Abstract
Both innate and adaptive immune responses participate in the control of murine cytomegalovirus (mCMV) infection. In some mouse strains, like BALB/c, the control of infection relies on the activities of CD8+ T cells. mCMV-specific CD8+ T-cell responses are unusual in that, even after mCMV has been controlled in the periphery, the numbers of circulating virus-specific CD8+ T cells remain high compared to those observed in other viral infections. To better understand the generation and maintenance of mCMV-specific CD8+ T-cell responses, we evaluated how antigen load and effector molecules, such as perforin (Prf) and gamma interferon (IFN-γ), influence these responses during acute infection in vivo. Viral burden affected the magnitude, but not the early kinetics, of antigen-specific CD8+ T-cell responses. Similarly, the magnitude of virus-specific CD8+ T-cell expansion was affected by Prf and IFN-γ, but contraction of antigen-specific responses occurred normally in both Prf- and IFN-γ-deficient mice. These data indicate that control of mCMV-specific CD8+ T-cell expansion and contraction is more complex than anticipated and, despite the role of Prf or IFN-γ in controlling viral replication, a full program of T-cell expansion and contraction can occur in their absence.
Adaptive immune responses are critical for the control of many viral and bacterial pathogens. CD8+ T cells contribute significantly to limiting viral replication during acute infection and are thought to be essential for the ongoing control of persistent infections. CD8+ T-cell responses are characterized by an expansion phase, during which rapid proliferation and differentiation of antigen-specific effector T cells occurs. Antigen-specific T cells then undergo contraction, whereby ∼95% of these cells are eliminated, leaving a small population of cells that persists as a stable pool of memory T cells (2, 16, 38). This pattern of expansion and contraction, which applies to most T-cell responses, has been shown to occur independently of ongoing antigen display and to be influenced by early inflammatory signals (3, 5, 22). It has also been postulated that perforin (Prf) and gamma interferon (IFN-γ) regulate the kinetics of CD8+ T-cell responses and that these activities are largely independent of the antimicrobial effector functions of these molecules (12, 13). Here, we investigated the kinetics of antigen-specific CD8+ T-cell responses in a model of cytomegalovirus (CMV) infection.
Infections with herpesviruses, such as CMV, are characterized by persistent infection and ultimately the establishment of latency. Under these conditions the virus remains with its host lifelong. Although in healthy subjects infection is generally asymptomatic, infection of immunocompromised hosts leads to severe disease. The high prevalence of human CMV (hCMV) infection, together with the ability of the virus to cause a chronic ongoing infection, makes hCMV a medically important pathogen. Murine CMV (mCMV) shares significant homology with hCMV, and the mouse model has been used extensively to define the contribution of various components of host immunity to controlling CMV infection. Control of mCMV requires the concerted activities of innate and adaptive immune effectors. In some mouse strains NK cells control mCMV during acute infection; however, cytotoxic CD8+ T cells are critical to control mCMV over time and to resolve disease arising from viral reactivation (25). In humans, although the T-cell response to hCMV seems to be broader than anticipated, IE1 and pp65 represent two of the most important viral antigens to which CD8+ T cells are directed (18, 19, 32). In the mouse, the best-characterized T-cell antigen is the H-2 Ld-restricted nonameric peptide YPHFMPTNL from the mCMV IE1 nonstructural protein (25, 37). Importantly, IE1-restricted CD8+ T cells can transfer protection against mCMV infection (23). CMV-specific CD8+ T cells have been studied extensively, both in humans and mice, but the precise requirements for the generation and maintenance of antiviral responses remain unclear. Recently, NK cells have been shown to promote early mCMV-specific CD8 T-cell responses by limiting IFN-α/β production (26). Unlike other viral infections, the number of virus-specific CD8+ T cells in primary CMV infection increases to an unusually high level (17). As viral loads decrease, virus-specific CD8+ T-cell responses contract, but the numbers of circulating virus-specific CD8+ T cells remain high compared to those observed in other viral infections (17, 20, 30).
The kinetics of antigen-specific CD8+ T-cell responses are programmed early after infection (3, 4), and effector molecules such as Prf and IFN-γ can play an important role in the homeostatic regulation of antigen-specific CD8+ T-cell responses in several models of infection. For example, in the absence of IFN-γ, lymphocytic choriomeningitis virus-specific CD8+ T cells show impaired expansion, as well as a considerable reduction in contraction (5). Analysis of the role of effector molecules on mCMV-specific CD8+ T-cell responses is complicated by the fact that mice lacking Prf or IFN-γ display increased susceptibility to mCMV (21, 33). Indeed, Prf-deficient hosts are highly susceptible to mCMV and normally succumb to infection. By reducing the dose of virus at the time of infection, we have developed a model in BALB/c mice in which overt viral replication and immunopathology are not observed in the absence of Prf or IFN-γ. Here we have characterized the effect of antigen load on the kinetics of antiviral CD8+ T-cell responses and have utilized this model to examine the role of NK cells, IFN-γ, and Prf in regulating mCMV-specific CD8+ T-cell responses. A full program of T-cell expansion and contraction occurred irrespective of antigen load and in the absence of Prf or IFN-γ. Importantly, NK cells, Prf, and IFN-γ were not required for the contraction of the mCMV-specific CD8+ T-cell response, despite affecting the magnitude of expansion.
MATERIALS AND METHODS
Mice.
Inbred BALB/c (H-2d, I-Ad) mice of 8 weeks of age were obtained from the Animal Resources Centre (Perth, Western Australia, Australia). BALB.B6-CT6 mice (27) were bred in the Animal Services Facility of the University of Western Australia. BALB/c.IFN-γ −/− (GKO) and BALB/c.Prf−/− (Prf−/−) mice were bred at the Peter MacCallum Cancer Centre. Mice with targeted gene deletions were either generated on a BALB/c background or backcrossed to a BALB/c background at least 10 times. During experimentation, all mice were housed under specific-pathogen-free conditions at the Animal Services Facility of the University of Western Australia. All animal experimentation was performed with the approval of the Animal Ethics and Experimentation Committee of the University of Western Australia and according to the guidelines of the National Health and Medical Research Council of Australia.
Cell lines and reagents.
Mouse embryonic fibroblasts were cultured in minimal essential medium (Gibco Life Sciences, Sydney, Australia) supplemented with neonatal calf serum (Gibco Life Sciences, Sydney, Australia).
Infection and in vivo monoclonal antibody administration.
Mice were infected intraperitoneally with either 104 or 102 PFU of mCMV-K181-Perth diluted in phosphate-buffered saline-0.05% fetal bovine serum. Control mice received an equal volume of phosphate-buffered saline-0.05% fetal bovine serum. For NK cell depletion studies, mice were injected intraperitoneally with PK136 monoclonal antibody (anti-NK1.1) or mouse immunoglobulin G 9E10 at days −2, 0, 2, and 4 and every 4 days after, as described elsewhere (28). Depletion of NK cells was confirmed by fluorescence-activated cell sorter (FACS) analysis.
Determination of viral titers.
Organs were prepared, and titers were determined as described elsewhere (36).
Isolation and phenotypic analysis of lymphocytes by flow cytometry.
Single-cell suspensions were prepared as described previously (1). Aliquots of cells were preincubated on ice for 30 min with EDTA-simple salt-fetal calf serum containing 20% normal goat serum. To detect IE1-specific T cells, allophycocyanin (APC)-conjugated T-cell receptor (TCR; H57-597; Biolegend) was used together with APC-Cy7-conjugated CD8α (53-6.7; Biolegend) and phycoerythrin (PE)-conjugated H-2-Ld-168-YPHFMPTNL-176 IE1 tetramer (ImmunoID Tetramers, University of Melbourne, Australia). 7-Amino-actinomycin D was incorporated in the final wash at 2 μg/ml to exclude dead cells from the analysis. The fluorescence-labeled preparations were analyzed on a FACSCanto (Becton Dickinson, San Jose, CA). Appropriately stained controls were used to check compensation for all fluorochromes used. Files of 2,000 IE1+ CD8+ events were collected and analyzed with the FlowJo software (Stanford University, CA).
Statistical analysis.
For statistical analysis, the two-tailed Mann-Whitney test was performed using Prism (GraphPad Software, San Diego, CA). All data are shown as means ± standard errors (SE).
RESULTS
Effect of antigen dose on IE1-specific CD8+ T-cell responses.
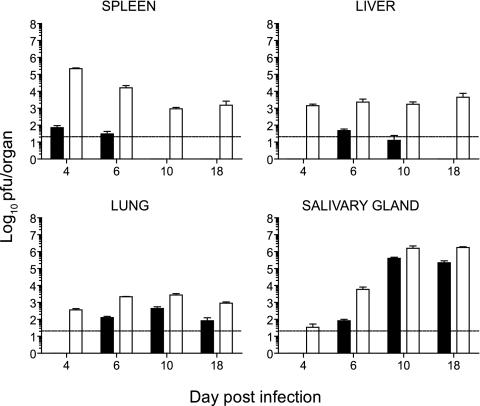
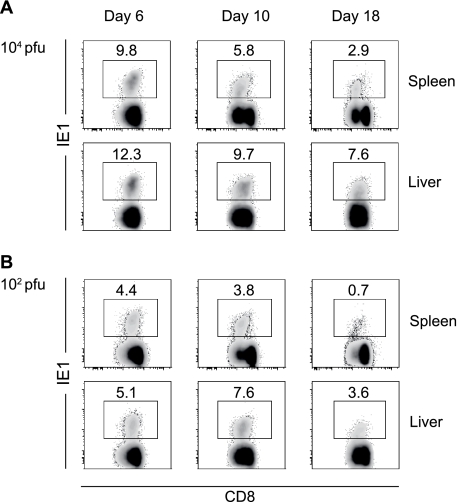
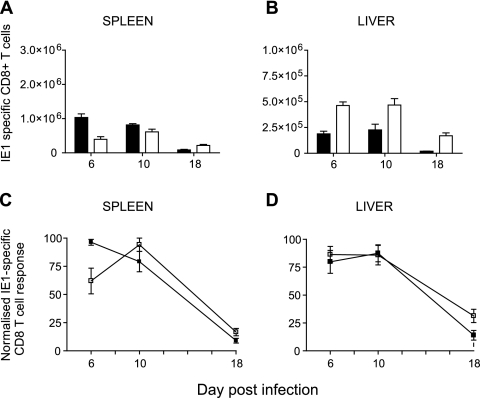
We established a model of infection in BALB/c mice whereby decreasing the viral inoculum to 102 PFU resulted in minimal viral replication in visceral organs compared to mice infected with our standard inoculum of 104 PFU (Fig. 1). Following infection with 102 PFU, viral titers in the spleen, liver, and lungs were significantly reduced compared to those observed in mice infected with 104 PFU of virus (Fig. 1). Despite the reduced viral replication in visceral organs, the virus did traffic and replicate in salivary glands, a site of viral persistence involved in horizontal transmission of CMV. In both 104 and 102 PFU infections, virus trafficked to the salivary gland with similar kinetics, but again viral titers were lower in mice infected with 102 PFU of virus (Fig. 1). Next, we sought to determine whether antigen dose affects the kinetics of antigen-specific CD8+ T-cell responses, including their expansion and contraction. BALB/c mice infected with mCMV mount a CD8+ T-cell response directed principally against the H-2-Ld-restricted peptide 168-176 from the IE1 protein (17, 30, 37). IE1-specific CD8+ T-cell responses were measured on days 6, 10, and 18 following infection of BALB/c mice with 104 (Fig. 2A) or 102 PFU of mCMV (Fig. 2B). IE1-specific CD8+ T cells were detected, in both spleen and liver, after infection with 102 PFU of mCMV (Fig. 2B), albeit at lower frequencies than those observed in mice infected with 104 PFU of virus (Fig. 2A). Thus, antigen-specific CD8+ T cells were effectively generated in mice infected with 102 PFU of virus, even though viral titers were low (Fig. 1). The frequencies of IE1-specific CD8+ T cells in the spleen and liver were higher in mice infected with the 104 PFU inoculum, suggesting that the magnitude of the antigen-specific T-cell response is affected by antigen burden. Analysis of IE1-specific CD8+ T-cell numbers over the course of infection was then undertaken to determine whether a full program of expansion and contraction did occur in mice infected with 102 PFU of virus. The numbers of IE1-specific CD8+ T cells in the spleen at day 6 after infection were higher in mice infected with 102 PFU than in mice infected with 104 PFU of mCMV (Fig. 3A). Since infection with 104 PFU of virus results in an overall loss of splenocytes, this result was not unexpected. In contrast, in the liver the number of IE1-specific CD8+ T cells was higher in mice infected with 104 PFU of virus at all the times tested (Fig. 3B). Thus, antigen dose did affect the magnitude of expansion of virus-specific CD8+ T cells, at least in the liver. Despite the differences in the numbers of IE1-specific CD8+ T cells observed in mice infected with 102 or 104 PFU of mCMV, a full program of expansion and contraction was observed in both the spleen (Fig. 3C) and liver (Fig. 3D) under either infection condition.
FIG. 1.
Viral titers in BALB/c mice following infection with 102 or 104 PFU of mCMV. Mice were infected with 102 (black bars) or 104 (white bars) PFU of mCMV, and spleen, liver, lung, and salivary gland titers were determined at various times after infection. The data presented are the means ± SE of five mice. Data are representative of at least two independent experiments. Viral titers were significantly different between 102 and 104 PFU infections at all time points in the spleen, liver, and lung (P < 0.005, except for day 10 results for the liver [P < 0.05]). In the salivary gland viral titers were significantly different at days 6 (P < 0.05) and 18 (P < 0.005).
FIG. 2.
Frequency of IE1-specific CD8+ T cells following infection of mice with 104 (A) or 102 (B) PFU of mCMV. Spleens and livers were harvested at days 6, 10, and 18 postinfection, and the frequency of IE1-specific T cells was determined by FACS staining with TCRβ-APC, CD8α-APC-Cy7, and H2-Ld IE tetramer-PE. Numbers in density plots indicate the frequencies of IE1-specific cells in the CD8+ T-cell pool. Density plots are representative of results observed in six mice from three independent experiments.
FIG. 3.
Kinetics of mCMV IE1-specific CD8+ T-cell responses in the spleen and liver. (A and B) The total number of IE1-specific CD8+ T cells in the spleens (A) and livers (B) of mice infected with 102 (black bars) or 104 (white bars) PFU of mCMV is presented as the combined data from two experiments with three mice per time point. Means ± SE are shown. (C and D) Kinetics of the response in the spleen (C) and liver (D) are shown as normalized IE1-specific CD8+ T-cell responses; the data are presented as a percentage, with the maximal number of IE1-specific CD8+ T cells set as 100%. Responses for mice infected with 102 (black squares) or 104 (white squares) PFU mCMV are shown. Combined data from two experiments with three mice per time point are presented. Means ± SE are shown.
Natural killer cells regulate the expansion of antigen-specific responses.
Having established that virus-specific CD8+ T cells undergo a full program of expansion and contraction in BALB/c mice infected with 102 PFU of virus, we utilized this model to define whether effector molecules affect the kinetics of IE1-specific CD8+ T-cell responses, especially the contraction phase, as shown in other models of viral infection. First, we evaluated the impact of NK cell-mediated activities. NK cells are rapidly activated in mCMV infection in both resistant and susceptible mouse strains. In resistant mouse strains, like C57BL/6, recognition of infected cells in a Ly49H-dependent manner results in their elimination. In contrast, in mouse strains like BALB/c, which lack Ly49H and are classified as mCMV susceptible, despite being activated, NK cells fail to recognize infected targets. Thus, the BALB/c model of mCMV infection provides a unique system to study whether NK cell-mediated responses can affect antigen-specific CD8+ T-cell responses in the absence of a cytolytic effector role on virus-infected targets.
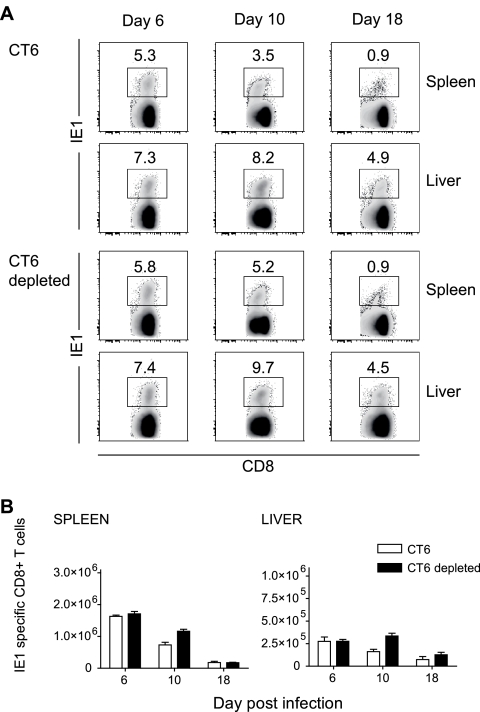
For these experiments we utilized the congenic BALB.B6-CT6 (CT6) mouse strain (27), which carries the NK1.1 locus and thus allows depletion of NK cells using the NK1.1-specific PK136 monoclonal antibody. Control or NK cell-depleted BALB.B6-CT6 mice were infected with 102 PFU of mCMV, and the kinetics of the IE1-specific CD8+ T-cell response were assessed. At day 6 after infection, the frequency of IE1-specific CD8+ T cells was equivalent in control and NK cell-depleted CT6 mice, in both the spleen and liver (Fig. 4A). Numbers of IE1-specific CD8+ T cells were also equivalent in control and NK cell-depleted CT6 mice at this time (Fig. 4B). At day 10 postinfection, mice lacking NK cells showed significantly higher frequencies (P < 0.05) (Fig. 4A) and numbers (P < 0.005) (Fig. 4B) of IE1-specific CD8+ T cells compared to control undepleted CT6 mice, in both the spleen and liver. The absence of NK cells, however, did not affect the contraction of virus-specific CD8+ T cells, and this phase of the response occurred normally in both the spleen and liver of NK cell-depleted mice (Fig. 4B). Thus, despite a slight delay, contraction of the IE1-specific CD8+ T-cell response occurs efficiently in the absence of NK cells.
FIG. 4.
Frequencies and numbers of IE1-specific CD8+ T cells in the spleens and livers of mice lacking NK cells. CT6 mice treated with an irrelevant immunoglobulin or with the anti-NK1.1 PK136 monoclonal antibody prior to infection with 102 PFU mCMV were used for these experiments. (A) Frequencies of IE1-specific CD8+ T cells at days 6, 10, and 18 after infection were determined by FACS staining with TCRβ-APC, CD8α-APC-Cy7, and H2-Ld IE tetramer-PE. Numbers in the density plots indicate the mean frequencies of IE1+ cells in the CD8 T-cell pool. Density plots are representative of six mice from two independent experiments with three mice per group. (B) Numbers of IE1-specific CD8+ T cells at days 6, 10, and 18 after infection; numbers represent the means ± SE of five to six mice from two independent experiments.
Kinetics of IE1-specific CD8+ T-cell responses elicited in the absence of Prf or IFN-γ.
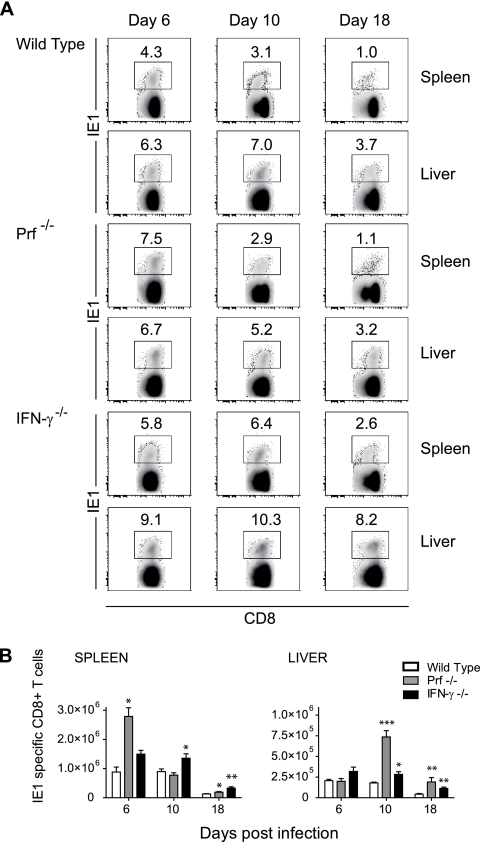
Next, we examined whether the kinetics of IE1-specific CD8+ T-cell responses were affected by Prf and IFN-γ, since these molecules affect the expansion and/or contraction of antigen-specific T-cell responses in other systems. BALB/c mice lacking either Prf (Prf−/−) or IFN-γ (IFN-γ−/−) were infected with 102 PFU of mCMV, and the frequencies and numbers of IE1-specific CD8+ T cells were measured at days 6, 10, and 18 after infection. Infection with this dose of virus did not cause overt pathogenesis in either Prf- or IFN-γ-deficient mice, and viral replication in visceral organs and the salivary gland was similar to that observed in wild-type mice (data not shown). The frequencies of IE1+ CD8+ T cells were significantly higher (P < 0.05) in the spleens and livers of IFN-γ-deficient mice at all time points, compared with wild-type mice (Fig. 5A). No significant differences were observed in the frequencies of IE1+ CD8+ T cells between wild-type and Prf−/− mice except at day 6 in the spleen (P < 0.05) (Fig. 5A). In comparison to wild-type mice, the number of IE1-specific CD8+ T cells was significantly higher in the spleens of Prf−/− mice at day 6 postinfection (Fig. 5B); no significant difference was observed at this time point in the liver (Fig. 5B). Significantly higher numbers of IE1-specific CD8+ T cells were also observed in the livers of Prf−/− mice at day 10 and in both the spleens and livers at 18 postinfection, compared to wild-type mice (Fig. 5B). In comparison to wild-type mice, IFN-γ−/− mice displayed significantly higher numbers of IE1-specific CD8+ T cells in both the spleen and the liver at days 10 and 18 postinfection (Fig. 5B). Analysis of the kinetics of contraction of IE1-specific CD8+ T-cell responses revealed that by day 18 postinfection, contraction occurred in both Prf- and IFN-γ-deficient mice in both the spleen and the liver. Together these data indicate that a full program of expansion and contraction of mCMV-specific CD8+ T-cell responses can occur in the absence of Prf and IFN-γ.
FIG. 5.
Frequencies and numbers of IE1-specific CD8+ T cells in the spleens and livers of mice lacking IFN-γ or Prf. BALB/c mice (wild type) or BALB/c mice lacking either IFN-γ (IFN-γ−/−) or Prf (Prf−/−) were infected with 102 PFU mCMV. (A) Frequencies of IE1-specific CD8+ T cells at days 6, 10, and 18 after infection were determined by FACS staining with TCRβ-APC, CD8α-APC-Cy7, and H2-Ld IE tetramer-PE. Numbers in the density plots indicate the mean frequency of IE1+ cells in the CD8 T-cell pool. (B) Numbers of IE1-specific CD8+ T cells in the spleen and liver at days 6, 10, and 18 after infection; numbers represent the means ± SE of six mice from two independent experiments for all times, except for day 6. *, P < 0.02; **, P < 0.005; ***, P < 0.0005.
DISCUSSION
hCMV is a major pathogen of humans. The reactivation of latent hCMV still poses a major health risk in immunocompromised patients, and a better understanding of the responses required to control this pathogen is therefore of immediate clinical relevance. The goal of the current study was to define the kinetics of mCMV-specific CD8+ T-cell responses elicited in the absence of Prf or IFN-γ. This was achieved by tracking the expansion and contraction phases of CD8+ T-cell responses elicited against the H2-Ld-restricted immunodominant mCMV IE1 epitope. This epitope represents the best-characterized antigen in the mCMV immune response (15, 17, 23, 25, 30, 37), and IE1-specific CD8+ T cells have been shown to transfer resistance to subsequent infections (15, 23). Furthermore, in hCMV infection antiviral CD8+ T-cell responses seem to be focused to IE1 and pp65, at least during primary acute infection (19). Accordingly, we focused our analysis on CD8+ T-cell responses restricted to the IE1 epitope. Interestingly, the IE1-specific CD8+ T-cell response displays some unusual characteristics, the most striking of which is the high number of circulating virus-specific CD8+ T cells that remain throughout the course of infection, even after the virus has been controlled at least in peripheral target organs (17, 30). Since virus-specific CD8+ T-cell responses influence the ability to control subsequent infection (15, 23) and contribute to the maintenance of viral latency and the control of viral reactivation (23, 31), a better understanding of the factors that underpin these responses will provide important insights into the management of hCMV. Indeed, the murine model of CMV infection in BALB/c mice closely mimics infection with hCMV in terms of the antigenicity of the viruses and the immunodominance of the major epitopes (18, 23, 32). Infection of BALB/c mice lacking IFN-γ or Prf with a standard sublethal dose of mCMV (104 PFU) leads to increased viral titers and/or mortality (N. Sumaria and M. A. Degli-Esposti, unpublished data), making it impossible to analyze the relevance of these molecules in the context of the generation and maintenance of antiviral CD8+ T-cell responses. We therefore established and characterized a model where infection is initiated by a lower viral inoculum (102 PFU). First, we assessed the impact of viral load on IE1-specific CD8+ T-cell responses. In other infection systems expansion of antigen-specific CD8+ T cells has been reported to be largely independent of antigen display (3, 22), despite the fact that high antigen loads can define the magnitude of expansion (3). Similarly, contraction of antigen-specific CD8+ T-cell responses is independent of duration of infection or antigen display (3, 5, 22). Unlike what is observed in the expansion phase, the magnitude of contraction is also independent of antigen display (3, 5); however, the timing of antigen display can affect the onset of antigen-specific CD8+ T-cell contraction (24). In our model of mCMV infection with a reduced viral dose (102 PFU), the magnitude of expansion of IE1-specific CD8+ T-cell responses was diminished, but a full program of expansion and contraction was observed, despite viral replication being below the level of detection in the principal target organs (spleen and liver) during the acute phase of infection.
Antigen-specific CD8+ T-cell kinetics, including expansion and contraction, can be regulated by Prf and IFN-γ. One of the principal sources of these effector molecules in viral infections, including mCMV infection, is NK cells. Indeed, NK cell-derived IFN-γ regulates the homeostasis of antigen-specific CD8+ T cells and has been reported to promote expansion, as well as regulate contraction (29).
In the absence of NK cells we observed higher frequencies and numbers of IE1-specific CD8+ T cells at day 10 postinfection. Although the waning of the response was slightly slower in the absence of NK cells, effective contraction was not impaired. Higher numbers of IE1-specific CD8+ T cells were also observed in mice lacking IFN-γ, supporting the notion that NK-cell derived IFN-γ affects the size of the IE1-specific CD8+ T-cell pool. IFN-γ has also been reported to play a critical role in the contraction of antigen-specific CD8+ T-cell responses in other models of infection (5). In our system IFN-γ only mildly affected the extent of contraction of the IE1-specific CD8+ T-cell response, and overall the response contracted by 60 to 80% by day 18 postinfection. A similar effect was reported after infection with vesicular stomatitis virus, a pathogen whose control does not require IFN-γ (8). In our model, in the absence of IFN-γ the lowest level of contraction was observed in the liver. Although it is possible that this effect is completely independent of viral control, it should be noted that even after low-dose infection, low-level viral replication was observed in the livers of IFN-γ-deficient mice (data not shown). Thus, it is most likely that the slightly reduced contraction observed in mCMV-infected IFN-γ−/− mice is due to the effect of IFN-γ on pathogen clearance, rather than a direct effect on antigen-specific CD8+ T cells. This conclusion concurs with the finding that IFN-γ is not necessary for the contraction of antigen-specific CD8+ T-cell responses in vesicular stomatitis virus infection (8) but is essential for contraction in Listeria sp. and lymphocytic choriomeningitis virus models (5), the latter being pathogens whose control heavily relies on the antimicrobial effects of this cytokine (5, 6). Furthermore, a defect in contraction was not observed in NK cell-depleted mice, indicating that the source of IFN-γ that affects IE1-specific CD8+ T-cell contraction is a cell type other than NK cells. Indeed, the main sources of IFN-γ from day 6 after mCMV infection are CD4+ and CD8+ T cells (data not shown).
The increased frequency and number of IE1-specific CD8+ T cells observed in IFN-γ-deficient mice indicate that IFN-γ interferes with the generation of antigen-specific CD8+ T cells. IFN-γ is generally thought to be essential for optimal antigen processing/presentation and, thus, the generation of maximal virus-specific T-cell responses (14). Indeed, IFN-γ is critical for the generation of IE1 nonamers (11). However, IFN-γ has also been reported to negatively regulate CD8+ T-cell responses (9). Analysis of the mechanisms by which IFN-γ can affect the generation of T-cell responses is in progress. Recent studies have defined and examined CD8 T-cell responses directed against a number of other viral epitopes, including those derived from m04, m45, m83, m84, and m164 (17, 30). These responses display the usual kinetics of expansion and contraction during acute infection, but the IE1- and m164-specific CD8+ T-cell responses show “memory inflation” and accumulate over time during the persistent and latent phases of infection (17, 30). The relevance of “memory inflation” remains unclear at present; however, CD8+ T-cell responses specific for “inflationary” mCMV epitopes display an effector memory phenotype which could possibly indicate that their activation and proliferation are induced over time by a continuous encounter with antigen (17, 30). Future studies, similar to the ones described here, investigating the role of NK cells, Prf, and IFN-γ on antiviral CD8+ T-cell responses specific for “inflationary” and “noninflationary” mCMV epitopes during the persistent/latent phases of infection will be of interest.
Like IFN-γ, Prf affected the magnitude of IE1-specific CD8+ T cells in the liver at day 10 postinfection. A slight reduction in the level of contraction was also observed in Prf-deficient mice but, as observed in IFN-γ-deficient mice, significant contraction still occurred in mice lacking Prf. These data indicate that the homeostasis of virus-specific CD8+ T cells is largely independent of Prf and IFN-γ and that a full program of expansion and contraction of mCMV-specific CD8+ T-cell responses can occur in the absence of either of these effector molecules.
In conclusion, although many of the features of antigen-specific CD8+ T-cell homeostasis are universal, our data suggest that analysis of antigen-specific T-cell responses and the factors that control their homeostasis requires careful interpretation specifically in the model under investigation. The finding that improved expansion of mCMV-specific CD8+ T cells is achieved in the absence of IFN-γ without a major defect in contraction may aid in the development of improved vaccine therapies. Strategies to improve the generation of hCMV-specific T cells ex vivo are being assessed (7, 10, 34, 35), and a better understanding of the factors that control the kinetics of these responses will aid the design of such protocols.
Acknowledgments
We are grateful to Robbert van der Most for stimulating and insightful discussions and critical reading of the manuscript. We also thank Helen Molder, Simone Ross, Shannon Griffiths, and the staff in the Animal Facilities at the University of Western Australia and the Peter MacCallum Cancer Centre for their support with animal breeding and maintenance.
This work was supported by grants and fellowships from the National Health and Medical Research Council of Australia, the Raine Foundation (to D.M.A.), and the Wellcome Trust (Overseas Senior Research Fellowship in Biomedical Science to M.A.D.-E).
Footnotes
Published ahead of print on 12 March 2008.
REFERENCES
- 1.Andrews, D. M., C. E. Andoniou, F. Granucci, P. Ricciardi-Castagnoli, and M. A. Degli-Esposti. 2001. Infection of dendritic cells by murine cytomegalovirus induces functional paralysis. Nat. Immunol. 21077-1084. [DOI] [PubMed] [Google Scholar]
- 2.Badovinac, V. P., and J. T. Harty. 2006. Programming, demarcating, and manipulating CD8+ T-cell memory. Immunol. Rev. 21167-80. [DOI] [PubMed] [Google Scholar]
- 3.Badovinac, V. P., B. B. Porter, and J. T. Harty. 2004. CD8+ T cell contraction is controlled by early inflammation. Nat. Immunol. 5809-817. [DOI] [PubMed] [Google Scholar]
- 4.Badovinac, V. P., B. B. Porter, and J. T. Harty. 2002. Programmed contraction of CD8+ T cells after infection. Nat. Immunol. 3619-626. [DOI] [PubMed] [Google Scholar]
- 5.Badovinac, V. P., A. R. Tvinnereim, and J. T. Harty. 2000. Regulation of antigen-specific CD8+ T cell homeostasis by perforin and interferon-gamma. Science 2901354-1358. [DOI] [PubMed] [Google Scholar]
- 6.Bartholdy, C., J. P. Christensen, D. Wodarz, and A. R. Thomsen. 2000. Persistent virus infection despite chronic cytotoxic T-lymphocyte activation in gamma interferon-deficient mice infected with lymphocytic choriomeningitis virus. J. Virol. 7410304-10311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carlsson, B., W. S. Cheng, T. H. Totterman, and M. Essand. 2003. Ex vivo stimulation of cytomegalovirus (CMV)-specific T cells using CMV pp65-modified dendritic cells as stimulators. Br. J. Haematol. 121428-438. [DOI] [PubMed] [Google Scholar]
- 8.Christensen, J. E., D. Wodarz, J. P. Christensen, and A. R. Thomsen. 2004. Perforin and IFN-gamma do not significantly regulate the virus-specific CD8+ T cell response in the absence of antiviral effector activity. Eur. J. Immunol. 341389-1394. [DOI] [PubMed] [Google Scholar]
- 9.Dalton, D. K., S. Pitts-Meek, S. Keshav, I. S. Figari, A. Bradley, and T. A. Stewart. 1993. Multiple defects of immune cell function in mice with disrupted interferon-gamma genes. Science 2591739-1742. [DOI] [PubMed] [Google Scholar]
- 10.Foster, A. E., D. J. Gottlieb, M. Marangolo, A. Bartlett, Y. C. Li, G. W. Barton, J. A. Romagnoli, and K. F. Bradstock. 2003. Rapid, large-scale generation of highly pure cytomegalovirus-specific cytotoxic T cells for adoptive immunotherapy. J. Hematother. Stem Cell Res. 1293-105. [DOI] [PubMed] [Google Scholar]
- 11.Geginat, G., T. Ruppert, H. Hengel, R. Holtappels, and U. H. Koszinowski. 1997. IFN-gamma is a prerequisite for optimal antigen processing of viral peptides in vivo. J. Immunol. 1583303-3310. [PubMed] [Google Scholar]
- 12.Haring, J. S., V. P. Badovinac, and J. T. Harty. 2006. Inflaming the CD8+ T cell response. Immunity 2519-29. [DOI] [PubMed] [Google Scholar]
- 13.Harty, J. T., and V. P. Badovinac. 2002. Influence of effector molecules on the CD8+ T cell response to infection. Curr. Opin. Immunol. 14360-365. [DOI] [PubMed] [Google Scholar]
- 14.Heise, M. T., M. Connick, and H. W. T. Virgin. 1998. Murine cytomegalovirus inhibits interferon gamma-induced antigen presentation to CD4 T cells by macrophages via regulation of expression of major histocompatibility complex class II-associated genes. J. Exp. Med. 1871037-1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holtappels, R., D. Thomas, J. Podlech, and M. J. Reddehase. 2002. Two antigenic peptides from genes m123 and m164 of murine cytomegalovirus quantitatively dominate CD8 T-cell memory in the H-2d haplotype. J. Virol. 76151-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaech, S. M., E. J. Wherry, and R. Ahmed. 2002. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2251-262. [DOI] [PubMed] [Google Scholar]
- 17.Karrer, U., S. Sierro, M. Wagner, A. Oxenius, H. Hengel, U. H. Koszinowski, R. E. Phillips, and P. Klenerman. 2003. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J. Immunol. 1702022-2029. [DOI] [PubMed] [Google Scholar]
- 18.Khan, N., M. Cobbold, R. Keenan, and P. A. Moss. 2002. Comparative analysis of CD8+ T cell responses against human cytomegalovirus proteins pp65 and immediate early 1 shows similarities in precursor frequency, oligoclonality, and phenotype. J. Infect. Dis. 1851025-1034. [DOI] [PubMed] [Google Scholar]
- 19.Khan, N., D. Best, R. Bruton, L. Nayak, A. B. Rickinson, and P. A. H. Moss. 2007. T cell recognition patterns of immunodominant cytomegalovirus antigens in primary and persistent infection. J. Immunol. 1784455-4465. [DOI] [PubMed] [Google Scholar]
- 20.Klenerman, P., and A. Hill. 2005. T cells and viral persistence: lessons from diverse infections. Nat. Immunol. 6873-879. [DOI] [PubMed] [Google Scholar]
- 21.Loh, J., D. T. Chu, A. K. O'Guin, W. M. Yokoyama, and H. W. T. Virgin. 2005. Natural killer cells utilize both perforin and gamma interferon to regulate murine cytomegalovirus infection in the spleen and liver. J. Virol. 79661-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mercado, R., S. Vijh, S. E. Allen, K. Kerksiek, I. M. Pilip, and E. G. Pamer. 2000. Early programming of T cell populations responding to bacterial infection. J. Immunol. 1656833-6839. [DOI] [PubMed] [Google Scholar]
- 23.Pahl-Seibert, M. F., M. Juelch, J. Podlech, D. Thomas, P. Deegen, M. J. Reddehase, and R. Holtappels. 2005. Highly protective in vivo function of cytomegalovirus IE1 epitope-specific memory CD8 T cells purified by T-cell receptor-based cell sorting. J. Virol. 795400-5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Porter, B. B., and J. T. Harty. 2006. The onset of CD8+-T-cell contraction is influenced by the peak of Listeria monocytogenes infection and antigen display. Infect. Immun. 741528-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reddehase, M. J. 2002. Antigens and immunoevasins: opponents in cytomegalovirus immune surveillance. Nat. Rev. Immunol. 2831-844. [DOI] [PubMed] [Google Scholar]
- 26.Robbins, S. H., G. Bessou, A. Cornillon, N. Zucchini, B. Rupp, Z. Ruzsics, T. Sacher, E. Tomasello, E. Vivier, U. H. Koszinowski, and M. Dalod. 2007. Natural killer cells promote early CD8 T cell responses against cytomegalovirus. PLoS Pathog. 31152-1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scalzo, A. A., M. G. Brown, D. T. Chu, J. W. Heusel, W. M. Yokoyama, and C. A. Forbes. 1999. Development of intra-natural killer complex (NKC) recombinant and congenic mouse strains for mapping and functional analysis of NK cell regulatory loci. Immunogenetics 49238-241. [DOI] [PubMed] [Google Scholar]
- 28.Scalzo, A. A., N. A. Fitzgerald, C. R. Wallace, A. E. Gibbons, Y. C. Smart, R. C. Burton, and G. R. Shellam. 1992. The effect of the Cmv-1 resistance gene, which is linked to the natural killer cell gene complex, is mediated by natural killer cells. J. Immunol. 149581-589. [PubMed] [Google Scholar]
- 29.Sercan, O., G. J. Hammerling, B. Arnold, and T. Schuler. 2006. Innate immune cells contribute to the IFN-gamma-dependent regulation of antigen-specific CD8+ T cell homeostasis. J. Immunol. 176735-739. [DOI] [PubMed] [Google Scholar]
- 30.Sierro, S., R. Rothkopf, and P. Klenerman. 2005. Evolution of diverse antiviral CD8+ T cell populations after murine cytomegalovirus infection. Eur. J. Immunol. 351113-1123. [DOI] [PubMed] [Google Scholar]
- 31.Simon, C. O., R. Holtappels, H. M. Tervo, V. Bohm, T. Daubner, S. A. Oehrlein-Karpi, B. Kuhnapfel, A. Renzaho, D. Strand, J. Podlech, M. J. Reddehase, and N. K. Grzimek. 2006. CD8 T cells control cytomegalovirus latency by epitope-specific sensing of transcriptional reactivation. J. Virol. 8010436-10456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sylwester, A. W., B. L. Mitchell, J. B. Edgar, C. Taormina, C. Pelte, F. Ruchti, P. R. Sleath, K. H. Grabstein, N. A. Hosken, F. Kern, J. A. Nelson, and L. J. Picker. 2005. Broadly targeted human cytomegalovirus-specific CD4+ and CD8+ T cells dominate the memory compartments of exposed subjects. J. Exp. Med. 202673-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tay, C. H., and R. M. Welsh. 1997. Distinct organ-dependent mechanisms for the control of murine cytomegalovirus infection by natural killer cells. J. Virol. 71267-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trivedi, D., R. Y. Williams, R. J. O'Reilly, and G. Koehne. 2005. Generation of CMV-specific T lymphocytes using protein-spanning pools of pp65-derived overlapping pentadecapeptides for adoptive immunotherapy. Blood 1052793-2801. [DOI] [PubMed] [Google Scholar]
- 35.Van den Bosch, G. A., P. Ponsaerts, G. Nijs, M. Lenjou, G. Vanham, D. R. Van Bockstaele, Z. N. Berneman, and V. F. Van Tendeloo. 2005. Ex vivo induction of viral antigen-specific CD8 T cell responses using mRNA-electroporated CD40-activated B cells. Clin. Exp. Immunol. 139458-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Dommelen, S. L., H. A. Tabarias, M. J. Smyth, and M. A. Degli-Esposti. 2003. Activation of natural killer (NK) T cells during murine cytomegalovirus infection enhances the antiviral response mediated by NK cells. J. Virol. 771877-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Volkmer, H., C. Bertholet, S. Jonjic, R. Wittek, and U. H. Koszinowski. 1987. Cytolytic T lymphocyte recognition of the murine cytomegalovirus nonstructural immediate-early protein pp89 expressed by recombinant vaccinia virus. J. Exp. Med. 166668-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wong, P., and E. G. Pamer. 2003. CD8 T cell responses to infectious pathogens. Annu. Rev. Immunol. 2129-70. [DOI] [PubMed] [Google Scholar]