative of pMT2 with multiple cloning sites between blunted PstI (blunted) and EcoRI sites downstream of the adenovirus major late promoter for metabolic labeling in Cos7 cells. The conserved domain II of the three HPV E7 proteins and the respective mutated residues characterized in this study are illustrated in Fig. 1.
Tissues, PHKs, and retroviral transduction.
Neonatal foreskins were obtained from the Newborn Nursery of the University of Alabama at Birmingham Hospital. Biopsies of normal larynx and of laryngeal papillomas were obtained from the Children's Hospital of Alabama. The normal cervix and vulvar dysplasia were obtained through University of Alabama at Birmingham Tissue Procurement. All tissues were obtained in compliance with University of Alabama at Birmingham Institutional Review Board regulations. Tissues used for in situ assays were fixed in buffered 10% formalin, paraffin embedded, and cut into 4-μm sections. PHKs were isolated from freshly collected foreskins and cultured in KSFM (Invitrogen, Carlsbad, CA) as described previously (65). Amphotrophic recombinant retroviruses were prepared as described previously (48) from GP+envAM12 (ATCC, Manassas, VA). Freshly collected retrovirus-containing media from producer cells were used to infect PHKs. Infected PHKs were selected with 1.5 μg/ml puromycin for 2 days (for pBabe vectors) or with 250 μg/ml G418 for 2 days (for pLC vectors) and then seeded onto 60-mm plates in KSFM. The cultures were treated at 70% confluence with 250 μM cycloheximide (CHX) (Sigma-Aldrich, St. Louis, MO) alone or in combination with 50 μM MG132 (Sigma-Aldrich) for 6 h immediately before harvest. Alternatively, PHKs were grown to approximately 90% confluence and treated with 2 mM CaCl2 for 48 h, as well as with CHX or MG132 for 6 h prior to harvest. Cultures of PHKs transduced with E7-ER were induced with 5 μM estradiol for 24 h prior to treatment with CHX, MG132, or both.
Organotypic raft cultures and cell cultures.
Organotypic cultures of PHKs transduced with E7, E7-ER, or with mutations were prepared as described previously (4). For all raft cultures, the medium was supplemented with 50 μg/ml bromodeoxyuridine (BrdU) for 12 h immediately before harvest to mark cells in S phase. The cultures were formalin fixed, paraffin embedded, and sectioned for in situ analysis. Alternatively, epithelial tissues were manually separated from the collagen bed and used for protein extraction. Cos7, HaCaT, HeLa, CaSki, and SiHa cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS). Organotypic cultures of CaSki and SiHa cells were prepared as with the PHK raft cultures.
Indirect immunofluorescence and microscopy.
Four-micrometer sections of formalin-fixed, paraffin-embedded tissues and raft cultures were deparaffinized and subjected to indirect immunofluorescence probing. p130 was probed overnight at 4°C with monoclonal Rb2 antibody (1:250 dilution, clone 10; BD Biosciences, Franklin Lakes, NJ), followed by treatment with a biotin-conjugated secondary antibody (1:75 dilution; Vector Laboratories, Burlingame, CA) for 1 h at room temperature and detected by streptavidin-conjugated horseradish peroxidase (HRP) and fluorescein-conjugated tyramide (TSA fluorescein system; PerkinElmer, Boston, MA). pRb was similarly detected with monoclonal anti-pRb antibody (1:250 dilution, clone G3-245; BD Biosciences, Franklin Lakes, NJ). BrdU was detected with a polyclonal sheep biotin-conjugated anti-BrdU antibody (1:150 dilution; Abcam, Cambridge, MA) for 1 h at room temperature followed by treatment with streptavidin-conjugated Texas red (1:75 dilution; Vector Laboratories). Slides were then mounted using Vectashield mounting medium with DAPI (4′,6′-diamidino-2-phenylindole; Vector Laboratories, Burlingame, CA) and analyzed with an Olympus Provis AX70 fluorescence microscope. The images were digitally captured with an Axiocam camera and Axiovision Image processing software (Carl Zeiss Micro-Imaging Inc., Thornwood, NY) through a triple filter for fluorescein isothiocyanate, Texas red, and DAPI (Chroma Technology Corp., Brattleboro, VT). Images were processed using Adobe Photoshop CS2 (Adobe Systems, San Jose, CA). Sections from a paraffin-embedded organotypic raft culture expressing H18 S32,S34D, previously prepared by Wei-Ming Chien (12), were probed as described above for p130 and BrdU detection.
Protein extraction, Western blotting, and coimmunoprecipitation.
Submerged PHKs were harvested with 3 mM EDTA in 1×phosphate-buffered saline (PBS) and lysed in mammalian cell lysis buffer (MCLB). MCLB base is 50 mM Tris, 5 mM EDTA, 100 mM NaCl, 10 mM NaF, and 0.5% NP-40 (pH 8.0). MCLB supplements included 1× protease inhibitor cocktail (catalog no. PA340; Sigma-Aldrich), 2 mM dithiothreitol, and 1 mM NaVO4. The epidermis from raft cultures was lysed manually with MCLB in a 2-ml chilled glass tissue grinder tube (catalog no. 885002-0002; Kontes Glass Co.) and pestle (catalog no. 85001-0002). Lysates were cleared by centrifugation at 7,826 × g for 10 min at 4°C. Protein concentrations of lysates were quantified by bicinchoninic acid assay (BCA protein assay reagent kit; Pierce, Rockford, IL), and equal amounts were analyzed. Samples were resolved by 5 to 15% gradient sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred to a nitrocellulose membrane for subsequent probing with murine monoclonal antibodies against pRB (1:300 dilution, clone Ab-11; Calbiochem, San Diego, CA), p130 (1:250 dilution, clone 10; BD Biosciences), or rabbit monoclonal anti-ER (1:250 dilution, clone SP1; Lab Vision Corp., Freemont, CA) in conjunction with the appropriate HRP-conjugated secondary antibody and ECL enhanced chemiluminescence detection system (GE Healthcare, Piscataway, NJ). Gel loading was determined by reprobing with HRP-conjugated goat monoclonal antiactin antibody (1:10,000, clone I-19; Santa Cruz Biotechnology, Santa Cruz, CA) or monoclonal goat anti-GAPDH (glyceraldehyde-3-phosphate dehydrogenase) antibody (1:250, clone V-18; Santa Cruz Biotechnology) in combination with HRP-conjugated donkey anti-goat secondary antibody (1:20,000; Santa Cruz Biotechnology). ECL signals were captured on HXR film (Hawkins X-Ray Supply, Oneonta, AL). The films were scanned and images processed using Adobe Photoshop 6.0 (Adobe Systems). Protein complexes containing E7-ER were coimmunoprecipitated from 1 mg of raft culture lysates with rabbit anti-ER polyclonal antibody (AB-16; Lab Vision Corporation), resolved by 5 to 15% SDS-PAGE, and blotted onto membranes. pRb, p130, or actin was detected as just described. ER was detected using monoclonal mouse anti-ER (1:250 dilution, clone F-10; Santa Cruz Biotechnology).
In vivo phosphorylation of E7-ER.
Ninety percent confluent Cos7 cells in six-well plates were transfected with pMTX vectors that express various HPV E7-ER fusion proteins by Lipofectamine 2000 (Invitrogen). After 6 h of incubation at 37°C in 5% CO2, the cells were washed with fresh DMEM-FBS and refreshed with 2 ml fresh medium containing 5 μM β-estradiol for 18 h. Cells were washed twice in PBS and cultured in 500 μl phosphate-free DMEM (Invitrogen)-10% dialyzed FBS and 300 μCi 32Pi (8,000 to 9,000 Ci/ml) and incubated for 6 h. Cells were harvested with 1 ml 3 mM EDTA in 1× PBS and pelleted in microcentrifuge tubes by centrifugation at 4,000 rpm for 6 min. Cells were then lysed in 220 μl modified MCLB (50 mM Tris-HCl [pH 8.0], 100 mM NaCl, 5 mM EDTA, 10 mM NaF, 0.5% NP-40) supplemented with phenylmethylsulfonyl fluoride (0.1 mM), NaVO4 (1 mM), 1× protease inhibitor cocktail (catalog no. P8340; Sigma-Aldrich), and 1× phosphatase inhibitor cocktail (catalog no. P2850; Sigma-Aldrich) on ice for 15 min. Lysates were cleared by centrifugation at 14,000 rpm for 15 min at 4°C. Eighty microliters of rabbit polyclonal anti-ER antibody (Ab-16; LabVision) was diluted with 160 μl of 50% glycerol. Two hundred microliters of lysate was added to 30 μl of antibody mix and gently agitated at 4°C for 1 h. Three hundred microliters of protein A Sepharose CL4 (Sigma) was washed three times with modified complete MCLB and resuspended in 500 μl modified (as described above) MCLB and 100 μl 50% glycerol. One hundred microliters of the suspension was added to each antibody-lysate mixture and rotated overnight at 4°C. The beads were washed three times in modified MCLB, centrifuged at 4,000 rpm for 30 s, and washed two more times with modified MCLB without any supplement. The beads were resuspended in 50 μl of 2× SDS protein loading buffer, boiled for 5 min, and resolved by 15% SDS-PAGE. The gel was dried and autoradiographed.
RESULTS
HPV infections clear the p130 normally present in squamous epithelia.
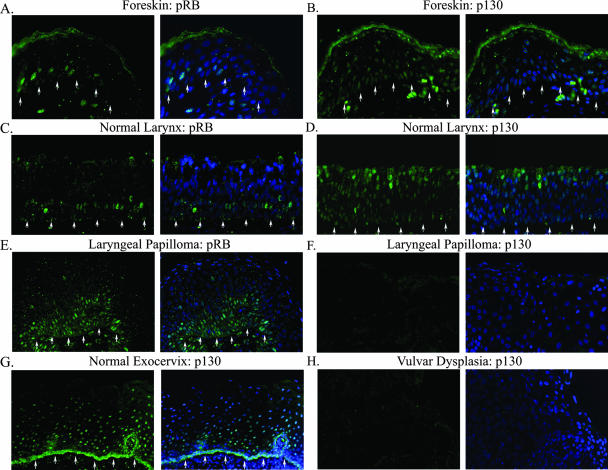
The distribution patterns of the pocket proteins p130 and pRb were separately examined by indirect immunofluorescence assays on 4-μm sections of formalin-fixed, paraffin-embedded biopsies of a normal larynx, a normal exocervix, several neonatal foreskins, five laryngeal papillomas, and a vulvar dysplasia. By PCR and by in situ hybridization, the laryngeal papillomas were previously determined to be positive for HPV-11, whereas the vulvar dysplasia was positive for HPV-16. p130 (green) was detected in most if not all of the differentiated spinous cells of foreskins (Fig. 2B), the normal larynx (Fig. 2D), and the normal exocervix (Fig. 2G). Signals were detected in the basal, presumably quiescent cells as well. In contrast, p130 was no longer detected in the differentiated strata in any of the laryngeal papillomas (Fig. 2F and data not shown) or in the vulvar dysplasia (Fig. 2H). These patient-derived tissues were positive for PCNA, indicating adequate tissue preservation (data not shown). Thus, p130 was destabilized by infections of the HR or LR HPV types. Unlike p130, pRb (green) was only observed in some of the parabasal transit-amplifying cells in the foreskins (Fig. 2A) and the normal larynx; there was no signal in the differentiated strata (Fig. 2C). We detected no pRB in the one case of vulvar dysplasia (data not shown). Interestingly, an intense pRB staining was observed in several layers of hyperproliferative parabasal cells in all five laryngeal papillomas examined (Fig. 2E and data not shown). Collectively, these results demonstrate that pRB is expressed only in proliferating transit-amplifying cells, consistent with previous reports (51).
FIG. 2.
Indirect immunofluorescence analyses of pRB and p130 proteins in normal tissues and HPV-infected lesions. Shown is pRB or p130 (green) in tissue sections from formalin-fixed and paraffin-embedded neonatal foreskin (A and B), normal laryngeal tissue (C and D), normal exocervical tissue (G), HPV-11-infected laryngeal papilloma (E and F), and HPV-16-infected vulvar dysplasia (H). Each image is presented twice. The left panels reveal the pocket protein staining, whereas the right panels show merged images with DAPI-stained nuclei (blue). Arrows indicate the basal stratum. For panels F and H, the basal stratum was out of view beyond the left lower corner. All images were captured with a ×20 objective lens but are presented at slightly different magnifications for best viewing.
HR and LR mucosotropic HPV E7 and the cutaneous HPV-1 E7 destabilize p130 in the differentiated keratinocytes of organotypic cultures.
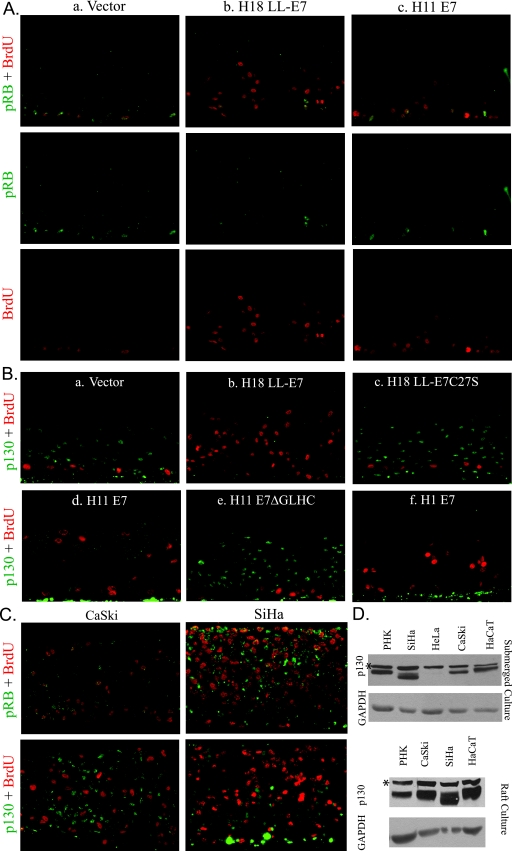
As we have previously described, raft cultures of vector-infected PHKs were characterized by S-phase (BrdU positive) basal and occasional parabasal cells, whereas cells in the differentiated strata were uniformly negative for cellular DNA synthesis (Fig. 3Aa and Ba). pRB was detected in the nuclei of the basal and occasional parabasal cells of these control raft cultures. Notably, the two signals often colocalized (Fig. 3Aa). The wild-type HPV-18 E7 (Fig. 3Ab and Bb) and HPV-11 E7 (Fig. 3Ac and Bd) promoted S-phase reentry in spinous cells, as indicated by BrdU incorporation. pRB was observed in a small number of basal as well as suprabasal cells (Fig. 3Ab and Ac) Again, pRB was usually localized to BrdU-positive cells.
FIG. 3.
Simultaneous detection of BrdU and pRB or p130 in organotypic raft cultures of cervical carcinoma cell lines and in PHKs expressing wild-type E7 or E7 mutated in the LxCxE motif. PHKs were acutely transduced with recombinant retroviruses that expressed the vector alone or the indicated HPV E7 (A and B). The CaSki and SiHa cervical carcinoma cell lines (C) express HPV-16 E6 and E7. All raft cultures were exposed to BrdU for 12 h immediately prior to harvest to mark cells in S phase. All sections were imaged at ×20 magnification. (A) pRB (green) plus BrdU incorporation (red) in separate and merged images. (B) p130 (green) and BrdU incorporation (red) in merged images. (C) Raft cultures derived from CaSki and SiHa cells. Upper panels show pRB (green) and BrdU incorporation (red); lower panels show p130 (green) and BrdU (red). (D) Western blots of total cell lysates from submerged or raft cultures of PHKs of indicated cell lines to detect p130. *, a nonspecific, cross-reactive protein band.
In contrast to pRB, p130 was detected in the nuclei of virtually all of the suprabasal cells in the control raft cultures of PHKs (data not shown) or PHKs transduced with the empty retroviral vector (Fig. 3Ba). In cultures expressing the wild-type HPV-18 E7, p130 was below detection in all cells (Fig. 3Bb). In similar experiments with HPV-11 E7, which induced S-phase reentry less efficiently than HPV-18 E7, p130 was significantly reduced relative to control raft cultures (Fig. 3Bd). The H18 LL-E7C27S and the H11 E7ΔGLHC mutations in the pocket protein binding motif did not induce S-phase reentry in the differentiated keratinocytes (4, 12), nor did they destabilize p130 in the differentiated strata (Fig. 3Bc and Be). These results show that E7 binding to pocket proteins is necessary for p130 destabilization and correlates with S-phase reentry in the differentiated strata. However, not all of the spinous cells were mobilized into S phase. This has been attributed to the E7-induced costablization of cyclin E/cdk2/p21cip1 and cyclin E/cdk2/p27kip1 into kinase-inactive complexes, incapable of promoting S-phase reentry (34, 50).
Anomalous among HPV E7 orthologs, the E7 protein of HPV-1 has a high affinity for pRB, as do the E7 proteins of the HR HPVs, but does not destabilize it (1, 15, 56). Furthermore, HPV-1 is unable to promote efficient S-phase entry in cell lines arrested in G1 by p16INK4A overexpression (31). Nevertheless, HPV-1 produces large amounts of virus particles in plantar warts (23). Thus, HPV-1 E7 offers an opportunity to examine a representative HPV genotype with a different tropism. In the spinous strata of HPV-1 E7-transduced PHK raft cultures, p130 was below detection and spinous cells in S phase were observed at a moderate frequency (Fig. 3Bf). Collectively, these results demonstrate that destabilization of p130 in the differentiated epithelium is a shared characteristic of E7 proteins from HPVs with diverse tissue tropisms and pathogenicities. Furthermore, p130 destabilization correlates with S-phase induction in the differentiated keratinocytes of the squamous epithelium.
p130 and pRB are destabilized in a fraction of cervical cancer cell lines in raft cultures.
We also prepared raft cultures of CaSki and SiHa cells, cervical carcinoma cell lines that contain integrated HPV-16 genomes and continuously express E6 and E7 genes at elevated levels in submerged cultures (2, 57, 63). A fraction of the CaSki and SiHa cells in stratified raft cultures incorporated BrdU (Fig. 3C). In CaSki cells, pRB was detected in a small subset of the BrdU-positive cells (Fig. 3C, top left). Unexpectedly, strong p130 signal was detected in many BrdU-negative cells (Fig. 3C, bottom left). In SiHa raft cultures, relatively strong p130 signal was detected in BrdU-negative cells in the lower strata. Contrary to CaSki cells, however, numerous suprabasal BrdU-negative SiHa cells had weak p130 but strong pRB signals (Fig. 3C, right panels). Cervical carcinoma-derived HeLa cells express HPV-18 E6/E7, but they did not stratify in raft culture for such analysis.
The presence of p130 in cervical carcinoma cells is quite unexpected. To investigate this issue further, lysates of submerged cultures of PHKs, HeLa, CaSki, SiHa, as well as HaCaT cells (an HPV-negative, immortalized epithelial cell line), were subjected to Western blot analyses (Fig. 3D, top panel). p130 was detected in all cells, except for HeLa. Both PHKs and CaSki expressed similar levels of p130 with similar mobility, probably a mixture of unphosphorylated and partially phosphorylated forms (8, 44). Unexpectedly, SiHa cells had a faint band of the expected mobility as well as a more intense band of faster migration. These expression patterns of p130 in CaSki and SiHa cells were also observed in the raft culture lysates (Fig. 3D, bottom panel). Also interesting to note is that p130 in HaCaT cells had the slowest migration rate, perhaps reflecting the highest phosphoryation of p130 among all the cell lines examined. Collectively, we conclude that, in a subset of the CaSki and SiHa cells, the E7 oncoprotein does not abolish p130 or pRB completely, possibly attributable to cell cycle-dependent CKII phosphorylation (43), which regulates its activity (see below).
The CKII phosphorylation motif of E7 is required for efficient p130 clearance in PHK raft cultures.
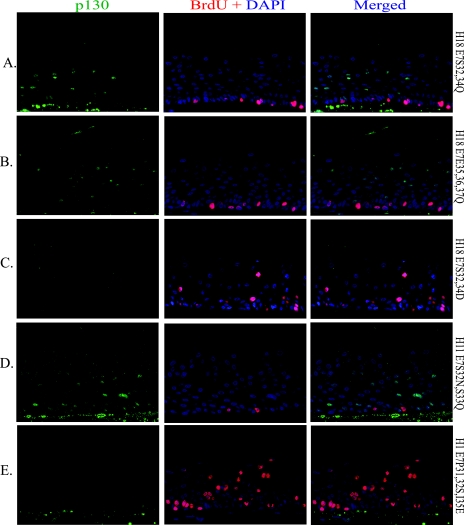
To assess the significance of CKII phosphorylation, we probed for p130 protein (green) and BrdU incorporation (red) in raft cultures of PHKs with E7 mutated in the CKII motif. Raft cultures expressing H18 E7S32,34Q, H18 E7E35,36,37Q, or H11 S32N,S33Q each destabilized p130 much less efficiently than the wild-type E7 (compare Fig. 4A and B to Fig. 3Bb and 4D to Fig. 3Bd). None of these mutations had the ability to induce S phase in spinous cells. BrdU incorporation was restricted to the basal cells. A phosphomimetic mutation, H18 E7S32,34D, cleared p130 with apparently similar efficiency to that of the wild type; but this mutation had a very limited ability to induce S phase (Fig. 4C). This result would strongly suggest that p130 must be destabilized below threshold levels for S-phase reentry to occur (see the next section).
FIG. 4.
Simultaneous detection of S-phase cells and p130 in organotypic cultures expressing E7 with gain- or loss-of-function mutations in the CKII recognition sequence or substrates. PHKs were acutely transduced with a retrovirus expressing the indicated E7 mutation. BrdU was added to the media for the last 12 h before harvest. p130 (green) and BrdU (red) incorporation are shown for substitution mutations in HPV-18 E7 (A, B, and C), HPV-11 E7 (D), or HPV-1 E7 (E). Left panels show p130 (green), middle panels show BrdU (red) plus DAPI, and right panels show merged images. All sections were imaged at ×20 magnification.
HPV-1 E7 has the conserved stretch of negatively charged residues downstream of the LxCxE motif but lacks the CKII substrates present in other HPV E7 orthologs (Fig. 1). In place of the CKII substrate serine residues, HPV-1 E7 possesses two proline residues (P31 and P32). We constructed and examined H1 E7P31,32S,I35E in PHK raft cultures. The mutations introduced two CKII substrates and replaced isoleucine with an acidic residue, thereby creating the conserved CKII consensus phosphorylation motif (SxxD/E; reviewed in reference 46). While p130 remained below detection, as in raft cultures transduced with the wild-type HPV-1 E7 (Fig. 4E), this mutation increased the number of spinous cells in S phase by 125% relative to the wild-type H1 E7 when the S-phase cells were counted across entire tissue sections of the two raft cultures (compare Fig. 4E and 3Bf). Collectively, these results strongly suggest that phosphorylation at the CKII substrates is important for efficient p130 clearance, which, in turn, is critical for differentiated keratinocytes to reenter S phase.
A conserved CKII phosphorylation motif of E7 enhances p130 destabilization in submerged PHK cultures.
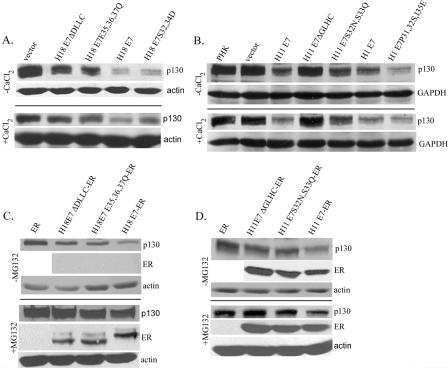
To compare the relative abilities of E7 proteins from various HPV types and mutations to destabilize p130, we performed Western blot analyses on lysates of submerged cultures of retrovirus-transduced PHKs. The p130 level was significantly reduced when the wild-type H18 E7 was expressed relative to when H18 E7ΔDLLC was present (Fig. 5A, top panel). The p130 level in PHKs expressing H18 E7E35,36,37Q was intermediate between that detected in cultures expressing the wild type and E7ΔDLLC. As in raft cultures (Fig. 4C), the expression of H18 E7S32,34D resulted in a significant reduction in p130, only slightly inferior to the wild-type E7 (Fig. 5A).
FIG. 5.
Binding and destabilization of p130 in PHKs expressing wild-type or mutated HPV E7 or E7-ER fusion proteins. PHKs were acutely transduced with a retrovirus which expressed the wild-type or mutated E7 (A and B) or E7-ER (C and D) or a control vector (empty or encoding the ER moiety) as indicated. (A and B) Lysates from subconfluent, proliferating PHK cultures (top panels) or 2 mM CaCl2-treated, confluent cultures (bottom panels) were Western blotted to reveal relative p130 levels. Actin or GAPDH was detected as a loading reference. Cultures in panel B were treated with 250 μM CHX for 6 h prior to harvest. (C and D) Western blot analyses of p130, E7-ER, and actin. The cultures were induced with 5 μM β-estradiol for 24 h prior to harvest and were further treated for 6 h with 250 μM CHX in the absence (top panels) or in the presence (bottom panels) of 50 μM MG132.
To examine p130 degradation of submerged cultures of PHKs expressing H11 and H1 E7, cells were treated with 250 μM CHX, an inhibitor of protein translation, for 6 h prior to harvest. Both E7 proteins destabilized p130 (Fig. 5B). Relative to wild-type H11 E7, the activity of H11 E7S32N,33Q was partially abrogated, whereas H11 E7ΔGLHC had no activity. Expression of H1 E7P31,32S,I35E destabilized p130 to a greater extent than wild-type H1 E7. The relative abilities of H18, H11, and H1 E7 to destabilize p130 were recapitulated in PHKs cultured under differentiating conditions (2 mM CaCl2) (59) (Fig. 5A and B, bottom panels). Collectively, these observations demonstrate that efficient E7-mediated p130 destabilization requires the pocket protein binding domain and is facilitated by a CKII motif.
HPV E7-ER fusion proteins maintain the activity to destabilize p130 in submerged cultures.
We have previously shown that H11 E7-ER and H16 E7-ER, in which E7 is fused to the carboxyl-terminal, ligand binding domain of the human ER, conditionally induce S-phase reentry in PHK raft cultures upon addition of hormone (4). These fusion proteins are readily detected by an antibody against the carboxyl domain of the ER, and their activities are revealed upon estrogen-induced nuclear entry. Importantly, the activity of the E7 fusion protein requires an intact LxCxE motif necessary for binding to the pocket proteins, whereas the ER alone has no activity (4). This system provides a means to compare the wild-type and mutated E7 proteins for their properties and activities in the absence of commercially available antibodies against the E7 of different HPV types. Thus, we constructed retroviruses that express the wild-type H18 E7-ER, H18 E7ΔDLLC-ER, and H18 E7E35,36,37Q-ER from the retroviral LTR. The wild-type H18 E7-ER promoted S-phase reentry in PHK raft cultures upon induction by estrogen, whereas mutated H18 E7-ER did not (data not shown), in agreement with results reported for native HPV-18 E7 and E7 mutations (12).
Subconfluent PHKs were acutely transduced with retroviruses expressing the wild-type or mutated H18 E7-ER fusion protein. The cells were treated with estrogen for 24 h and with 250 μM CHX for 6 h prior to harvest, and then Western blot analyses were performed on the lysates. The results revealed that wild-type H18 E7-ER reduced p130 relative to the PHK cultures or cultures infected with the control vector. In contrast, H18 E7ΔDLLC-ER and H18 E7E35,36,37Q-ER were very inefficient (Fig. 5C, top panels). Although we detected the ER alone (data not shown), we did not detect E7-ER in this experiment, due to the very short half-life of this E7 protein (our unpublished observation) combined with the inhibition of protein synthesis by CHX. However, similar levels of H18 E7-ER proteins were observed when the cultures were treated with the proteasome inhibitor MG132. MG132 also stabilized p130 in the presence of E7-ER (Fig. 5C, bottom panels), indicating that both E7-ER and p130 were degraded by proteasomes.
We also conducted similar experiments with subconfluent, proliferating PHK cultures transduced with H11 E7-ER, H11 E7ΔGLHC-ER, or H11 E7S32N,S33Q-ER. The proteins were expressed at comparable levels. Only the wild-type H11 E7 destabilized p130, whereas the mutant forms had little activity (Fig. 5D, top panels). Again, increased p130 was observed in the presence of MG132 (Fig. 5D, bottom panels). These results show that E7-ER maintains the function of wild-type E7 to destabilize p130. Furthermore, the difference in p130 stability in the presence of wild-type or mutant forms of E7 cannot be attributed to a difference in protein abundance. It is also interesting to note that, despite a higher HPV-11 E7-ER protein level relative to HPV-18 E7-ER (using cellular protein as a reference), HPV-11 E7-ER was less effective at destabilizing p130 (Fig. 5C to D), in agreement with the observation made in raft cultures (Fig. 3Bb and Bd).
The CKII motif mediates E7 phosphorylation in vivo.
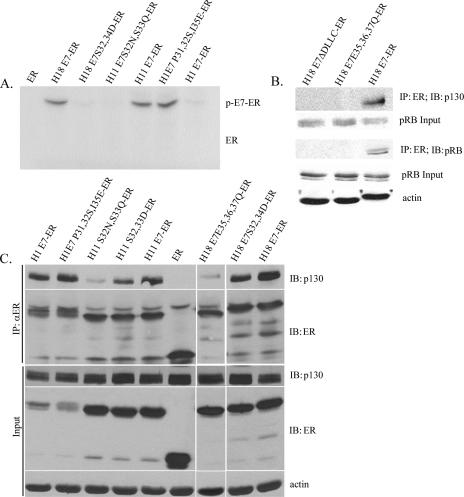
The wild-type HPV-18 E7, but not E7 mutated at the CKII substrates or binding motif, is phosphorylated in vivo (12). To investigate whether HPV-11 and HPV-1 E7 P31,32S,I35E proteins are phosphorylated in vivo, as strongly suggested by the previous mutational analyses, we constructed plasmid expression vectors of H18 E7-ER, H11 E7-ER, H1 E7-ER, and corresponding CKII substrate gain-or-loss mutations for expression in Cos7 cells. These transfected cells were metabolically labeled with 32Pi. The E7-ER proteins were immunoprecipiated with an antibody against ER. An autoradiogram of the immunoprecipitate after SDS-PAGE is presented in Fig. 6A. We did not detect phosphorylation on the ER moiety alone. HPV-11 E7-ER was phosphorylated, but phosphorylation was abolished in the CKII substrate mutation H11 E7S32N,S33Q-ER. HPV-1 E7, which lacks CKII substrates, was not phosphorylated. In contrast, phosphorylation was detected in HPV-1 E7P31,32S,I35E-ER, into which the canonical CKII substrates were introduced. HPV-18 E7-ER was phosphorylated, whereas HPV-18 E7S32,34D-ER was not, in agreement with our previous studies of native E7 proteins (12). These results demonstrate that H11 E7 and H1 E7P31,32S,I35E are indeed phosphorylated at the CKII motif in vivo and that the various phenotypes attributable to these proteins correlate with their phosphorylation potential.
FIG. 6.
Phosphorylation of HPV E7 at the CKII motif increases binding to p130 in vivo. (A) E7 phosphorylation in vivo requires the CKII substrates. Lysates of Cos7 cells expressing ER or the indicated E7-ER fusions metabolically labeled with 32Pi were immunoprecipitated (IP) with an anti-ER (αER) antibody. The immunoprecipitates were analyzed by SDS-PAGE, and the autoradiogram is presented. “ER” indicates the expected migration of the ER moiety. “p-E7-ER” indicates the migration of the phosphorylated E7-ER fusion. (B and C) The presence of CKII substrates increases binding to p130. E7-ER was immunoprecipitated from total lysates of PHK raft cultures. IB, immunoblotting. (B) Immunoprecipitates were probed for p130 or pRB. Input lysates were probed for p130, pRB, and actin. (C) Immunoprecipitates were probed with p130 or ER antibody. Input lysates were probed with p130, ER, and actin.
The CKII phosphorylation motif of E7 enhances p130 binding in PHK raft cultures.
Next we investigated whether the phosphorylation potential of HPV-18 E7 by CKII affects the association with p130. Lysates of estrogen-induced raft cultures of PHKs transduced with H18 E7-ER or mutations were immunoprecipitated with anti-ER rabbit polyclonal antibody. Coimmunoprecipitated p130 was detected only in cultures expressing the wild-type H18 E7-ER but not in the precipitate from cultures expressing H18 E7ΔDLLC-ER or H18 E7E35,36,37Q-ER (Fig. 6B). Similarly, pRb was detected only in precipitates from lysates of raft cultures expressing the wild-type H18 E7-ER. These observations demonstrate that CKII phosphorylation enhances E7 binding to pocket proteins, and in the absence of stable binding, p130 degradation is inefficient. Similar results were obtained when p130 binding by HPV-11 E7-ER, HPV-1 E7-ER, or mutations therein was examined in raft culture (Fig. 6C). Much less p130 was coimmunoprecipitated with an HPV-11 phospho-null mutation, E7S32N,S33Q-ER, whereas phosphomimetic HPV-11 E7S32,33D-ER and HPV-18 E7S32,34D-ER mutations restored much of the wild-type activity. Interestingly, HPV-1 E7-ER, which lacks CKII substrates but bears negatively charged amino acids in conserved positions downstream of the LxCxE motif (Fig. 1), was also able to bind p130 very effectively. Consistent with the phenotypes enhanced by introducing CKII substrates (Fig. 4E and 5B), the H1 E7 P31,32S,I35E mutation exhibited a higher p130 binding than the wild type. HPV-18 E7-ER and HPV-18 E7E35,36,37Q-ER served as strong and weak p130 binding partner controls in this experiment. These results demonstrate that p130 interaction with E7 is stabilized by phosphorylation at the CKII motif immediately downstream of the LxCxE pocket protein binding domain.
DISCUSSION
E7 expression of both HR and LR HPV genotypes induces S-phase reentry in postmitotic, differentiated keratinocytes to facilitate productive viral DNA amplification. Western blot analyses indicate that p130 binding and destabilization are shared properties among HR and LR HPV E7 proteins (19, 58, 62, 66). In the present study, we characterized this virus-host interaction in detail; our results support this model and further demonstrate that p130, not pRB, is responsible for maintaining homeostasis of the squamous epithelium, preventing the differentiated cells from reentry into S phase. Genetic studies show that pRB is required for the cells to exit the cell cycle to undergo differentiation, whereupon p130 is expressed (51, 52). We detected p130 throughout the differentiated strata in normal squamous epithelia from several body sites as well as in basal, presumably quiescent cells (Fig. 2). Strikingly, p130 was below the level of detection in HPV-infected lesions (Fig. 2). Furthermore, expression of the E7 gene from representative members of several HPV species, including the mucosotrophic HR HPV-18 and the LR HPV-11 as well as the cutaneous HPV-1, destabilizes p130, leading to S-phase reentry in a subset of the differentiated cells (Fig. 3). The extent of p130 degradation correlated with the relative efficiencies of S-phase reentry. These conclusions were verified by mutational analyses of these three phylogenically diverse E7 orthologs (Fig. 3 to 5). In contrast, pRB was detected only in parabasal cells in normal tissues and in benign lesions infected by LR HPV, the E7 of which is limited in its ability to destabilize pRB (Fig. 2). We suggest these are cycling cells since, in PHK raft cultures, pRB can be detected in a subpopulation of S-phase cells in the presence or absence of E7 (Fig. 3). This interpretation is consistent with previous studies that demonstrate pRB localization to DNA replication centers (5, 36).
Our conclusion with regard to p130 would agree with a recent report by Litvochick et al. (42). These authors reported that in quiescent cells, p130 was present in a complex called DREAM which contains E2F4, DP, LIN9, and MuvB proteins, suppressing over 800 genes, the majority of which are involved in cell cycle progression. Previously, we have observed that numerous genes involved in DNA replication and associated metabolism were transactivated in the microarray analysis of rafts expressing HPV-18 E6 and E7 (29). We have also made an interesting observation that, under certain circumstances, pRB appears to substitute for p130 functionally. We found that the SiHa cell line, which expresses HPV-16 E6 and E7 proteins, could be heterozygous in its p130 alleles. One of the alleles encodes a smaller protein (Fig. 3), which resembles the 116-kDa form of p130 previously observed in Burkitt's lymphoma and acute lymphoblastic lymphoma (16). This shorter form might be defective. This putative deficiency in p130 could explain the compensatory increase in pRB expression primarily in BrdU-negative cells in SiHa raft cultures relative to raft cultures of CaSki cells that do not have the shortened form of p130 (Fig. 3).
Our conclusion regarding p130 is also consistent with that of Ueno et al. who demonstrated the ability of short hairpin RNA against p130 to promote S-phase reentry in a fraction of differentiated PHKs in organotypic cultures (62). However, as to how E7 modulates the pocket proteins in the PHK raft cultures, there are several differences between our studies and those of Ueno et al. who examined individual antigens by immunohistochemistry but no mechanistic studies. The differences include tissue histology, the distribution and signal intensities of p130 in the presence of HPV-18 or HPV-11 E7 relative to the control, and the localization of BrdU-positive cells in the presence or absence of E7. In addition, Ueno et al. had only analyzed one E7 mutation which was not informative with regard to the E7-p130 interaction.
By in situ double-immunofluorescence assays of raft cultures (Fig. 3 and 4) and biochemical analyses of submerged and raft cultures (Fig. 5 and 6), we showed that, as expected, the pocket protein binding motif is critical for E7-mediated p130 destabilization, in agreement with the findings by Zhang et al. (66). In addition, our results demonstrate that CKII-mediated phosphorylation of HPV-18 and HPV-11 E7 is necessary for effective binding to and destabilization of p130 as well as for S-phase reentry (Fig. 4 to 6). Indeed, the cocrystal structure of the pRB pocket domain and an LxCxE-containing HPV-16 E7 peptide suggests that negatively charged residues downstream of the LxCxE binding motif might help stabilize their interaction (39). The significance of CKII phosphorylation is further underscored by properties of the H1 E7P31,32S,I35E mutation, into which CKII substrates have been introduced. This mutation augmented the binding to and destabilization of p130, resulting in an elevated efficiency of S-phase induction compared to the wild-type HPV-1 E7 (Fig. 3 to 6). Along the same line, the HPV-18 E7 phosphomimetic mutation is slightly inferior to the wild-type E7 in p130 binding and destabilization (Fig. 5 and 6). This discrepancy is possibly attributable to a difference in charge and size between the phosphorylated serines and aspartic acid residues. This subtle difference nevertheless leads to a dramatic reduction in its ability to induce S-phase reentry in the differentiated cells (12) (Fig. 3 and 4). The wild-type HPV-1 E7, however, has the capacity to bind p130 in the absence of phosphorylation. We postulate two possible reasons for this ability. First, HPV-1 E7 has an aspartic acid residue immediately preceding the LxCxE motif known to stabilize E7 binding of pRB (33, 54). Second, two proline residues, which follow closely downstream of the LxCxE motif, may have brought the stretch of conserved negatively charged residues into a configuration functionally analogous to the phosphorylated CKII substrates relative to the preceding LxCxE motif (Fig. 1). Collectively, we have clearly demonstrated that the ability of E7 to induce S-phase reentry in the differentiated epithelium is dependent on the pocket protein binding motif and that phosphorylation of the serine residues by CKII is instrumental for efficient binding to the pocket protein, resulting in subsequent p130 destabilization.
These findings and our previous studies in combination with those from other laboratories now explain why the LR HPVs and the benign cutaneous HPV-1, the E7 proteins of which do not destabilize pRB, amplify viral DNA in differentiated keratinocytes as well as do HR HPVs, the E7 proteins of which do efficiently target pRb for proteasomal degradation. In essence, p130 is the key regulator preventing cell cycle reentry in differentiated keratinocytes, and p130-mediated repression must be surmounted by E7 for induction of S phase. In contrast, efficient pRB destabilization, a property unique to HR HPV E7, accounts for its ability to immortalize primary keratinocytes in collaboration with HR HPV E6 in vitro and to initiate neoplastic progression in patients.
Acknowledgments
This work was supported by USPHS grant CA83679. N. J. Genovese is the recipient of a fellowship from the Basic Mechanisms in AIDS Pathogenesis Training, grant T32 AI07493.
DNA sequencing was conducted by the Center for AIDS Research Core Facility. Special thanks goes to Sami Banerjee for assistance with protein resolution and Western analysis. We thank Saleem Khan for the pBR322 HPV1a plasmid.
Footnotes
Published ahead of print on 5 March 2008.
REFERENCES
- 1.Alunni-Fabbroni, M., T. Littlewood, L. Deleu, S. Caldeira, M. Giarre, M. Dell' Orco, and M. Tommasino. 2000. Induction of S phase and apoptosis by the human papillomavirus type 16 E7 protein are separable events in immortalized rodent fibroblasts. Oncogene 192277-2285. [DOI] [PubMed] [Google Scholar]
- 2.Baker, C. C., W. C. Phelps, V. Lindgren, M. J. Braun, M. A. Gonda, and P. M. Howley. 1987. Structural and transcriptional analysis of human papillomavirus type 16 sequences in cervical carcinoma cell lines. J. Virol. 61962-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baker, G. L., M. W. Landis, and P. W. Hinds. 2005. Multiple functions of D-type cyclins can antagonize pRb-mediated suppression of proliferation. Cell Cycle 4330-338. [PubMed] [Google Scholar]
- 4.Banerjee, N. S., N. J. Genovese, F. Noya, W.-M. Chien, T. R. Broker, and L. T. Chow. 2006. Conditionally activated E7 proteins of high-risk and low-risk human papillomaviruses induce S phase in postmitotic, differentiated human keratinocytes. J. Virol. 806517-6524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barbie, D. A., B. A. Kudlow, R. Frock, J. Zhao, B. R. Johnson, N. Dyson, E. Harlow, and B. K. Kennedy. 2004. Nuclear reorganization of mammalian DNA synthesis prior to cell cycle exit. Mol. Cell. Biol. 24595-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barbosa, M. S., C. Edmonds, C. Fisher, J. T. Schiller, D. R. Lowy, and K. H. Vousden. 1990. The region of the HPV E7 oncoprotein homologous to adenovirus E1a and SV40 large T antigen contains separate domains for Rb binding and casein kinase II phosphorylation. EMBO J. 9153-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berezutskaya, E., and S. Bagchi. 1997. The human papillomavirus E7 oncoprotein functionally interacts with the S4 subunit of the 26 S proteasome. J. Biol. Chem. 27230135-30140. [DOI] [PubMed] [Google Scholar]
- 8.Bhattacharya, S., J. Garriga, J. Calbó, T. Yong, D. S. Haines, and X. Graña. 2003. SKP2 associates with p130 and accelerates p130 ubiquitylation and degradation in human cells. Oncogene 222443-2451. [DOI] [PubMed] [Google Scholar]
- 9.Calbó J., M. Parreño, E. Sotillo, T. Yong, A. Mazo, J. Garriga, and X. Graña. 2002. G1 cyclin/cyclin-dependent kinase-coordinated phosphorylation of endogenous pocket proteins differentially regulates their interactions with E2F4 and E2F1 and gene expression. J. Biol. Chem. 27750263-50274. [DOI] [PubMed] [Google Scholar]
- 10.Cheng, L., F. Rossi, W. Fang, T. Mori, and D. Cobrinik. 2000. Cdk2-dependent phosphorylation and functional inactivation of the pRB-related p130 protein in pRB(−), p16INK4A(+) tumor cells. J. Biol. Chem. 27530317-30325. [DOI] [PubMed] [Google Scholar]
- 11.Cheng, S., D.-C. Schmidt-Grimminger, T. Murant, T. R. Broker, and L. T. Chow. 1995. Differentiation-dependent up-regulation of the human papillomavirus E7 gene reactivates cellular DNA replication in suprabasal differentiated keratinocytes. Genes Dev. 92335-2349. [DOI] [PubMed] [Google Scholar]
- 12.Chien, W. M., J. N. Parker, D.-C. Schmidt-Grimminger, T. R. Broker, and L. T. Chow. 2000. Casein kinase II phosphorylation of the human papillomavirus-18 E7 protein is critical for promoting S-phase entry. Cell Growth Differ. 11425-435. [PubMed] [Google Scholar]
- 13.Chow, L. T., and T. R. Broker. 2006. Mechanisms and regulation of papillomavirus DNA replication, p. 53-71. In M. S. Campo (ed.), Papillomavirus research: from natural history to vaccines and beyond. Caister Academic Press, Norwich, United Kingdom.
- 14.Chow, L. T., and T. R. Broker. 2007. Human papillomavirus RNA transcription, p. 109-131. In R. L. Garcea and D. DiMaio (ed.), The papillomaviruses. Springer, New York, NY.
- 15.Ciccolini, F., G. Di Pasquale, F. Carlotti, L. Crawford, and M. Tommasino. 1994. Functional studies of E7 proteins from different HPV types. Oncogene 92633-2638. [PubMed] [Google Scholar]
- 16.Cinti, C., P. P. Claudio, C. M. Howard, L. M. Neri, Y. Fu, L. Leoncini, G. M. Tosi, N. M. Maraldi, and A. Giordano. 2000. Genetic alterations disrupting the nuclear localization of the retinoblastoma-related gene RB2/p130 in human tumor cell lines and primary tumors. Cancer Res. 60383-389. [PubMed] [Google Scholar]
- 17.Classon, M., and N. Dyson. 2001. p107 and p130: versatile proteins with interesting pockets. Exp. Cell Res. 264135-147. [DOI] [PubMed] [Google Scholar]
- 18.Cobrinik, D. 2005. Pocket proteins and cell cycle control. Oncogene 242796-2809. [DOI] [PubMed] [Google Scholar]
- 19.Collins, A. S., T. Nakahara, A. Do, and P. F. Lambert. 2005. Interactions with pocket proteins contribute to the role of human papillomavirus type 16 E7 in the papillomavirus life cycle. J. Virol. 7914769-14780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DeGregori, J., and D. G. Johnson. 2006. Distinct and overlapping roles for E2F family members in transcription, proliferation and apoptosis. Curr. Mol. Med. 6739-748. [DOI] [PubMed] [Google Scholar]
- 21.de Villiers, E.-M., C. Fauquet, T. R. Broker, H.-U. Bernard, and H. zur Hausen. 2004. Classification of papillomaviruses. Virology 32417-27. [DOI] [PubMed] [Google Scholar]
- 22.Dyson, N., P. M. Howley, K. Münger, and E. Harlow. 1989. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science 243934-937. [DOI] [PubMed] [Google Scholar]
- 23.Egawa, K., A. Iftner, J. Doorbar, Y. Honda, and T. Iftner. 2000. Synthesis of viral DNA and late capsid protein L1 in parabasal spinous cell layers of naturally occurring benign warts infected with human papillomavirus type 1. Virology 268281-293. [DOI] [PubMed] [Google Scholar]
- 24.Firzlaff, J. M., D. A. Galloway, R. N. Eisenman, and B. Luscher. 1989. The E7 protein of human papillomavirus type 16 is phosphorylated by casein kinase II. New Biol. 144-53. [PubMed] [Google Scholar]
- 25.Firzlaff, J. M., B. Luscher, and R. N. Eisenman. 1991. Negative charge at the casein kinase II phosphorylation site is important for transformation but not for Rb protein binding by the E7 protein of human papillomavirus type 16. Proc. Natl. Acad. Sci. USA 885187-5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Flores, E. R., B. L. Allen-Hoffmann, D. Lee, and P. F. Lambert. 2000. The human papillomavirus type 16 E7 oncogene is required for the productive stage of the viral life cycle. J. Virol. 746622-6631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frolov, M. V., and N. J. Dyson. 2004. Molecular mechanisms of E2F-dependent activation and pRB-mediated repression. J. Cell Sci. 1172173-2181. [DOI] [PubMed] [Google Scholar]
- 28.Gage, J. R., C. Meyers, and F. O. Wettstein. 1990. The E7 proteins of the nononcogenic human papillomavirus type 6b (HPV-6b) and of the oncogenic HPV-16 differ in retinoblastoma protein binding and other properties. J. Virol. 64723-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garner-Hamrick., P. A., J. M. Fostel, W.-M. Chien, N. S. Banerjee, L. T. Chow, T. R. Broker, and C. Fisher. 2004. Global effects of human papillomavirus type 18 E6/E7 in an organotypic keratinocyte culture system. J. Virol. 789041-9050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Genovese, C., D. Trani, M. Caputi, and P. P. Claudio. 2006. Cell cycle control and beyond: emerging roles for the retinoblastoma gene family. Oncogene 255201-5209. [DOI] [PubMed] [Google Scholar]
- 31.Giarrè, M., S. Caldeira, I. Malanchi, F. Ciccolini, M. J. Leão, and M. Tommasino. 2001. Induction of pRb degradation by the human papillomavirus type 16 E7 protein is essential to efficiently overcome p16INK4a-imposed G1 cell cycle arrest. J. Virol. 754705-4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haigis, K., J. Sage, J. Glickman, S. Shafer, and T. Jacks. 2006. The related retinoblastoma (pRb) and p130 proteins cooperate to regulate homeostasis in the intestinal epithelium. J. Biol. Chem. 281638-647. [DOI] [PubMed] [Google Scholar]
- 33.Heck, D. V., C. L. Yee, P. M. Howley, and K. Münger. 1992. Efficiency of binding the retinoblastoma protein correlates with the transforming capacity of the E7 oncoproteins of the human papillomaviruses. Proc. Natl. Acad. Sci. USA 894442-4446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jian, Y., B. A. Van Tine, W. M. Chien, G. M. Shaw, T. R. Broker, and L. T. Chow. 1999. Concordant induction of cyclin E and p21cip1in differentiated keratinocytes by the human papillomavirus E7 protein inhibits cellular DNA synthesis. Cell Growth Differ. 10101-111. [PubMed] [Google Scholar]
- 35.Jones, D. L., D. A. Thompson, and K. Münger. 1997. Destabilization of the RB tumor suppressor protein and stabilization of p53 contribute to HPV type 16 E7-induced apoptosis. Virology 23997-107. [DOI] [PubMed] [Google Scholar]
- 36.Kennedy, B. K., D. A. Barbie, M. Classon, N. Dyson, and E. Harlow. 2000. Nuclear organization of DNA replication in primary mammalian cells. Genes Dev. 142855-2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Korenjak, M., and A. Brehm. 2005. E2F-Rb complexes regulating transcription of genes important for differentiation and development. Curr. Opin. Genet. Dev. 15520-527. [DOI] [PubMed] [Google Scholar]
- 38.Lechler, T., and E. Fuchs. 2005. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature 437275-280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee, J. O., A. A. Russo, and N. P. Pavletich. 1998. Structure of the retinoblastoma tumour-suppressor pocket domain bound to a peptide from HPV E7. Nature 391859-865. [DOI] [PubMed] [Google Scholar]
- 40.Leng, X., M. Noble, P. D. Adams, J. Qin, and J. W. Harper. 2002. Reversal of growth suppression by p107 via direct phosphorylation by cyclin D1/cyclin-dependent kinase 4. Mol. Cell. Biol. 222242-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leone, G., F. Nuckolls, S. Ishida, M. Adams, R. Sears, L. Jakoi, A. Miron, and J. R. Nevins. 2000. Identification of a novel E2F3 product suggests a mechanism for determining specificity of repression by Rb proteins. Mol. Cell. Biol. 203626-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Litovchick, L., S. Sadasivam, L. Florens, X. Zhu, S. K. Swanson, S. Velmurugan, R. Chen, M. P. Washburn, X. S. Liu, and J. A. DeCaprio. 2007. Evolutionarily conserved multisubunit RBL2/p130 and E2F4 protein complex represses human cell cycle-dependent genes in quiescence. Mol. Cell 26539-551. [DOI] [PubMed] [Google Scholar]
- 43.Massimi, P., and L. Banks. 2000. Differential phosphorylation of the HPV-16 E7 oncoprotein during the cell cycle. Virology 276388-394. [DOI] [PubMed] [Google Scholar]
- 44.Mayol, X., J. Garriga, and X. Graña. 1995. Cell cycle-dependent phosphorylation of the retinoblastoma-related protein p130. Oncogene 11801-808. [PubMed] [Google Scholar]
- 45.McLaughlin-Drubin, M. E., J. L. Bromberg-White, and C. Meyers. 2005. The role of the human papillomavirus type 18 E7 oncoprotein during the complete viral life cycle. Virology 33861-68. [DOI] [PubMed] [Google Scholar]
- 46.Meggio, F., and L. A. Pinna. 2003. One-thousand-and-one substrates of protein kinase CK2? FASEB J. 17349-368. [DOI] [PubMed] [Google Scholar]
- 47.Merola, E., E. Mattioli, C. Minimo, W. Zuo, C. Rabitti, M. Cicala, R. Caviglia, L. Pollice, A. Gabbrielli, A. Giordano, and P. P. Claudio. 2006. Immunohisto-chemical evaluation of pRb2/p130, VEGF, EZH2, p53, p16, p21waf-1, p27, and PCNA in Barrett's esophagus. J. Cell Physiol. 207512-519. [DOI] [PubMed] [Google Scholar]
- 48.Morgenstern, J. P., and H. Land. 1990. Advanced mammalian gene transfer: high titre retroviral vectors with multiple drug selection markers and a complementary helper-free packaging cell line. Nucleic Acids Res. 183587-3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Münger, K., B. A. Werness, N. Dyson, W. C. Phelps, E. Harlow, and P. M. Howley. 1989. Complex formation of human papillomavirus E7 proteins with the retinoblastoma tumor suppressor gene product. EMBO J. 84099-4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Noya, F., W.-M. Chien, T. R. Broker, and L. T. Chow. 2001. p21cip1 degradation in differentiated keratinocytes is abrogated by costabilization with cyclin E induced by human papillomavirus E7. J. Virol. 756121-6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paramio, J. M., S. Lain, C. Segrelles, E. B. Lane, and J. L. Jorcano. 1998. Differential expression and functionally co-operative roles for the retinoblastoma family of proteins in epidermal differentiation. Oncogene 17949-957. [DOI] [PubMed] [Google Scholar]
- 52.Ruiz, S., C. Segrelles, A. Bravo, M. Santos, P. Perez, H. Leis, J. L. Jorcano, and J. M. Paramio. 2003. Abnormal epidermal differentiation and impaired epithelial-mesenchymal tissue interactions in mice lacking the retinoblastoma relatives p107 and p130. Development 1302341-2353. [DOI] [PubMed] [Google Scholar]
- 53.Ruiz, S., M. Santos, C. Segrelles, H. Leis, J. L. Jorcano, A. Berns, J. M. Paramio, and M. Vooijs. 2004. Unique and overlapping functions of pRb and p107 in the control of proliferation and differentiation in epidermis. Development 1312737-2748. [DOI] [PubMed] [Google Scholar]
- 54.Sang, B. C., and M. S. Barbosa. 1992. Single amino acid substitutions in “low-risk” human papillomavirus (HPV) type 6 E7 protein enhance features characteristic of the “high-risk” HPV E7 oncoproteins. Proc. Natl. Acad. Sci. USA 898063-8067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Scheffner, M., K. Münger, J. M. Huibregtse, and P. M. Howley. 1992. Targeted degradation of the retinoblastoma protein by human papillomavirus E7-E6 fusion proteins. EMBO J. 112425-2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schmitt, A., J. B. Harry, B. Rapp, F. O. Wettstein, and T. Iftner. 1994. Comparison of the properties of the E6 and E7 genes of low- and high-risk cutaneous papillomaviruses reveals strongly transforming and high Rb-binding activity for the E7 protein of the low-risk human papillomavirus type 1. J. Virol. 687051-7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Seedorf, K., T. Oltersdorf, G. Krämmer, and W. Röwekamp. 1987. Identification of early proteins of the human papilloma viruses type 16 (HPV 16) and type 18 (HPV 18) in cervical carcinoma cells. EMBO J. 6139-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Smith-McCune, K., D. Kalman, C. Robbins, S. Shivakumar, L. Yuschenkoff, and J. M. Bishop. 1999. Intranuclear localization of human papillomavirus 16 E7 during transformation and preferential binding of E7 to the Rb family member p130. Proc. Natl. Acad. Sci. USA 966999-7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stanley, J. R., and S. H. Yuspa. 1983. Specific epidermal protein markers are modulated during calcium-induced terminal differentiation. J. Cell Biol. 961809-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stoler, M. H., and T. R. Broker. 1986. In situ hybridization detection of human papillomavirus DNAs and messenger RNAs in genital condylomas and a cervical carcinoma. Hum. Pathol. 171250-1258. [DOI] [PubMed] [Google Scholar]
- 61.Tedesco, D., J. Lukas, and S. I. Reed. 2002. The pRb-related protein p130 is regulated by phosphorylation-dependent proteolysis via the protein-ubiquitin ligase SCF(Skp2). Genes Dev. 162946-2957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ueno, T., K. Sasaki, S. Yoshida, N. Kajitani, A. Satsuka, H. Nakamura, and H. Sakai. 2006. Molecular mechanisms of hyperplasia induction by human papillomavirus E7. Oncogene 254155-4164. [DOI] [PubMed] [Google Scholar]
- 63.Van Tine, B. A., J. C. Kappes, N. S. Banerjee, J. Knops, L. Lai, R. D. M. Steenbergen, C. L. J. M. Meijer, P. J. F. Snijders, P. Chatis, T. R. Broker, P. T. Moen, Jr., and L. T. Chow. 2004. Clonal selection for transcriptionally active viral oncogenes during progression to cancer. J. Virol. 7811172-11186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walboomers, J. M., M. V. Jacobs, M. M. Manos, F. X. Bosch, J. A. Kummer, K. V. Shah, P. J. Snijders, J. Peto, C. J. Meijer, and N. Muñoz. 1999. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J. Pathol. 18912-19. [DOI] [PubMed] [Google Scholar]
- 65.Wilson, J. L., S. C. Dollard, L. T. Chow, and T. R. Broker. 1992. Epithelial-specific gene expression during differentiation of stratified primary human keratinocyte cultures. Cell Growth Differ. 3471-483. [PubMed] [Google Scholar]
- 66.Zhang, B., W. Chen, and A. Roman. 2006. The E7 proteins of low- and high-risk human papillomaviruses share the ability to target the pRB family member p130 for degradation. Proc. Natl. Acad. Sci. USA 103437-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zou, N., J.-S. Liu, S.-R. Kuo, T. R. Broker, and L. T. Chow. 1998. The carboxyl-terminal region of the human papillomavirus type 16 E1 protein determines E2 protein specificity during DNA replication. J. Virol. 723436-3441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.zur Hausen, H. 2002. Papillomaviruses and cancer: from basic studies to clinical application. Nat. Rev. Cancer 2342-350. [DOI] [PubMed] [Google Scholar]