Abstract
We previously have demonstrated that insulin and insulin-like growth factor-I (IGF-I) down-regulate growth hormone (GH) binding in osteoblasts by reducing the number of surface GH receptors (GHRs). The present study was undertaken to investigate the mechanism of GHR down-regulation. Treatment with 5 nM insulin or IGF-I for 18 hr significantly decreased surface GH binding to 26.4 ± 2.9% and 23.0 ± 2.7% of control (mean ± SE; P < 0.05), respectively. No corresponding reductions in the mRNA level and total cellular content of GHR were found, nor was the rate of receptor internalization affected. The effects on GHR translocation were assessed by measuring the reappearance of GH binding of whole cells after trypsinization to remove the surface receptors. GH binding of control cultures significantly increased (P < 0.05) over 2 hr after trypsinization, whereas no recovery of binding activity was detected in insulin and IGF-I-treated cultures, indicating that GHR translocation was impaired. Studies on the time course of GHR down-regulation revealed that surface GH binding was reduced significantly by 3-hr treatment (P ≤ 0.0005), whereas GHR translocation was completely abolished by 75–90 min with insulin and IGF-I. The inhibition of receptor translocation by insulin, but not IGF-I, was attenuated by wortmannin. In conclusion, insulin and IGF-I down-regulated GH binding in osteoblasts by acutely impairing GHR translocation, with their effects exerted through distinct postreceptor signaling pathways.
Growth hormone (GH) stimulates peripheral tissue growth through a dual effector mechanism involving the endocrine action of insulin-like growth factor-I (IGF-I) produced from the liver (1) and paracrine action of locally produced IGF-I (2). GH exerts its actions through binding to specific high affinity receptors (GHRs) in the liver and in extrahepatic tissues (3). The factors and mechanisms regulating GHRs in the liver have been the subject of intensive investigation and, in particular, in relation to the effects of nutrition (3, 4). These studies show that hepatic GHRs are strongly and positively regulated by nutrition (5) and insulin (6), with evidence of regulation at the transcriptional level (7).
Very little is known about the factors and mechanisms involved in the regulation of GHRs in extrahepatic tissues. Using osteoblasts as a model of extrahepatic tissues, we have recently reported that insulin and IGF-I act through their respective receptors to down-regulate GHRs (8). The effect of insulin is diametrically opposite to its effect on GHRs in the liver, suggesting that GHRs in hepatic and extrahepatic tissues may be regulated differently. Moreover, the negative effect of IGF-I on GHRs indicates the existence of a peripheral feedback loop limiting the action of GH in peripheral tissues. The present study was undertaken to investigate the mechanisms by which insulin and IGF-I down-regulate GHRs.
MATERIALS AND METHODS
Cell Culture.
The osteoblast-like cell line, UMR106.06, was a generous gift from T. J. Martin (St. Vincent’s Hospital, Melbourne, Australia). The cells were routinely grown in monolayer culture at 37°C in 5% CO2/95% air in Eagle’s minimum essential medium (EMEM) supplemented with 10% fetal bovine serum, 25 mM Hepes, l-glutamine, and penicillin/streptomycin. All reagents for cell culture were purchased from Cytosystems (Sydney, Australia).
In all subsequent studies, monolayer cultures were treated in triplicate with 5 nM human insulin (Wellcome) or human IGF-I (GroPep, Adelaide, Australia) in EMEM with 0.2% BSA (EMEM/0.2% BSA) at 37°C in 5% CO2/95% air for 18 hr, unless otherwise stated. These concentrations of insulin and IGF-I previously were shown to cause maximal inhibition of GH binding to the cells (8).
GH Binding to Cell Monolayers.
The GH binding assay with cell monolayers has been described previously (8). Briefly, recombinant human GH (9) was radiolabeled with Na125I (ARI, Sydney, Australia) by the Iodogen (Pierce) method to a specific activity of 20–30 μCi/μg (10). Monolayer cultures at a density of 4 × 104 cells/cm2 were set up in 6-well plates (Flow Laboratories) and treated with insulin or IGF-I. After two washes with PBS, 1 ml of EMEM/0.2% BSA containing 125I-labeled GH (2 × 105 cpm) was added, and the binding assay was allowed to proceed at 22°C for 2 hr. Nonspecific binding of the radioligand was estimated by adding 10 μg/ml of unlabeled GH. The assay was terminated by washing the cultures five times with ice-cold PBS containing 0.2% BSA (PBS/0.2% BSA). Cells were detached with trypsin/EDTA and counted with a hemocytometer. Cell lysates were prepared by solubilizing in 0.5 M NaOH and 0.1% Triton X-100, and radioactivity in the lysates was counted on a γ-counter (Packard). Specific binding of the radioligand was calculated as the difference of total and nonspecific binding and corrected for cell number in the wells.
Northern Analysis.
The effects of insulin and IGF-I on the abundance of GHR mRNA were quantified by Northern analysis using a cDNA probe (pRat1–20) encompassing the region from position −7 to 794 of the rat GHR mRNA (11). Monolayer cultures at a density of 1.3 × 104 cells/cm2 were set up in 10-cm dishes (Flow Laboratories), followed by treatment with 5 nM insulin or IGF-I for 18 hr. Total RNA was isolated using 4 M guanidinium isothiocyanate followed by cesium chloride gradient centrifugation (12) and was quantified by absorbance at 260 nm. Integrity of the RNA was assessed by visual inspection of the 18S and 28S rRNA bands stained with ethidium bromide after agarose-formaldehyde gel electrophoresis.
Thirty micrograms of total RNA was separated by electrophoresis in 1% agarose gel with 6% formaldehyde and transferred to Hybond-N membrane (Amersham) by capillary immobilization (12). The membrane was prehybridized and hybridized in Church buffer (0.5 M Na2HPO4, pH 7.2/7% SDS/0.5% BSA/1 mM EDTA) (13) as described previously (11). The GHR probe was radiolabeled with [α-32P]dCTP by random priming. Hybridization was performed at 60°C for 18 hr, followed by washing with 2× standard saline citrate (SSC)/0.1% SDS at 65°C. Bands were visualized by autoradiography. RNA load was corrected for the levels of β-actin mRNA and 18S rRNA by reprobing the membrane with a [α-32P]UTP-labeled riboprobe encompassing a 358-nt fragment of the rat β-actin gene or with a [γ-32P]ATP-labeled 30-bp oligoprobe of 18S rRNA. All autoradiographs were quantified with a densitometer (Molecular Dynamics).
Western Analysis.
The content of immunoreactive GHR protein was assessed by Western analysis using an anti-rabbit GHR antiserum (14). Briefly, cell monolayers were set up and treated with insulin or IGF-I in the same way as in the Northern studies described above. The treated cultures were solubilized in SDS-containing buffer. Protein content of the lysates was measured by the Bradford method and normalized for gel loading. The samples were separated by SDS/PAGE (15) and blotted to a nitrocellulose membrane (Schleicher & Schuell). The membrane was treated sequentially with blocking buffer (20 mM Tris⋅HCl, pH 7.4/150 mM NaCl/5% skim milk powder/0.1% Tween 20), the anti-GHR antiserum (1:300 in blocking buffer) or preimmunized rabbit serum, and donkey anti-rabbit Ig-horseradish peroxidase. The bands were visualized using the enhanced chemiluminescence method (Amersham) and quantified by densitometry.
Total GHR.
To estimate the total cellular content of functional GHRs, GH binding activity of surface and intracellular membranes derived from cell sonicates was measured as follows. Cell monolayers were set up in 10-cm dishes and treated with insulin or IGF-I for 18 hr. The cells were detached by scraping in 1 ml PBS containing a mixture of protease inhibitors (1 trypsin inhibitor unit/ml of aprotinin/10 mM benzamidine/0.2 mM phenylmethylsulfonyl fluoride) obtained from Sigma. After centrifugation, the packed cells were resuspended in 2 ml of ice-cold inhibitor mixture and disrupted by sonication. The cell sonicates were centrifuged at 150,000 × g for 15 min at 4°C to yield pellets of total cellular membranes. Protein content of the membrane fractions was determined by the bicinchoninic acid assay (Pierce).
To measure GH binding, the membrane pellets at a protein concentration of 0.5–1.0 mg/ml were resuspended in EMEM/0.2% BSA. The membrane suspensions were incubated in triplicate with 125I-labeled GH (2 × 105 cpm/ml) at 22°C for 2 hr. Nonspecific binding was defined as the binding in the presence of 10 μg/ml unlabeled GH. At the end of the assay, the membrane suspensions were centrifuged, washed twice with ice-cold PBS/0.2% BSA, and counted on a γ-counter.
Surface GHR.
As the GH binding assay with cell monolayers measures the sum of surface-bound and internalized radioligand, the effects of insulin and IGF-I on the component of cell surface binding was measured by undertaking the binding assay at low temperature to prevent GHR internalization (16, 17). Monolayer cultures were set up in 6-well plates, treated with insulin or IGF-I, and assayed for GH binding as described above, except that the binding assay was carried out at 4°C for 2 hr.
Intracellular GHR.
To measure the content of intracellular GHRs, monolayer cultures were treated with insulin or IGF-I, and then incubated with 0.1 g/liter trypsin at 22°C for 15 min to degrade GHRs on the cell surface. The trypsinization was terminated by adding an equal volume of 2 trypsin inhibitor units/ml of aprotinin in EMEM/0.2% BSA. Cells were centrifuged and resuspended in 2 ml of ice-cold inhibitor mixture, and sonicated and centrifuged at 150,000 × g to yield the membrane fractions. GH binding to the membrane fractions was assayed as described above for total GHR.
Internalization of GHR.
Internalization of GHR was assessed by the method of Roupas and Herington (17). After treatment with insulin or IGF-I, GH binding assay with monolayer cultures was carried out at 22°C for 2 hr as described above. At the end of the binding assay, the cultures were washed with ice-cold PBS/0.2% BSA, followed by treatment with 50 mM HCl in the same buffer at 4°C for 1 min. The acid treatment has been shown to remove more than 90% of the surface-bound radioligand (17), which was confirmed in our laboratory. After three washes with PBS/0.2% BSA, the cells were lysed with 0.5 M NaOH and 0.1% Triton X-100. Radioactivity in the lysates was measured on a γ-counter. The cell-associated radioactivity of the acid-treated cultures reflects the level of radioligand internalized with GHR. Parallel cultures were set up but not treated with acid to determine the levels of total incorporation of radioactivity.
Translocation of GHR.
The rate of GHR translocation from the intracellular pool to the cell surface was measured as the recovery of GH binding to whole cells after stripping of surface receptors by trypsinization (18). Monolayer cultures in 10-cm dishes were treated with insulin or IGF-I, followed by incubation with 0.1 g/liter trypsin for 15 min. After removal of trypsin, the cells were resuspended in EMEM/0.2% BSA, dispensed at a concentration of 6–8 × 105 cells/well to 6-well plates and allowed to recover at 22°C for 2 hr with continuous shaking. At times 0, 0.5 and 2 hr of the recovery period, 125I-labeled GH (2 × 105 cpm/ml) with and without unlabeled GH (10 μg/ml) was added in triplicate. The incubation was continued at 22°C for 30 min before radioactivity bound to the cells was measured.
Time Course of Inhibition.
To investigate the time course of inhibition of GH binding, cell monolayers in 6-well plates were incubated in serum-free conditions for 18 hr, and then treated with insulin or IGF-I for 1.5, 3, 6, and 24 hr before monolayer GH binding was assayed as described above. The time course of impairment of GHR translocation was studied in a similar manner, except that the effect on receptor translocation was measured after treatment with insulin or IGF-I for 30, 45, 60, 75, 90, and 180 min.
Effects of Wortmannin and PD098059.
Phosphatidylinositol 3-kinase (PI3K) (19–23) and mitogen-activated protein kinase kinase (24, 25) are known to mediate the biological actions of insulin and IGF-I. To investigate whether the inhibitory actions of the two growth factors are mediated by these kinases, wortmannin, a PI3K inhibitor (Sigma) and PD098059, a mitogen-activated protein kinase kinase inhibitor (Calbiochem–Novabiochem) were used to abolish the kinase activity. Briefly, monolayer cultures were incubated in serum-free conditions for 18 hr, and then treated at 37°C for 0.5 hr with 100 nM wortmannin, 10 μM PD098059, or the dimethyl sulfoxide vehicle at a final concentration of 0.01% before the addition of insulin or IGF-I. Treatment with the two growth factors was allowed to proceed at 37°C for 1.5 hr, followed by the GHR translocation assay.
Statistical Analyses.
All points in the experiments were measured in triplicate, or as indicated. All experiments were repeated at least three times unless otherwise stated, and mean ± SE of the results from the experiments are presented. The degree of significance of differences between groups is calculated using Student’s t test and ANOVA (StatView 4.02, Abacus Concepts, Berkeley, CA) where appropriate and set at P < 0.05.
RESULTS
We confirm our previous findings (8) that 18-hr treatment with insulin and IGF-I significantly reduced GH binding of cell monolayers to 26.4 ± 2.9% and 23.0 ± 2.7% of control, respectively (P < 0.05).
Biosynthesis of GHRs.
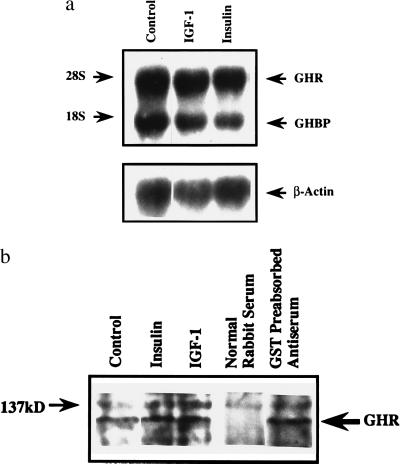
The effects of insulin and IGF-I on the abundance of GHR mRNA were assessed by Northern analysis. Two bands corresponding to the GHR and GH binding protein were detected using the pRat1–20 probe (Fig. 1a). When normalized to β-actin mRNA, the abundance of GHR mRNA for cultures treated with insulin (86.3 ± 11.0% of control; n = 3) and IGF-I (103.4 ± 23.0%) was not different from that of control. Similar results were obtained when the GHR mRNA content was normalized to 18S rRNA (data not shown).
Figure 1.
Northern and Western studies of GHR. (a) Northern analysis. Total RNA was isolated from the cultures after treatment with insulin or IGF-I, and abundance of mRNA for GHR, GH binding protein (GHBP), and β-actin was quantified by Northern analysis. Positions of the 18S and 28S rRNA were shown. (b) Western analysis. Total protein content was harvested from control and treated cultures and was studied using Western blotting technique with an anti-GHR antiserum. Position of the 137-kDa molecular mass marker was shown. Similar results were obtained in a repeated experiment.
The total cellular content of immunoreactive GHR protein was examined by Western analysis (14). A 120-kDa band was identified for the control and treated cultures (Fig. 1b). The intensity of this band was not affected by preabsorption of the anti-GHR antiserum with glutathione S-transferase fusion protein and was absent when preimmunized rabbit serum was used, thus confirming the specificity of the antiserum. The intensity of the 120-kDa band was not changed by treatment with insulin and IGF-I (108.3% and 96.9% of control, respectively; n = 2), suggesting that content of immunoreactive GHR protein was not affected by the treatment.
GHR Distribution.
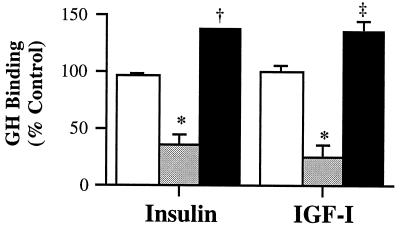
The effects of insulin and IGF-I on the total cellular content of functional GHRs were next assessed by measuring the specific binding of GH to surface and intracellular membranes. Specific binding of the membrane fractions was not affected by insulin and IGF-I, with the levels being 96.0 ± 2.2% and 99.6 ± 6.1% of control, respectively (Fig. 2). These findings confirm the results of Western analysis that down-regulation of GH binding by the two growth factors was not associated with a reduction in the total cellular content of GHRs.
Figure 2.
GHR distribution. After treatment with insulin or IGF-I, total (□), surface (░⃞), and intracellular GH binding (▪) were determined as described in Materials and Methods. The control values for total, surface, and intracellular GH binding were 2,459 ± 209 cpm/mg, 112 ± 20 cpm/106 cells, and 1,723 ± 103 cpm/mg, respectively. Vs. control, ∗, P < 0.05; ‡, P < 0.005; and †, P = 0.0001.
The effects of insulin and IGF-I on GH binding confined to the cell surface were investigated by undertaking the binding assay at 4°C to prevent GHR internalization (18). Treatment with the two growth factors significantly reduced surface GH binding to 36.7 ± 7.6% and 26.4 ± 9.6% of control, respectively (P < 0.05; Fig. 2). The corresponding effects on intracellular GHR content were assessed by undertaking parallel binding studies of total cellular membranes after trypsination to remove cell surface receptors. As shown in Fig. 2, after treatment with insulin and IGF-I, GH binding to membrane preparations of the cultures stripped of surface receptors increased significantly to 137.6 ± 0.5% (P < 0.0001) and 135.2 ± 9.3% of control (P < 0.005), respectively. Thus, the collective data show that these two growth factors reduced surface GH binding and increased intracellular receptor content without changing the total cellular content of GHRs.
GHR Turnover.
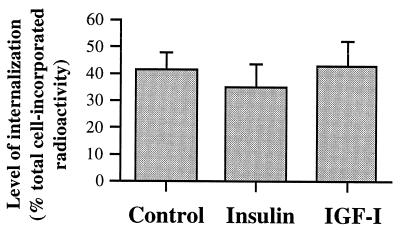
To address the possibility that reduction in GH binding at the cell surface may have arisen from an increased rate of internalization of surface GHRs, the proportion of 125I-labeled GH incorporated into the cells after removing the surface-bound radioligand with acid was measured. At the end of 2-hr incubation, the proportion of radioactivity internalized after treatment with insulin (34.3 ± 8.8% of total radioactivity incorporated; Fig. 3) or IGF-I (42.8 ± 9.3%) was not significantly different from control (41.3 ± 6.0%). Thus, the GHR internalization was not affected by the two growth factors.
Figure 3.
GHR internalization. GH binding assay was undertaken at 22°C for 2 hr. At the end of the binding assay, the surface-bound radioligand was removed by washing with 50 mM HCl at 4°C. The remaining cell incorporated radioactivity accounted for the amount of radioligand internalized with GHRs.
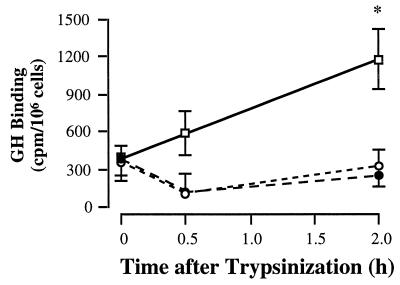
To investigate whether insulin and IGF-I down-regulated surface GH binding by preventing translocation of GHRs from the intracellular pool to the cell surface, the time course of reappearance of binding activity on the cell surface after removal of surface GHRs with trypsin was measured (Fig. 4). In control culture, specific GH binding increased by 596 ± 98 cpm/106 cells (range 210–770 cpm/106 cells, n = 5) 2 hr after trypsinization. In contrast, binding activity of the treated cultures showed no significant change over the 2-hr recovery period. These findings indicate that insulin and IGF-I impaired translocation of GHRs to the cell surface.
Figure 4.
GHR translocation. GHR translocation was measured as the recovery of GH binding on the cell surface after removal of the surface GHR by trypsinization. The trypsinized cells were allowed to recover at 22°C for the time periods indicated, followed by GH binding assay. Results from a representative experiment were shown, and similar findings were obtained in four other experiments. □, Control; •, insulin; ○, IGF-I. Vs. 0 hr, ∗, P < 0.05.
Time Course of Down-Regulation.
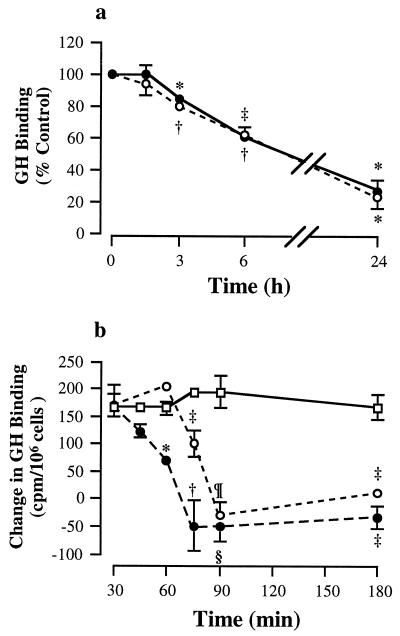
The time course of down-regulation of GH binding and GHR translocation was studied next. Both insulin and IGF-I caused a time-dependent reduction of surface GH binding (Fig. 5a). The reduction was significant by 3-hr treatment to 84.9 ± 1.5% and 79.3 ± 1.1% of control for insulin (P = 0.0005) and IGF-I (P < 0.0001), respectively. The specific binding fell progressively to 27.2 ± 7.0% and 23.6 ± 7.3% (P = 0.0005) after 24-hr treatment with insulin and IGF-I, respectively. No significant difference was found between the effects of the two growth factors.
Figure 5.
Time course of inhibition. (a) Surface GH binding. Cultures were incubated in serum-free conditions for 18 hr, treated with insulin or IGF-I for the indicated time periods, and assayed for surface GH binding of cell monolayers. •, insulin; ○, IGF-I. GH binding of the control at time 0 was 1,065 ± 92 cpm/106 cells. Vs. control, ‡, P = 0.001; ∗, P = 0.0005, and †, P < 0.0001. (b) GHR translocation. Cultures were incubated in serum-free conditions for 18 hr, followed by treatment with insulin or IGF-I for the durations as indicated. Cells were then briefly trypsinized, and recovery of surface GH binding was measured 2 hr after trypsinization. □, control; •, insulin; ○, IGF-I. Vs. control at the same time point, ‡, P = 0.02; †, P = 0.007; ∗, P = 0.0006; ¶, P = 0.0002; and §, P = 0.0001.
The time course of inhibition of GHR translocation was investigated by studying the effects of insulin and IGF-I treatment for 30, 45, 60, 75, 90, and 180 min. As shown in Fig. 5b, GH binding of control cultures increased by 165–200 cpm/106 cells over the 2-hr recovery period. Insulin treatment for 60 min significantly reduced the recovery of GH binding to 42.7 ± 6.1% of control (P = 0.0006), whereas treatment for 75 min completely suppressed recovery of binding. In contrast, IGF-I treatment for 60 min had no significant effect. Partial inhibition (51.9 ± 10.5% of control; P = 0.01) was detected after 75-min treatment with IGF-I and complete impairment by 90 min.
Effects of Wortmannin and PD098059.
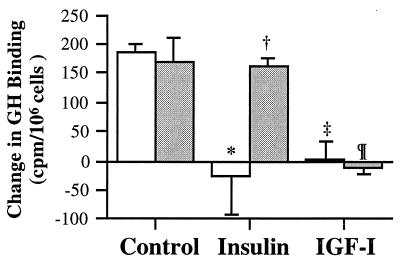
The involvement of PI3K in the inhibition of GHR translocation by insulin and IGF-I was examined by pretreating the cells with 100 nM wortmannin for 0.5 hr. As observed in the time-course study, treatment with the two growth factors for 1.5 hr completely blocked the recovery of surface GH binding, with the levels of GH binding of insulin- and IGF-I-treated cultures (−27 ± 65 and 2 ± 32 cpm/106 cells, respectively) being significantly (P < 0.05) lower than that of untreated cultures (186 ± 14 cpm/106 cells, Fig. 6). Pretreatment with wortmannin had no significant effect on control (170 ± 40 cpm/106 cells) and IGF-I-treated cultures (−11 ± 12 cpm/106 cells), but significantly increased surface GH binding of insulin-treated cultures (160 ± 16 cpm/106 cells; P < 0.05) to a level not significantly different from control cultures. Thus, the data suggest that these two growth factors use different postreceptor signaling pathways for their inhibitory action. Pretreatment with 10 μM PD098059 did not affect the effects of insulin and IGF-I on GH binding (data not shown).
Figure 6.
Effect of wortmannin. Cultures were treated with dimethyl sulfoxide vehicle (□) or 100 nM wortmannin (░⃞) at 37°C for 0.5 hr, followed by treatment with insulin or IGF-I for 1.5 hr. Then, cells were briefly trypsinized, and recovery of surface GH binding was measured 2 hr after trypsinization. Vs. dimethyl sulfoxide control, ∗, P < 0.05 and ‡, P < 0.01. vs. dimethyl sulfoxide insulin; †, P < 0.05. vs. wortmannin control; ¶, P = 0.01.
DISCUSSION
The present study confirms our earlier findings that insulin and IGF-I down-regulate GH binding in osteoblasts (8). These two growth factors did not affect the mRNA abundance or the total cellular content of GHRs, indicating that the mechanism was not exerted at the transcriptional or translational level. Both insulin and IGF-I did not alter the rate of internalization of the surface receptor. Rather, they prevented translocation of GHRs from the intracellular pool to the cell surface, which resulted in reduced surface GH binding and increased intracellular GHR content, with total receptor content unchanged. The impairment of GHR translocation was rapid, occurring within 75–90 min of treatment. The effect of insulin was attenuated by wortmannin, suggesting that inhibition of GHR translocation by insulin is mediated by the PI3K signaling pathway. In contrast, the effect of IGF-I was not affected by wortmannin and PD098095, suggesting that PI3K and mitogen-activated protein kinase kinase are not involved in IGF-I action.
The inhibition of GHR translocation to the cell surface by insulin and IGF-I was acute and preceded the fall in surface GH binding, which was detectable only after 3 hr of treatment. We previously have reported that decreased GH binding arises from reduced number of receptor with no change in binding affinity (8). The sequence of events strongly suggests that the reduction in the number of surface receptors resulted from inhibition of translocation. The lag time between onset of inhibition of GHR translocation and surface GH binding is likely to reflect the time it takes the receptors to be removed from the cell surface by internalization, which occurs constitutively (26), and before the decrease in GH binding becomes detectable.
Previous studies investigating the mechanism by which GHR in the liver is regulated have provided strong evidence for transcriptional control. These studies have shown that modulation of hepatic GH binding by insulin, GH, estrogens, and nutritional status is accompanied by parallel changes in the abundance of GHR mRNA (7, 27–30). In the present study, the effect of insulin on the GHR status in osteoblasts is opposite to its positive effect on hepatic GHRs (6) and occurs with no change in GHR mRNA abundance. These differences in insulin effects between hepatocytes and osteoblasts may reflect tissue specific regulation.
The inhibition of translocation identified in osteoblasts has not been previously considered as a mechanism of GHR regulation, so that it is not known whether this mechanism exists in the liver. However, indirect evidence indicates that such a mechanism also may exist in another extrahepatic tissue. It has been reported in IM-9, a human lymphocyte cell line, that phorbol diesters induce a fall in GH binding without changing the total cellular content of GHRs (31). In this same cell line, the tyrosine phosphatase inhibitor, vanadate, reduced both GH and insulin receptors on the cell surface (32). Further investigation of the insulin receptor showed that the fall in surface receptors was accompanied by an accumulation of an intracellular pool of the receptor. These findings are analogous to ours and implicate an effect of tyrosine kinase. Further studies need to be done to determine whether GHR translocation is a regulatory mechanism within all GHR-expressing tissues in general or only within certain extrahepatic tissues.
The finding that IGF-I did not affect the abundance of GHR mRNA in osteoblasts is not in agreement with a recent report by Slootweg et al. (33). The reason for the disagreement is not clear although they used a different subclone of the osteoblast cell line (UMR 106.01). In that study, solution hybridization/ribonuclease protection assay was used to measure the levels of GHR mRNA from crude preparations of total nucleic acid, although gel studies for quantifying the abundance of the protected fragment were not performed. In our Northern study, only two bands corresponding to the mRNA of GHR and GH binding protein were detected, confirming the specificity of the rat cDNA probe (11). In addition to the Northern results, we also demonstrated that the total cellular content of immunoreactive and functional GHR was not affected by insulin or IGF-I. Our collective data consistently indicate that the down-regulation of GHR by IGF-I did not arise from a reduction in the biosynthesis of the receptor.
Translocation of intracellular proteins such as GLUT4 to the cell surface represents a well recognized intracellular response of insulin-sensitive cells to the action of insulin and is a rapid mechanism that subserves the metabolic needs of the cells. Because of the close interplay between GH and insulin in regulating substrate metabolism with the actions of one opposing but complementing those of the other (34), it is tempting to speculate that regulation of GHR translocation by insulin may be a means by which insulin can rapidly modulate the metabolic actions of GH in extrahepatic tissues.
Although translocation has not been previously described as a mechanism for GHR regulation, the ability of insulin to regulate protein distribution to the cell surface has been described for the receptors of IGF-II and transferrin (35). These receptors recycle as component proteins of GLUT4-containing vesicles, so that they share the same recycling kinetics as GLUT4 in response to insulin. The insulin effect on GHRs is unique because unlike in the IGF-II and transferrin receptors, inhibition rather than recruitment of transport to the cell surface occurs, suggesting that the mechanism does not involve an association with GLUT4-containing vesicles. The present report represents an example of negative regulation of receptor translocation by insulin to the cell surface and raises for consideration that other receptor types may be negatively regulated in this way.
Whereas both insulin and IGF-I appear to induce similar, rapid inhibitory effects on GHR translocation, the mechanisms by which these are achieved are different. By using receptor-specific antibodies, we previously have demonstrated that the effects of insulin and IGF-I are exerted through their own receptors (8). The effect of insulin occurs shortly but consistently before that of IGF-I by about 15 min. Additionally, brief treatment for 1.5 hr with insulin results in a transient reduction of GH binding of longer duration than with IGF-I (unpublished observations). We also present evidence that the postreceptor signaling mechanism required for these actions is different; the studies with wortmannin suggest that the inhibition of GHR translocation by insulin, but not IGF-I, is mediated by PI3K.
The postreceptor signaling mechanism required for inhibiting GHR translocation is not known, although the inhibitor data suggests an involvement of PI3K for insulin, but not IGF-I, with no involvement of mitogen-activated protein kinase kinase. Some insights can be gained from studies on the translocation of GLUT4. GLUT4 translocation to the cell surface after activation of insulin receptor is dependent on regional aggregations of the actin microfilament network, which forms tracks for trafficking intracellular vesicles and organelles (36, 37). A cascade of postreceptor signaling events, including phosphorylation of insulin receptor substrate-1 (IRS-1) and activation of PI3K and Rac, are associated with insulin-induced translocation of the glucose transporter (38–40). The present findings with wortmannin strongly suggest that the same signaling cascade at least to PI3K activation may be involved in GHR regulation. IRS-1 phosphorylation also occurs as a postreceptor signaling event for the IGF-I receptor (41). However, the findings with wortmannin suggest that the pathways for insulin and IGF-I linked to the translocation of GHRs diverge beyond IRS-1.
In conclusion, insulin and IGF-I down-regulate GHRs in osteoblasts by acutely preventing the translocation of intracellular receptors to the cell surface, and not by reducing the receptor biosynthesis. This is a novel mechanism and level of GHR regulation. Inhibition of GHR translocation to the cell surface may subserve acute regulation of the metabolic actions of GH. The role that translocation plays in regulating GH action in other tissues, including the liver, is not known and deserves further study. This mechanism of negatively regulating functional receptor availability may have general significance beyond the GHR.
Acknowledgments
We thank Erefili Peters, Samantha O’Connor, Nathan Doyle, and Christine Wells for excellent technical assistance, and Dr. Tiina Iismaa for helpful advice. We also thank Professor T. J. Martin for providing the UMR106.06 cells. This work was supported by a grant from the National Health and Medical Research Council of Australia and was presented in part at the 77th Annual Meeting of the U.S. Endocrine Society, June 14–17, 1995, Washington, D.C.
ABBREVIATIONS
- GH
growth hormone
- GHR
growth hormone receptor
- IGF-I
insulin-like growth factor-I
- EMEM
Eagle’s minimum essential medium
- PI3K
phosphatidylinositol 3-kinase
References
- 1.Daughaday W H. Perspect Biol Med. 1989;32:194–211. doi: 10.1353/pbm.1989.0006. [DOI] [PubMed] [Google Scholar]
- 2.Isaksson O G P, Jansson J-O, Gause I A M. Science. 1982;216:1237–1239. doi: 10.1126/science.7079756. [DOI] [PubMed] [Google Scholar]
- 3.Waters M J. In: Handbook of Physiology. Kostyo J, editor. Vol. 5. London: Oxford Univ. Press; 1997. in press. [Google Scholar]
- 4.Barnard R, Waters M J. J Endocrinol. 1997;153:1–14. doi: 10.1677/joe.0.1530001. [DOI] [PubMed] [Google Scholar]
- 5.Maes M, Maiter D, Thissen J-P, Underwood L E, Ketelslegers J-M. Trends Endocrinol Metab. 1991;2:92–97. doi: 10.1016/s1043-2760(05)80003-7. [DOI] [PubMed] [Google Scholar]
- 6.Baxter R C, Bryson J M, Turtle J R. Endocrinology. 1980;107:1176–1181. doi: 10.1210/endo-107-4-1176. [DOI] [PubMed] [Google Scholar]
- 7.Menon R K, Stephan D A, Rao R H, Shen-Orr Z, Downs L S, Jr, Roberts C T, Jr, LeRoith D, Sperling M A. J Endocrinol. 1994;142:453–462. doi: 10.1677/joe.0.1420453. [DOI] [PubMed] [Google Scholar]
- 8.Leung K-C, Rajkovic I A, Peters E, Markus I, Van Wyk J J, Ho K K Y. Endocrinology. 1996;137:2694–2702. doi: 10.1210/endo.137.7.8770888. [DOI] [PubMed] [Google Scholar]
- 9.Ho K Y, Weissberger A J, Stuart M C, Day R O, Lazarus L. Clin Endocrinol. 1989;30:335–345. doi: 10.1111/j.1365-2265.1989.tb00431.x. [DOI] [PubMed] [Google Scholar]
- 10.Ho K K Y, Valiontis E, Waters M J, Rajkovic I A. J Clin Endocrinol Metab. 1993;76:302–308. doi: 10.1210/jcem.76.2.8432772. [DOI] [PubMed] [Google Scholar]
- 11.Baumbach W R, Horner D L, Logan J S. Genes Dev. 1989;3:1199–1205. doi: 10.1101/gad.3.8.1199. [DOI] [PubMed] [Google Scholar]
- 12.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 13.Church G M, Gilbert W. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lobie P E, Wood T J J, Chen C M, Waters M J, Norstedt G. J Biol Chem. 1994;269:31735–31746. [PubMed] [Google Scholar]
- 15.Laemmli U K. Nature (London) 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 16.Murphy L J, Lazarus L. Endocrinology. 1984;115:1625–1632. doi: 10.1210/endo-115-4-1625. [DOI] [PubMed] [Google Scholar]
- 17.Roupas P, Herington A C. Endocrinology. 1987;120:2158–2165. doi: 10.1210/endo-120-5-2158. [DOI] [PubMed] [Google Scholar]
- 18.Roupas P, Herington A C. Mol Cell Endocrinol. 1988;57:93–99. doi: 10.1016/0303-7207(88)90037-8. [DOI] [PubMed] [Google Scholar]
- 19.Kanai F, Ito K, Todaka M, Hayashi H, Kamohara S, Ishii K, Okada T, Hazeki O, Ui M, Ebina Y. Biochem Biophys Res Commun. 1993;195:762–768. doi: 10.1006/bbrc.1993.2111. [DOI] [PubMed] [Google Scholar]
- 20.Myers M G, Jr, Sun X J, Cheatham B, Jachna B R, Glasheen E M, Backer J M, White M F. Endocrinology. 1993;132:1421–1430. doi: 10.1210/endo.132.4.8384986. [DOI] [PubMed] [Google Scholar]
- 21.Kotani K, Yonezawa K, Hara K, Ueda H, Kitamura Y, Sakaue H, Ando A, Chavanieu A, Calas B, Grigorescu F, Nishiyama M, Waterfield M D, Kasuga M. EMBO J. 1994;13:2313–2321. doi: 10.1002/j.1460-2075.1994.tb06515.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okada T, Kawano Y, Sakakibara T, Hazeki O, Ui M. J Biol Chem. 1994;269:3568–3573. [PubMed] [Google Scholar]
- 23.Jullien D, Heydrick S J, Gautier N, Van Obberghen E, Le Marchand-Brustel Y. Diabetes. 1996;45:869–875. doi: 10.2337/diab.45.7.869. [DOI] [PubMed] [Google Scholar]
- 24.Lazar D F, Wiese R J, Brady M J, Mastick C C, Waters S B, Yamauchi K, Pessin J E, Cuatrecasas P, Saltiel A R. J Biol Chem. 1995;270:20801–20807. doi: 10.1074/jbc.270.35.20801. [DOI] [PubMed] [Google Scholar]
- 25.Coolican S A, Samuel D S, Ewton D Z, McWade F J, Florini J R. J Biol Chem. 1997;272:6653–6662. doi: 10.1074/jbc.272.10.6653. [DOI] [PubMed] [Google Scholar]
- 26.Roupas P, Herington A C. Mol Cell Endocrinol. 1989;61:1–12. doi: 10.1016/0303-7207(89)90184-6. [DOI] [PubMed] [Google Scholar]
- 27.Mullis P E, Lund T, Patel M S, Brook C G D, Brickell P M. Mol Cell Endocrinol. 1991;76:125–133. doi: 10.1016/0303-7207(91)90267-v. [DOI] [PubMed] [Google Scholar]
- 28.Postel-Vinay M-C, Cohen-Tanugi E, Charrier J. Mol Cell Endocrinol. 1982;28:657–669. doi: 10.1016/0303-7207(82)90153-8. [DOI] [PubMed] [Google Scholar]
- 29.Straus D S, Takemoto C D. Mol Endocrinol. 1990;4:91–100. doi: 10.1210/mend-4-1-91. [DOI] [PubMed] [Google Scholar]
- 30.Gabrielsson B G, Carmignac D F, Flavell D M, Robinson I C A F. Endocrinology. 1995;136:209–217. doi: 10.1210/endo.136.1.7828533. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki K, Suzuki S, Saito Y, Ikebuchi H, Terao T. J Biol Chem. 1990;265:11320–11327. [PubMed] [Google Scholar]
- 32.Torossian K, Freedman D, Fantus I G. J Biol Chem. 1988;263:9353–9359. [PubMed] [Google Scholar]
- 33.Slootweg M C, Ohlsson C, Salles J-P, de Vries C P, Netelenbos J C. Endocrinology. 1995;136:4210–4217. doi: 10.1210/endo.136.10.7545101. [DOI] [PubMed] [Google Scholar]
- 34.Davidson M B. Endocr Rev. 1987;8:115–131. doi: 10.1210/edrv-8-2-115. [DOI] [PubMed] [Google Scholar]
- 35.Kandror K V, Pilch P F. Am J Physiol. 1996;271:E1–E14. doi: 10.1152/ajpendo.1996.271.1.E1. [DOI] [PubMed] [Google Scholar]
- 36.Adams R J, Pollard T D. Nature (London) 1986;322:754–756. doi: 10.1038/322754a0. [DOI] [PubMed] [Google Scholar]
- 37.Kuznetsov S A, Langford G M, Weiss D G. Nature (London) 1992;356:722–725. doi: 10.1038/356722a0. [DOI] [PubMed] [Google Scholar]
- 38.Cheatham B, Kahn C R. Endocr Rev. 1995;16:117–142. doi: 10.1210/edrv-16-2-117. [DOI] [PubMed] [Google Scholar]
- 39.Nobes C D, Hall A. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 40.Ridley A J. Curr Biol. 1996;6:1256–1264. doi: 10.1016/s0960-9822(02)70711-2. [DOI] [PubMed] [Google Scholar]
- 41.LeRoith D, Werner H, Beitner-Johnson D, Roberts C T., Jr Endocr Rev. 1995;16:143–163. doi: 10.1210/edrv-16-2-143. [DOI] [PubMed] [Google Scholar]