Abstract
Growth factors can influence lineage determination of neural crest stem cells (NCSCs) in an instructive manner, in vitro. Because NCSCs are likely exposed to multiple signals in vivo, these findings raise the question of how stem cells would integrate such combined influences. Bone morphogenetic protein 2 (BMP2) promotes neuronal differentiation and glial growth factor 2 (GGF2) promotes glial differentiation; if NCSCs are exposed to saturating concentrations of both factors, BMP2 appears dominant. By contrast, if the cells are exposed to saturating concentrations of both BMP2 and transforming growth factor β1 (which promotes smooth muscle differentiation), the two factors appear codominant. Sequential addition experiments indicate that NCSCs require 48–96 hrs in GGF2 before they commit to a glial fate, whereas the cells commit to a smooth muscle fate within 24 hr in transforming growth factor β1. The delayed response to GGF2 does not reflect a lack of functional receptors; however, because the growth factor induces rapid mitogen-activated protein kinase phosphorylation in naive cells. Furthermore, GGF2 can attenuate induction of the neurogenic transcription factor mammalian achaete-scute homolog 1, by low doses of BMP2. This short-term antineurogenic influence of GGF2 is not sufficient for glial lineage commitment, however. These data imply that NCSCs exhibit cell-intrinsic biases in the timing and relative dosage sensitivity of their responses to instructive factors that influence the outcome of lineage decisions in the presence of multiple factors. The relative delay in glial lineage commitment, moreover, apparently reflects successive short-term and longer-term actions of GGF2. Such a delay may help to explain why glia normally differentiate after neurons, in vivo.
The diverse cell types of complex tissues such as the blood and the brain are thought to be generated from self-renewing multipotent progenitors called stem cells (1). These stem cells must generate progeny of different phenotypes, in the correct proportions, sequence, and location. The manner in which this is accomplished is not understood. It is clear that the local microenvironment of stem cells has an important influence on their behavior. In most systems, however, the identity of key environmental influences and the nature of their effects on stem cells is not clear.
Previously, we isolated and characterized self-renewing multipotent progenitor cells from the mammalian neural crest (2). In vitro, these neural crest stem cells (NCSCs) are able to generate autonomic neurons, Schwann (glial) cells, and smooth muscle (SM)—three cell types derived from the neural crest in vivo (3). Subsequently, we identified three growth factors that promote differentiation along each of these three lineages, respectively: bone morphogenetic protein 2 (BMP2), glial growth factor 2 [GGF2, a neuregulin (4)], and transforming growth factor β1 (TGF-β1) (5, 6). Clonal analysis and serial observation of identified cells has suggested that each of these factors acts instructively rather than selectively on NCSCs (5, 6) [although some of the factors may do both (7)]. In other words, GGF2, BMP2, and TGF-β1 individually directed the differentiation rather than the survival or proliferation of the majority of individual identified NCSCs plated at clonal density. The neural crest thus represents one of the few systems in which instructive lineage determination signals for multipotential stem cells have been identified (for review, see ref. 1).
It is likely that in vivo NCSCs and other stem cells are exposed to multiple environmental signals. It is, therefore, important to understand how the stem cells integrate such competing influences. We now have performed experiments in which NCSCs are exposed to different combinations of instructive signals. In principle, four outcomes to such experiments are possible: (i) the influence of one signal could dominate over others; (ii) the signals could exert equivalent influences, producing a mixture of lineage-committed progeny; (iii) the signals could nullify each others’ influence, thereby inhibiting differentiation; or (iv) the combination of signals could generate new differentiated phenotypes not seen with any individual signal alone. Our results indicate that each of the first two possible outcomes can be obtained, depending upon the specific combination and concentration of signals tested. These data therefore suggest that stem cell fate is not solely determined by what factors are present in the environment but also is influenced by cell-intrinsic differences in the relative sensitivity and timing of responses to different environmental signals.
MATERIALS AND METHODS
NCSC Cultures.
NCSCs were obtained and cultured at clonal density on a poly-d-lysine/fibronectin substrate as described (2, 6). Briefly, trunk neural tubes from embryonic day 10.5 rat fetuses were isolated by using collagenase dissociation (2). Tubes were plated on a fibronectin substrate in L15-CO2 medium containing 10% chicken embryo extract (CEE) and additives as described (2). Neural crest cells were obtained from trypsinized primary explants and re-plated on a poly-d-lysine/fibronectin substrate (2) in medium containing 15% CEE, and grown in a 6% CO2/94% air atomosphere. The cells were allowed to attach for 6–8 hr before adding supplementary growth factors. Mixing of growth factors was always performed before addition to the culture medium. In experiments involving treatment with TGF-β1, or TGF-β1 followed by BMP2, colonies were established on a fibronectin-only substrate because this allowed better survival of cells. Qualitatively similar results were obtained on pdL/FN, however.
Sources of the growth factors were as described (5, 6). The activities of new lots of each factor were determined by dose–response experiments. Saturating responses were obtained at 1 nM GGF2, 2 nM BMP2, and 20 pM TGF-β1, consistent with our previous results (5, 6). The effects of these factors on differentiation was assessed after 5 days, a time at which spontaneous differentiation of NCSCs to neurons and SM is not yet detectable (2, 6).
Immunocytochemistry.
Mouse mAbs to low-affinity neurotrophin receptor (p75LNTR), mammalian achaete-scute homolog 1 (MASH1), glial fibrillary acidic protein, and α-SM actin (αSMA) and rabbit polyclonal antibody to peripherin were used as described (2, 5, 6). Not all SMs are αSMA+ (6); in such cases, SMs were identified on the basis of their characteristic morphology and lack of immunoreactivity for neuronal, glial, and NCSC markers (6). To assess the inhibitory effects of GGF and TGF-β on neuronal differentiation, “neuronal” colonies were conservatively defined as containing at least one peripherin+ process-bearing cell (although typical neuronal colonies usually contained many more than one neuron). Some neuronal colonies contained SM cells but no LNTR+ cells (6). Neuron-only and neuronal colonies were scored in aggregate for this analysis. SM colonies contained only SM cells (6).
For the mitogen-activated protein kinase (MAPK) phosphorylation assay, 250-1,000 NCSCs were plated in the center of a 12-well Corning dish. After an 8- to 10-hr incubation in control medium (2), growth factors were added to the wells without removing the dish from the incubator. An equal volume of carrier solution without factor was added to some wells as a control. Dishes were agitated to ensure even distribution of the growth factor. (This entire procedure took 1–2 min.) The timer was started and dishes were fixed 10, 20, and 60 min later. Activated MAPK was detected with a rabbit polyclonal antibody (Promega; 1 μg/ml) by the manufacturer’s recommendations. Staining was visualized with an horseradish peroxidase-conjugated secondary antibody and nickelous sulfate/3,3′-diaminobenzidine histochemistry.
RESULTS
NCSCs Exhibit Dosage-Sensitive Interactions Between GGF2 or TGF-β1 and BMP2.
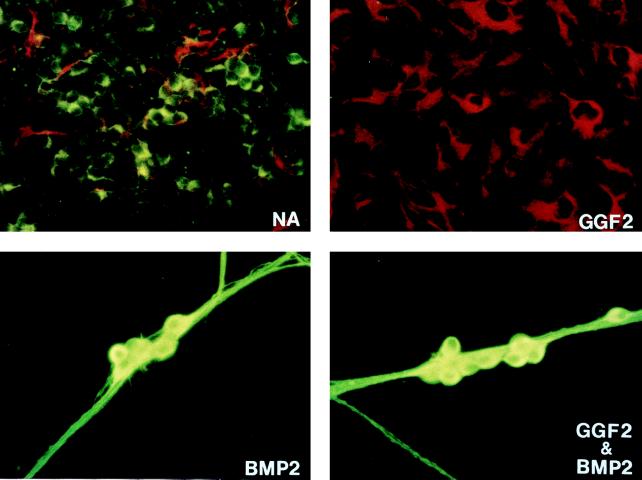
We first examined the ability of two signals that individually promote nonneurogenic fates, GGF2 and TGF-β1, to interfere with autonomic neurogenesis promoted by BMP2. Initially, we used concentrations of each growth factor at or just below saturation for their respective differentiation effects on NCSCs (5, 6). Strikingly, in the presence of BMP2 plus GGF2, glial differentiation was suppressed, and the majority (65%) of colonies were neuronal (Fig. 1, BMP2+GGF2, and Fig. 2A, compare GGF2 vs. BMP2+GGF2, hatched and black bars). A similar result was obtained even if the concentration of GGF2 was increased 5-fold (to 5 nM), a concentration well above saturation for both glial differentiation and Schwann cell mitogenesis (5) (Fig. 2A, compare 5xGGF2 vs. BMP2+5xGGF2, black and hatched bars). We noted a slight increase in the percentage of SM colonies in BMP2+GGF2 compared with BMP2 alone (Fig. 2A, open bars). This may reflect an effect of GGF2 to weakly inhibit the neurogenic activity of BMP2 (see below); however, the trend did not continue with increasing concentrations of GGF2 (Fig. 2A, BMP2+5XGGF2, open bars). Nevertheless, this result does not alter the basic conclusion that gliogenesis but not neurogenesis is blocked in the presence of saturating doses of both GGF2 and BMP2.
Figure 1.
BMP2 is dominant over GGF2 at saturating concentrations of both factors. (A) NCSCs were grown for 15 days at clonal density under the indicated conditions and analyzed by immunocytochemistry for expression of glial fibrillary acidic protein (GFAP; red/phycoerythrin) and peripherin (green/fluorescein isothiocyanate). Due to the large size of the colonies, only parts of colonies are depicted. Both neurons and glia have differentiated in control (no addition) cultures (NA). In GGF2 (1 nM)-treated cultures, only GFAP+ glia are seen, but in either BMP2 (2 nM) or BMP2&GGF2, peripherin+ neurons, but not glia, are observed.
Figure 2.
Dosage-sensitive interactions between GGF2 or TGF-β1 and BMP2. (A) Quantitative analysis of NCSC differentiation in saturating concentrations of GGF2 (1 nM) and BMP2 (2 nM). Colonies were analyzed after 5 days, by which time BMP2 has induced overt neuronal differentiation (6), by triple labeling with antibodies to p75LNTR, peripherin, and α-SM actin. LNTR+colonies (hatched bars) contained no neurons. Peripherin+ colonies (solid bars) contained neurons and in some cases SM cells, but no LNTR+ nonneuronal cells; SM colonies (open bars) contained only SM cells. Note that no LNTR+ colonies developed in BMP2 plus any concentration of GGF2 tested. 5xGGF2, 5 nM GGF2; 5xBMP2, 10 nM BMP2. The results represent the mean ± SEM of two experiments; ≈20 colonies per condition per experiment were analyzed. (B) Quantitative analysis of NCSC differentiation in saturating concentrations of BMP2 (2 nM) and TGF-β1 (20 pM). Cultures were assayed as described for A.
A very different result was obtained when NCSCs were cultured in BMP2 plus TGF-β1. In this case, a response intermediate to that obtained with either factor alone was observed: roughly half the colonies were neuronal, and the remainder contained SM (Fig. 2B, BMP2+TGF-β1, compare solid vs. open bars). By contrast, in BMP2 alone, 75–80% of colonies contained neurons and the remainder were SM (Fig. 2B, BMP2, solid bars), whereas in TGF-β1 alone >90% of colonies were SM (Fig. 2B, TGF-β1, open bars). Undifferentiated NCSCs, identified by expression of p75LNTR, were not observed in any of these conditions (Fig. 2B, hatched bars), although the majority of control colonies consisted of such cells (data not shown). By this criterion, therefore, most or all NCSCs have exited the stem cell state in the presence of TGF-β1, BMP2, or both factors. Increasing the concentration of TGF-β1 by 5-fold (100 pM) led to an increase in the percentage of SM colonies and a corresponding drop in the proportion of neuronal colonies (Fig. 2B, compare BMP2+TGF-β1 vs. BMP2+5xTGF-β1, open vs. solid bars). Increasing the concentration of BMP2 5-fold (to 10 nM) only slightly increased the proportion of neuronal colonies (Fig. 2B, compare BMP2+TGF-β1 vs. 5xBMP2+TGF-β1). These data suggest that when NCSCs are grown in BMP2+TGF-β1, the influence of the two factors is codominant and reflects, at least to some extent, their relative concentrations.
The foregoing observations suggested that NCSCs integrate the combined influences of GGF2 and BMP2 vs. TGF-β1 and BMP2 very differently. To determine whether the apparent dominance of BMP2 over GGF2 was absolute or was dependent on the concentration of BMP2, we asked whether GGF2 (at a saturating dose of 1 nM) could inhibit neurogenesis promoted by subsaturating doses of BMP2. At 50-fold lower concentrations of BMP2 (40 pM), GGF2 was indeed able to reduce the percentage of neuronal colonies, from 77 ± 4 to 39 ± 5% (Table 1). However, even at 20 pM BMP2, GGF2 did not completely suppress neurogenesis (Table 1).
NCSCs Lose Neurogenic Capacity with Different Kinetics in GGF2 vs. TGF-β1.
The preceding results raised the question of why GGF2 is relatively weaker than TGF-β1 in its ability to interfere with neurogenesis promoted by BMP2. On the one hand, this could reflect differences in the relative sensitivity of the cells to these growth factors. On the other hand, it could reflect differences in the temporal windows in which NCSCs are competent to respond to the different factors. For example, although NCSCs are able to respond to BMP2 within 6–12 hr, as assayed by induction of the autonomic lineage-specific (8) transcription factor MASH1 (6), NCSCs or their progeny may only acquire GGF2 responsiveness after several days in vitro. If, in subsaturating doses of BMP2, commitment to neurogenesis occurs stochastically and asynchronously (9), some NCSCs would escape the influence of BMP2 during the first few days of culture. These uncommitted cells then could acquire responsiveness to GGF2. In contrast, in saturating concentrations of BMP2, all cells would commit to a neuronal fate before they had a chance to become GGF2-responsive, so that no effect of the latter factor would be observed.
To distinguish these possibilities, we carried out experiments in which NCSCs were exposed sequentially to GGF2 and BMP2 or to TGF-β1 and BMP2. We measured the kinetics with which the cells lost neurogenic capacity by assaying their ability to express MASH1 protein [which is essential for autonomic neurogenesis (10)] in response to a subsequent addition of BMP2. Because neither Schwann cells nor SM cells express MASH1 when exposed to BMP2 (N.M.S., unpublished results), the loss of MASH1 inducibility provides an indirect index of the kinetics of commitment of NCSCs to these nonneuronal lineages.
When BMP2 and TGF-β1 were added simultaneously, the proportion of colonies expressing MASH1 after 24 hr was already reduced, from about 85% to 45% (Fig. 3A, 0 hr, hatched bar), consistent with the fact that TGF-β1 reduced the extent of overt neuronal differentiation measured after 5 days of growth in the presence of both factors (Fig. 2B, BMP2+TGF-β1). If BMP2 was added 24 hr after TGF-β1, moreover, only ≈15% of colonies expressed MASH1 (Fig. 3A, 24 hr, hatched bar). If BMP2 was added 48 hr after TGF-β1, virtually none of the colonies expressed MASH1 (Fig. 3A, 48 hr, hatched bar). By contrast, in control cultures grown without TGF-β1, delayed addition of BMP2 at any of these times induced MASH1 in most colonies (Fig. 3A, solid bars). Thus, NCSCs rapidly lose responsiveness to BMP2 if preincubated in TGF-β1. The kinetics of this change, and an estimated cell cycle time of 12–18 hr for NCSCs (2), further implies that NCSCs or their immediate progeny are responsive to TGF-β1 in the same time window as they are responsive to BMP2 (6). Consistent with this idea, if NCSCs were, conversely, first cultured in BMP2 for 24 hr and then exposed to TGF-β1, the extent of SM differentiation promoted by TGF-β1 was greatly attenuated (data not shown).
Figure 3.
NCSCs lose BMP2 responsiveness in TGF-β1 more rapidly than they do in GGF2. (A) Hatched bars: BMP2 (2 nM) was added simultaneously with (0 hr) or 24 or 48 hr after addition of TGF-β1 (20 pM). The proportion of colonies containing any MASH1+ cells was determined 24 hr later by staining with anti-MASH1 antibody. Solid bars: BMP2 was added at the indicated times to control cultures grown without TGF-β1. No MASH1 expression was detected in cultures grown in TGF-β1 alone (data not shown). (B) Analogous experiment in which BMP2 was added simultaneously with or at the indicated times after addition of GGF2 (1 nM). No MASH1 expression was detected in cultures grown in GGF2 alone (data not shown). Note that almost 50% of the colonies contain some MASH1+ cells even if BMP2 is added 48 hr after GGF2. If BMP2 was added after 96 hr in GGF2, ≈45% of colonies still contained some MASH1+ cells (hatched bars). (However, only ≈5% of cells within these colonies were MASH1+ vs. 50% MASH1+ cells per colony in cultures grown without GGF2 for 96 hr.) Thus most cells within colonies lose the ability to express MASH1 in response to BMP2 after 48–96 hr in GGF2. Data represent the mean ± SEM of two experiments; ≈20 colonies were analyzed per experiment for each time and condition shown.
A very different kinetic profile was observed if NCSCs first were grown in GGF2 alone and subsequently assayed for expression of MASH1 after a 24-hr exposure to BMP2. In this case, preincubation in GGF2 for 24 hr produced no apparent diminution in the extent of MASH1 induction elicited by subsequent exposure to BMP2 (Fig. 3B, 24 hr, solid vs. hatched bars). Furthermore, even after 48 and 96 hr of exposure to GGF2, a significant fraction of colonies expressed MASH1 in response to BMP2 (Fig. 3B, 48 and 96 hr, hatched bars; although by 96 hr, the proportion of MASH1+ cells within these colonies was reduced to ≈5%). Importantly, in control conditions, the ability to respond to delayed addition of BMP2 by induction of MASH1 was retained for at least 96 hr (Fig. 3B, solid bars). Therefore, the loss of MASH1 inducibility in the presence of GGF2 was indeed caused by the growth factor and was not simply a spontaneous gradual loss of BMP2 responsiveness by NCSCs. However, this loss of neurogenic capacity in GGF2 required 48–96 hr of exposure to the growth factor, whereas it occurred in less than 24 hr in TGF-β1.
We addressed the possibility that selective cell death during the preincubation in GGF2 could account for the loss of BMP2 responsiveness. However, after 72 hr in GGF2, only 30 ± 10% of colonies contained any dead cells (identified by 4,6-diamidino-2-phenylindole staining for fragmented apoptotic nuclei), and within these colonies only 3.5% of cells were dead. By contrast during this period, 55–60% of colonies have lost the ability to respond to BMP2 by expression of MASH1 (Fig. 3B), and within individual colonies, 85–90% of cells fail to express MASH1 in response to BMP2. These data argue that a selective death of BMP2-responsive cells is unlikely to explain the loss of MASH1 inducibility by NCSCs cultured in GGF2. Rather, the loss of BMP2 responsiveness likely reflects the gradual commitment of the cells to a nonneurogenic (e.g., glial) fate.
Freshly Isolated NCSCs Exhibit a Rapid Direct Response to GGF2.
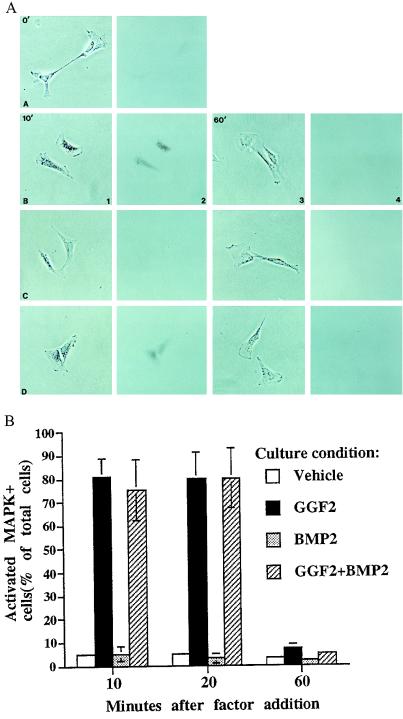
One simple explanation for the delayed loss of neurogenic capacity in GGF2 would be that NCSCs are not competent to respond to the growth factor during their first 48 hr in vitro. To address this question, we asked whether GGF2 can cause phosphorylation of MAPK in freshly isolated NCSCs, by using an antibody that recognizes only the doubly phosphorylated active form of MAPK (MAPK/PO4) (11). Within 10 min of exposure to GGF2 (the earliest time tested), MAPK/PO4 was detected in ≈80% of the cells (Fig. 4 A, sections B1 and B2, and B). In many cells, MAPK/PO4 appeared concentrated in the nucleus (Fig. 4A, section B2). Expression of MAPK/PO4 was sustained for 20 min after GGF2 addition (Fig. 4B), but by 60 min was no longer detectable (Fig. 4 A, sections B3 and B4, and B). Induction of MAPK phosphorylation by GGF2 was not blocked by simultaneous addition of BMP2 (Fig. 4 A, sections D1 and D2 and B), indicating that the dominant influence of BMP2 over GGF2 (Figs. 1 and 2A) is not due to a complete suppression of early GGF2-mediated signal transduction events. These data reveal that freshly isolated NCSCs respond directly to GGF2. Therefore, the delayed loss of neurogenic capacity in GGF2 (Fig. 3B) cannot be explained by a delayed acquisition of functional GGF2 receptors.
Figure 4.
GGF2 induces rapid phosphorylation of MAPK in freshly isolated NCSCs. Columns 2 and 4 represent bright-field views of the phase-contrast views in columns 1 and 3, respectively. Cells in row A were fixed before any factor was added; activated MAPK is not expressed in cells at this time. Cells in row B received GGF2 (1 nM), cells in row C received BMP2 (2 nM), and cells in row D were treated with GGF2+BMP2. Cells in columns 1 and 2 were treated with growth factor(s) for 10 min, and cells in columns 3 and 4 were treated with growth factor(s) for 60 min. (B) Quantification of induction of phosphorylated MAPK by GGF2 in NCSCs. The results represent the mean ± SEM of two experiments.
GGF2 Inhibits Induction of MASH1 by Subsaturating Doses of BMP2.
The ability of GGF2 to induce rapid MAPK phosphorylation in NCSCs left open the question of whether the growth factor influences the differentiative behavior of the cells during the first 24–48 hr in vitro. For example, the cells might lack other components necessary for glial lineage commitment that only become expressed after 48 hr of culture in GGF2. However, early molecular markers of committed glial cells are currently unavailable. We therefore asked whether GGF2 could interfere with early events in neuronal differentiation, by using induction of MASH1 expression after 24 hr as a short-term assay for the neurogenic influence of BMP2.
As expected, GGF2 (1 nM) had no effect on MASH1 induction after 24 hr in a saturating concentration of BMP2 (Table 1), consistent with its inability to block overt neuronal differentiation after 5 days in a similar dose of BMP2 (Fig. 2A). However, GGF2 did significantly attenuate induction of MASH1 by subsaturating doses of BMP2. For example, in 20 pM BMP2, 76 ± 4% of colonies contained some MASH1+ cells, but this was reduced to 30 ± 2% in the presence of 1 nM GGF2 (Table 1). These data indicate that GGF2 is able to exert a negative influence on neurogenesis by NCSCs within the first 24 hr in culture. Interestingly, however, the attenuation of MASH1 induction by GGF2 was not as great as the attenuation of neuronal differentiation: for example, at 40 pM BMP2, GGF2 reduced the proportion of neuronal colonies from 77 ± 4 to 39 ± 5%, whereas it produced a statistically insignificant reduction in the proportion of MASH1+ colonies at this BMP2 concentration (77 ± 4 vs. 69 ± 9%; Table 1). This suggests that the inhibitory effect of GGF2 on BMP2-induced neurogenesis cannot be explained solely by its ability to inhibit induction of MASH1 expression; perhaps, for example, GGF2 also inhibits the function of MASH1, or the expression of other factors necessary for neuronal differentiation.
Taken together, the results in Table 1 and Fig. 3B imply that the short-term inhibition by GGF2 of MASH1 induction at low doses of BMP2 is insufficient to commit NCSCs to a glial fate. To demonstrate this, cells first were grown for 24 hr in 1 nM GGF2 plus a low dose of BMP2, then challenged with a second addition of BMP2 at 2 nM, and assayed 24 hr later for induction of MASH1. No significant diminution in the extent of MASH1 induction by this second addition of saturating BMP2 (data not shown) resulted, confirming that the cells had not committed to a glial fate during the preincubation period despite the initial inhibition of MASH1 induction. This result was identical to that obtained when NCSCs were cultured in GGF2 alone for 24 hr and then challenged with 2 nM BMP2 (Fig. 3B), indicating that the presence of a low dose of BMP2 in the mixed factor preincubation did not interfere with the effect of GGF2. These data imply that although GGF2 is able to exert an inhibitory effect on the neurogenic influence of BMP2 within the first 24 hr in vitro, the action revealed by this negative effect is not sufficient to commit the cells to a glial fate. Such an irreversible restriction appears to depend on longer-term consequences of GGF2 action, which require 48–96 hr of continuous exposure to the growth factor.
DISCUSSION
In this study, we have used neural crest cells as a model system to understand how multipotent stem cells integrate the influences of competing instructive signals for lineage determination. Our results indicate that two determinants of nonneuronal fates, TGF-β1 and GGF2, differ markedly in their ability to override the neurogenic influence of BMP2: at saturating concentrations of all ligands, the influence of BMP2 is dominant over that of GGF2, whereas it appears codominant with that of TGF-β1. Moreover, NCSCs commit to SM or neuronal fates in TGF-β1 and BMP2, respectively, much more rapidly than they do to a glial fate in GGF2. These results indicate that intrinsic biases in NCSCs can affect the outcome of lineage decisions made in the presence of multiple competing instructive signals.
TGF-β1, but not GGF2, is able to inhibit induction of MASH1 by saturating concentrations of BMP2 (Fig. 3, 0 hr). However, at a 100-fold lower concentration of BMP2, GGF2 is able to partially inhibit induction of MASH1 (Table 1). These data suggest that NCSCs differ in their intrinsic relative sensitivities to different instructive growth factors. They also suggest that the signal transduction pathways for these factors may be linked by reciprocal inhibitory interactions that differ in their relative strength. The molecular basis of these differences is unknown. It could reflect the relative number of receptors per cell for each growth factor, the threshhold levels of signaling necessary to elicit their respective biological responses, or other regulatory mechanisms.
The difference in the ability of TGF-β1 and GGF2 to compete with BMP2 is not simply a function of relative dosage sensitivities, however, but also is influenced by differences in the relative kinetics of commitment to the SM and glial fates. NCSCs undergo a restriction in their neurogenic capacity within 24 hr in TGF-β1, whereas this restriction is not apparent until after >48 hr in GGF2. Our results set some constraints on the mechanisms underlying this apparent delay in glial lineage commitment. Specifically, it cannot be due simply to a delayed expression of functional receptors for GGF2, because the growth factor is able to both activate phosphorylation of MAPK and also to inhibit induction of MASH1 by low doses of BMP2; both effects are detected in the first 24 hr in vitro.
Other explanations therefore are necessary to account for the delayed loss of neurogenic capacity in GGF2. One parsimonious model is that it involves loss of a critical signaling component for BMP2, such as the receptors. If GGF2 caused a rapid inhibition of BMP2 receptor expression, for example, then the time required for the cells to lose BMP2 responsiveness would be determined by the initial number of receptors on the cells and the kinetics of degradation of these receptors. A testable prediction of this model is that constitutive expression of BMP receptors in NCSCs should prevent loss of neurogenic capacity in GGF2. Another possibility is that NCSCs have to undergo a certain number of cell divisions in GGF2 to lose BMP2 responsiveness. Testing such a model is less straightforward, however, because mitotic inhibitors appear toxic to NCSCs (N.M.S., unpublished results).
Why do NCSCs lose neurogenic capacity more rapidly in TGF-β1 than in GGF2? As TGF-β1 and BMP2 use related signal transduction pathways (12), these pathways may operate with similar kinetics and compete for shared components. For example, a family of proteins called Smads has recently been identified as mediating signal transductions events specific to TGF-β and BMP2/4 (13). One of these proteins, Smad4/DPC4, is thought to serve as a common heterodimeric partner for other Smads specific to TGF-β or BMP2 (14, 15). Competition for limiting amounts of this common partner could thus explain the ability of TGF-β to inhibit BMP2-specific signaling, an hypothesis testable by overexpression of Smad4/DPC4.
The data presented herein shed light on the mechanism of GGF2 action on NCSCs. Previously, the effects of GGF2 were assayed after 6–15 days of culture, by examining the expression of phenotypic markers specific for neurons (or their precursors) and glia (5). From such data it was not possible to determine when GGF2 first influences NCSCs and whether this influence is primarily positive (to promote gliogenesis), negative (to inhibit neurogenesis), or both. The ability of GGF2 to attenuate MASH1 induction by low doses of BMP2 provides evidence that GGF2 can exert an inhibitory influence on neurogenesis by NCSCs in the first 24 hr of culture. This does not exclude the possibility that GGF2 also exerts early positive effects on gliogenesis, although molecular markers of such effects have yet to be identified. Moreover, our results indicate that these short-term influences of GGF2 are insufficient to commit NCSCs to a glial fate; therefore, other unknown events must occur upon further exposure to GGF2 that cause an irreversible loss of neurogenic capacity. It will be interesting to determine whether factors that promote gliogenesis by central nervous system stem cells (16–18) also exert such sequential effects.
Finally, our results may help to explain why, in vivo, neurogenesis invariably precedes gliogenesis (19). NCSCs commit to a neuronal fate in response to BMP2 far more rapidly than they commit to a glial fate in GGF2. Furthermore, the influence of BMP2 is dominant over that of GGF2 when both factors are present at saturating amounts. Thus if migrating neural crest cells encountered an environment containing both BMP2 and GGF2, they likely would differentiate into neurons first. This immediately raises the problem of what mechanism would prevent the entire pool of crest cells from differentiating into neurons. We have suggested (5) that a negative feedback signal derived from neurons, possibly mediated by GGF, could inhibit neurogenesis in neighboring uncommitted cells. If the local concentration of BMP2 declined with time to subsaturating levels, our results suggest that GGF2 would be able to inhibit the influence of this neurogenic signal to promote gliogenesis in the remaining stem cell pool. Alternatively, multiple negative feedback signals may act in concert to override neuronal inducing signals in vivo. Expression of activated forms of NOTCH inhibits neurogenesis, but not gliogenesis, by some multipotent progenitor cells in vitro (20). It will be interesting to determine whether ligands for NOTCH proteins, such as Delta-1 (21), function cooperatively with factors such as GGF2 to suppress neurogenesis in NCSCs.
Table 1.
Suppression of neuronal differentiation and induction of MASH1 by GGF2 at subsaturating doses of BMP2
| Culture condition | % neuronal colonies | % MASH1+ colonies |
|---|---|---|
| BMP2 (2 nM) | 81 ± 2 | 95 ± 5 |
| BMP2 (2 nm) + GGF2 (1 nM) | 86 ± 2 | 94 ± 6 |
| BMP2 (40 pM) | 77 ± 4 | 77 ± 4 |
| BMP2 (40 pM) + GGF2 (1 nM) | 39 ± 5 | 69 ± 9 |
| BMP2 (20 pM) | 62 ± 7 | 76 ± 4 |
| BMP2 (20 pM) + GGF2 (1 nM) | 17 ± 3 | 30 ± 2 |
| BMP2 (4 pM) | 6 ± 3 | 39 ± 6 |
| BMP2 (4 pM) + GGF2 (1 nM) | 0 | 7 ± 3 |
| Control | 0 | 5 ± 1 |
| GGF2 (1 nM) | 0 | 0 |
Neural crest cells were grown at clonal density in the presence of BMP2 at the indicated concentrations, either alone or in the presence of 1 nM GGF2. The cultures for determining neuronal colonies were fixed after 5 days and the percentage of neuronal (i.e., neuron containing) colonies determined after staining for peripherin. The cultures for MASH1+ colonies were fixed after 24 hr and immunostained for MASH1; the percentage of total colonies containing ≥1 MASH1+ cell was scored. The results represent the mean ± SEM of two experiments, in which 25–30 colonies per experiment were scored.
Acknowledgments
We thank Scott Fraser, Paul Sternberg, Kai Zinn, Paul Patterson, Sean Morrison, and Ron McKay for helpful suggestions and comments on an early version of the manuscript. This work was supported by the National Institutes of Health and a grant from the Muscular Dystrophy Association. D.J.A. is an Investigator of the Howard Hughes Medical Institute.
ABBREVIATIONS
- NCSC
neural crest stem cell
- BMP2
bone morphogenetic protein 2
- TGF-β1
transforming growth factor β1
- GGF2
glial growth factor 2
- MASH1
mammalian achaete-scute homolog 1
- MAPK
mitogen-activated protein kinase
- SM
smooth muscle
- LNTR
low-affinity neurotrophin receptor
References
- 1.Morrison S J, Shah N M, Anderson D J. Cell. 1997;88:287–298. doi: 10.1016/s0092-8674(00)81867-x. [DOI] [PubMed] [Google Scholar]
- 2.Stemple D L, Anderson D J. Cell. 1992;71:973–985. doi: 10.1016/0092-8674(92)90393-q. [DOI] [PubMed] [Google Scholar]
- 3.Le Douarin N M. The Neural Crest. Cambridge, U.K.: Cambridge Univ. Press; 1982. [Google Scholar]
- 4.Marchionni M A, Goodearl A D J, Chen M S, Bermingham-McDonogh O, Kirk C, et al. Nature (London) 1993;362:312–318. doi: 10.1038/362312a0. [DOI] [PubMed] [Google Scholar]
- 5.Shah N M, Marchionni M A, Isaacs I, Stroobant P W, Anderson D J. Cell. 1994;77:349–360. doi: 10.1016/0092-8674(94)90150-3. [DOI] [PubMed] [Google Scholar]
- 6.Shah N M, Groves A, Anderson D J. Cell. 1996;85:331–343. doi: 10.1016/s0092-8674(00)81112-5. [DOI] [PubMed] [Google Scholar]
- 7.Dong Z, Brennan A, Liu N, Yarden Y, Lefkowitz G, Mirsky R, Jessen K R. Neuron. 1995;15:585–596. doi: 10.1016/0896-6273(95)90147-7. [DOI] [PubMed] [Google Scholar]
- 8.Lo L, Guillemot F, Joyner A L, Anderson D J. Perspect Dev Neurol. 1994;2:191–201. [PubMed] [Google Scholar]
- 9.Gusella J, Geller R, Clarke B, Weeks V, Housman D. Cell. 1976;9:221–229. doi: 10.1016/0092-8674(76)90113-6. [DOI] [PubMed] [Google Scholar]
- 10.Guillemot F, Lo L-C, Johnson J E, Auerbach A, Anderson D J, Joyner A L. Cell. 1993;75:463–476. doi: 10.1016/0092-8674(93)90381-y. [DOI] [PubMed] [Google Scholar]
- 11.Marshall C J. Curr Opin Genet Dev. 1994;4:82–89. doi: 10.1016/0959-437x(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 12.Massagué J. Cell. 1996;85:947–950. doi: 10.1016/s0092-8674(00)81296-9. [DOI] [PubMed] [Google Scholar]
- 13.Graff J M, Bansal A, Melton D A. Cell. 1996;85:479–487. doi: 10.1016/s0092-8674(00)81249-0. [DOI] [PubMed] [Google Scholar]
- 14.Lagna G, Hata A, Hemmati-Brivanlou A, Massagué J. Nature (London) 1996;383:832–836. doi: 10.1038/383832a0. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Musci T, Derynck R. Curr Biol. 1997;7:270–276. doi: 10.1016/s0960-9822(06)00123-0. [DOI] [PubMed] [Google Scholar]
- 16.Gross R E, Mehler M F, Mabie P C, Zang Z, Santschi L, Kessler J A. Neuron. 1996;17:595–606. doi: 10.1016/s0896-6273(00)80193-2. [DOI] [PubMed] [Google Scholar]
- 17.Johe K K, Hazel T G, Muller T, Dugich-Djordjevic M M, McKay R D G. Genes Dev. 1996;10:3129–3140. doi: 10.1101/gad.10.24.3129. [DOI] [PubMed] [Google Scholar]
- 18.Qian X, Davis A A, Goderie S K, Temple S. Neuron. 1997;18:81–93. doi: 10.1016/s0896-6273(01)80048-9. [DOI] [PubMed] [Google Scholar]
- 19.Carr V M, Simpson S B. J Comp Neurol. 1978;182:727–740. doi: 10.1002/cne.901820410. [DOI] [PubMed] [Google Scholar]
- 20.Nye J S, Kopan R, Axel R. Development (Cambridge, UK) 1994;120:2421–2430. doi: 10.1242/dev.120.9.2421. [DOI] [PubMed] [Google Scholar]
- 21.Henrique D, Adam J, Myat A, Chitnis A, Lewis J, Ish-Horowicz D. Nature (London) 1995;375:787–790. doi: 10.1038/375787a0. [DOI] [PubMed] [Google Scholar]