Abstract
Calgranulins are a family of powerful chemoattractants, which have been implicated as biomarkers in inflammatory diseases. To determine how different respiratory diseases affect the expression of calgranulins, we measured the expression of S100A8/A9 and S100A12 in bronchoalveolar lavage fluid (BALF) of ARDS patients and healthy volunteers by ELISA. Analysis of calgranulin expression revealed a high level of S100A12 in the lavages of patients suffering from ARDS compared to controls (p< 0.001). Based on the hypothesis that the increased expression of S100A12 relative to the S100A8/A9 heterodimer was a characteristic of respiratory diseases with neutrophilic inflammation, we measured calgranulin expression in BALF of cystic fibrosis (CF) patients. Despite similarly elevated levels of S100A8/A9, S100A12 was significantly higher in ARDS compared to CF BALF (p<0.001). The differential expression of calgranulins was unique for inflammatory markers, as an array of cytokines did not differ between CF and ARDS patients.
Since ARDS is an acute event and CF a chronic inflammation with acute exacerbations, we compared calgranulin expression in sputum obtained from CF and patients with chronic obstructive lung disease (COPD). Levels of S100A12 and S100A8/9 were elevated in CF sputum compared to COPD sputum, but the ratio of S100A12 to S100A8/A9 was similar in COPD and CF and reflected more closely that seen in healthy controls. The results indicate that the regulation of human calgranulin expression and the ratio of S100A8/A9 to S100A12 may provide important insights in the mechanism of respiratory inflammation.
Keywords: neutrophils, inflammation, acute respiratory distress syndrome, S100A12
Introduction
Recent evidence in mammals has implicated calgranulins as important mediators of neutrophil influx during infections (1-3). Calgranulins (S100A8, S100A9, and S100A12) are members of the S100 family of small calcium-binding proteins (4) and are expressed predominantly in the cytosol of neutrophils and monocytes. Calgranulins exist as homodimers; but S100A8 and S100A9 can also form a heterodimer (S100A8/A9), which is the predominant form found in the cytosol and the extracellular space. S100A8, S100A9, and S100A12 stimulate neutrophil and monocyte chemotaxis, adhesion to fibrinogen and diapedesis in vitro, as well as neutrophil migration to inflammatory sites in vivo (3, 5-7).
Calgranulin expression varies between cell types and stimuli. For instance, while S100A8 expression is increased in macrophages following acute LPS stimulation, S100A9 responds to additional stimuli, including peptidoglycans, expressed by Gram positive bacteria (2, 8). Evidence from S100A9-deficient mice also indicates that the presence of S100A9 protein is required for the translation of S100A8 mRNA, while S100A12, which is only expressed in humans but absent in mice, is known to be induced by IL-6 (9). Acute Respiratory Distress Syndrome (ARDS), is associated with elevated IL-6 levels (10) and conceivably this IL-6 response could enhance the expression of S100A12 during the inflammatory cascade that occurs in the lung following an acute injury.
ARDS is a frequent complication in trauma patients and constitutes one of the leading causes of mortality in critically ill patients (11-13). ARDS is characterized by a heightened inflammatory response secondary to severe lung injury (14-16). The severity and outcome of ARDS is thought to be determined by the balance of activated neutrophils and the secretion of cytokines and chemokines. Reports suggest that the neutrophil influx in ARDS may be secondary to the lung injury rather than the cause of it (17, 18); but neutrophils contribute actively to the destruction of the lung. Understanding the molecular basis that regulates neutrophil activation and influx into the airways may therefore be important in limiting the lung injury through these inflammatory mediators. To determine how calgranulin expression is altered during ARDS, we analyzed calgranulin expression in patients suffering from acute and chronic pulmonary diseases.
Calgranulins have also been described in the pathogenesis of cystic fibrosis (CF), a disease characterized by excessive neutrophil driven inflammation in the lung and by chronic infection. The calgranulin heterodimer S100A8/A9 has been reported as the CF antigen, which was found to be up-regulated in the serum and sputum of cystic fibrosis patients (19). More recently, S100A12 was also found to be elevated in the serum and sputum of cystic fibrosis patients during acute exacerbations (20), which might implicate S100A12 in acute pulmonary disease.
Since S100A8 and S100A9 form a heterodimer, but are independent of S100A12, we hypothesized that the ratio of S100A12 to S100A8/A9 could differ between acute and chronic respiratory infections. S100A12 expression is of particular interest in acute respiratory diseases as it is induced by IL-6, which is one of the cytokines implicated in the pathogenesis of ARDS (10). Since mice lack functional S100A12, we compared S100A12 expression with S100A8/A9 in patients suffering from acute and chronic respiratory diseases.
Materials and Methods
Patient recruitment and characteristics
Patients admitted to the trauma Intensive Care Unit (ICU) were eligible for inclusion in this study if ARDS, defined as PaO2/FIO2 ratio <200, developed during their stay in the ICU. Additional ARDS patients were identified from the pulmonary / critical care unit using the American-European Consensus Conference definition (21). All patients underwent clinically indicated bronchoscopy within the first 4 days of meeting ARDS criteria.
BAL fluid was obtained from cystic fibrosis patients undergoing clinically indicated bronchoscopy for increased respiratory symptoms suggestive of new infection and who were unable to produce sputum. Diagnosis of CF had been confirmed according to consensus criteria, including a positive sweat test (22). Two of the BALF samples from CF patients were not infected; the remaining BALF had infections with various gram-positive and gram-negative organisms, including two children with P. aeruginosa infections. Since a subsequent comparison with COPD patients was necessary and bronchoscopy is risky and rarely indicated for clinical reasons in COPD, we also collected sputum from clinically stable patients with CF at completion of intravenous antibiotic treatment.
COPD patients with mild to moderate disease severity, as defined by Global Institute for Chronic Obstructive Lung Disease (GOLD score ≤2) (23) were recruited to undergo sputum induction. Five of the patients continued to be active smokers. One patient was on oral corticosteroid therapy (prednisone), while the other patients were on combination of medicines including short and long acting beta agonist therapy. All patients were clinically stable and had not experienced any exacerbations of their COPD within the preceding three months. All COPD patients were pre-treated with beta-agonist (albuterol) prior to sputum induction.
Healthy adult controls were recruited for the collection of BALF as part of a GCRC-sponsored protocol on lung inflammation and injury in asthma and ARDS. Healthy controls for sputum induction were recruited as part of a study described previously (24). Informed consent, according to the Institutional Review Board, was obtained from all patients and healthy controls.
BALF and sputum processing
Bronchoscopy on Medical ICU patients was performed in either the lingula or right middle lobe using four 50 ml aliquots of sterile saline. For comparison, BALF from healthy volunteers was obtained as described (25). Bronchoscopy and bronchoalveolar lavage on trauma patients was performed in the affected area of the lung using three 50 ml aliquots of sterile saline. Bronchoscopy in children with CF was performed under sedation or general anesthesia and BAL was performed by instilling 2-3 aliquots of 1ml/kg (max 30cc) sterile saline and immediately aspirating the fluid through the bronchoscope (26). The BAL was performed in the most affected segment selected by clinical findings on X-ray or bronchoscopy. The total BALF was centrifuged (200 × g for 10 minutes) to isolate the cells. The cell free supernatants were stored at −20°C for ELISA. Induced sputum was performed and processed as previously described (27).
Measurement of calgranulins in bronchoalveolar lavage
ELISA assays for calgranulin expression in the lung lavage were done as previously described (28). Briefly, for the ELISA measurement of S100A8 and S100A9, Costar High Binding 96-well plates (Corning, NY) were coated overnight at 4°C with 100 μl/well purified rabbit IgG against S100A8 or S100A9 diluted to a concentration of 1 μg/ml in 0.1 M carbonate buffer, pH 9.6. The wells were blocked with PBS/0.1% Tween 20/2% BSA (150 μl/well) for 30 min at room temperature. The samples and standards (100 μl) were added and incubated for 45 min at room temperature. The plates were washed three times with PBS/0.1% Tween 20, and were incubated with rat IgG (100 μl/well) against S100A8 or S100A9 diluted in PBS/0.1% Tween 20/2% BSA (1/10,000) for 45 min at room temperature. The plates were then washed three times in PBS/0.1% Tween 20. To reveal the immune complex, peroxidase-conjugated goat anti-rat IgG (H + L) (minimum cross-reaction to rabbit serum proteins) (100 μl/well), at a dilution of 1/10,000, was added and incubated 45 min at room temperature. The plates were washed three times and 100 μl/well TMB-S substrate was added according to the manufacturer's instructions. The ODs were read at 500 nm. The lower limit of quantification was determined as 4 ng/ml for both S100A8 and S100A9.
For the ELISA assays to measure the S100A8/A9 heterodimer, 96-well plates were coated overnight at 4°C with purified anti-S100A9 rabbit IgG (μl/100 well) diluted 1 μg/ml in 0.1 M carbonate buffer, pH 9.6. The wells were blocked with PBS/0.1% Tween 20/2% BSA (150 μl/well) for 30 min at room temperature. The samples and standards (100 μl) were added and incubated for 45 min at room temperature. The plates were washed three times with PBS/0.1% Tween 20 then incubated with 100 μl/well anti-S100A8 rat IgG diluted in PBS/0.1% Tween 20/2% BSA (1/10,000) for 45 min at room temperature. The plates were next washed three times in PBS/0.1% Tween 20 and incubated with 100 μl/well peroxidase-conjugated goat anti-rat IgG at a dilution of 1/10,000 for 45 min at room temperature. After three washes, 100 μl/well TMB-S substrate were added according to the manufacturer's instructions. The ODs were read on a plate reader at 500 nm. The lower limit of quantification of this assay was determined as 10 ng/ml. All three ELISAs were tested using excess amounts (100 times) of S100A8, S100A9, or S100A8/A9 proteins and were shown to be specific under the conditions reported here. In addition, spiking studies revealed that no inhibitors in the BALF and sputum interfered with the ELISA readouts.
Statistics
Sputum and bronchoalveolar lavage measurements for S100 proteins were normalized against total protein content in the samples as indicated. Calgranulin concentration in BAL fluid is reported as median and range for the patient groups. Nonparametric analysis (Mann Whitney test) was used to compare the calgranulin expression between the test groups, with significance defined as p<0.05. Data were log transformed only for graphical presentation.
Results
S100A12 levels are elevated in the BALF of ARDS patients compared to CF patients
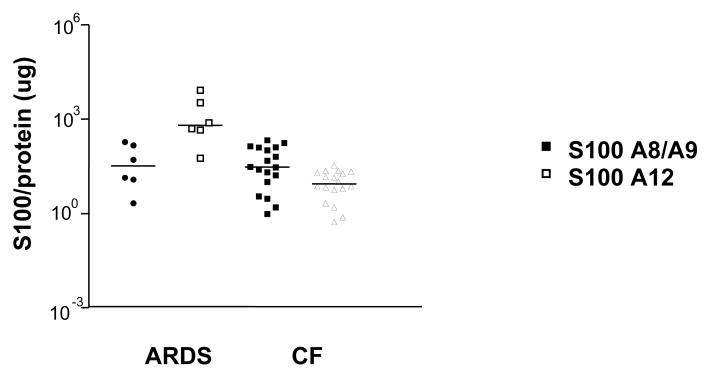
The heterodimer S100A8/A9 has been reported to mediate the neutrophil influx following an acute LPS stimulation (29) and is also up-regulated in the sputum of CF patients, who have neutrophil dominated lung inflammation (19). When we compared S100A8/A9 levels in CF and ARDS patients, we found similar levels of calgranulin heterodimer normalized to total BAL protein in both groups of patients (Figure 1, closed symbols).
Figure 1. S100A8/A9 and S100A12 expression in the BALF.
S100A12 (open symbols) and S100A8/A9 (closed symbols) were measured in the BALF of ARDS patients and CF patients. Results are presented in log-scale, corrected for total protein with the median values for each subject group indicated.
By contrast, the expression of S100A12 protein, which is associated with acute exacerbations in CF patients (20), was significantly higher in BALF of ARDS patients compared to that measured in BALF of CF patients (Fig. 1, open symbols) (median 98.2, range 0.9 - 682) vs. CF (29.7, range 0.96-212.9) (p=0.0004). S100 proteins were also measured in BAL obtained from healthy control individuals. Comparison of S100, non-normalized to total BAL protein, showed that both patient groups had significantly higher levels of S 100 A8/A9 and of S100A12 than healthy controls, who had a median (range) 19.4 (9 - 77) ng/ml and 1,819 (236 – 17,853) ng/ml (p<0.001), respectively. The data for the comparison of the raw measurements is shown in the online supplement.
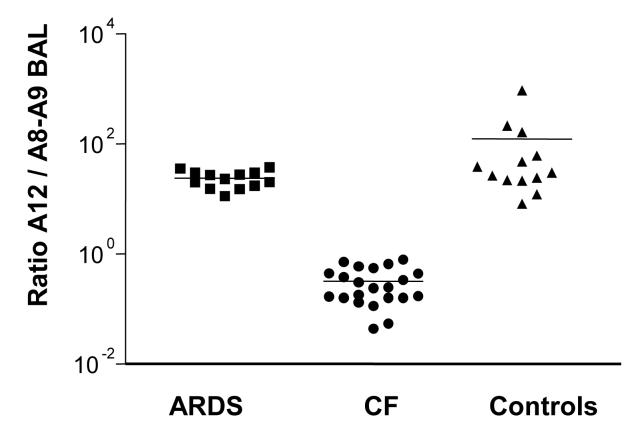
As production and likely also the function of S100A12 differs from that of the S100A8/A9 heterodimer, we compared the ratios of these two proteins (30). The ratio of S100A12 to S100A8/A9 in the BAL of ARDS patients was not significantly different from healthy controls (p=0.112). Compared to ARDS patients and healthy controls, the ratio S100A12 to S100A8/A9 was significantly lower in cystic fibrosis patients (p<0.001) (Fig. 2). Thus the profile of calgranulin expression differs significantly between CF and ARDS patients, despite the fact that both diseases show an elevation of S100A12 and S100 A8/A9. However when stratifying patients with ARDS by etiology (6 patients with direct vs. 7 patients with indirect lung injury), we observed no difference in either S100A12 or S100A8/A9 or their ratio to each other (data not shown).
Figure 2. Ratio of calgranulin expression in the BALF.
The relative expression ratio of S100A12 to S100A8/A9 was determined in the BALF of ARDS patients, CF patients, and healthy volunteers. Results are presented on log-scale, with the median values for each subject group indicated.
To determine whether the difference observed in the calgranulin ratio between CF and ARDS was specific for calgranulins or shared by other inflammatory markers, the expression of multiple inflammatory mediators was determined. We measured an array of cytokines expressed in BALF samples during ARDS and CF. These cytokines and the S100 proteins were corrected for total protein in BALF. The following cytokines were analyzed: RANTES, IL-6, IL-8, IP-10, TNF-α and ICAM. With the exception of IL-8, which was significantly higher in CF (p=0.005), these cytokines were expressed at similar levels in the BAL fluid of ARDS and CF patients (data not shown). The difference in the ratio of S100A8/A9 to S100A12 observed in CF and ARDS patients is therefore unique to calgranulins and not shared by other inflammatory mediators.
Sputum calgranulin expression in chronic respiratory diseases
ARDS and CF exacerbations are acute inflammatory diseases, in which neutrophils are thought to contribute significantly to inflammation. To assess if the calgranulin profile differed in chronic and acute lung inflammation, we compared stable CF to stable COPD patients. For ethical reasons, we did not perform bronchoscopic lavages, but used sputum collected from COPD and CF patients. Our control samples were induced sputa obtained form healthy controls.
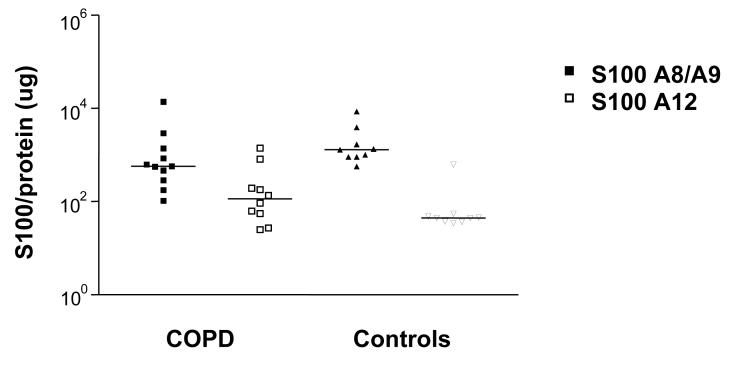
We measured calgranulin expression in the sputum of CF patients after a course of intravenous antibiotics to ensure that the lung inflammation was stabilized and reflected better the chronic inflammation of stable COPD. For the raw data, levels of S100A8/A9 were similar in COPD and healthy controls ((median, range) 581 (116 – 4,648) and (668 (448 – 16,122) ng/ml, respectively). By contrast, they were significantly elevated in the sputum of CF patients 15,155 (5,996 – 20,428) ng/ml) (p<0.001) (Figure 3). Similarly, S100A12 expression in the sputum of CF patients was significantly elevated (p<0.001) (median 12,473 (4,047 – 20,993) ng/ml) compared to the COPD patients (172 (28 – 4,693) ng/ml) and the healthy control group 31 (8 – 1,158) ng/ml). We also normalized S100 proteins to total protein in sputum from CF patients and therefore total protein was only measured in 4 samples. Normalization of the calgranulin levels to total protein content revealed a similar pattern in COPD and healthy subjects as in the raw measurements. Normalization was difficult for CF patients as we had insufficient sputum to measure protein in 4 samples, and protein concentrations were at the lower limit of detection in 3 of the remaining 4 samples. When we accounted for the dilution, these values were in the detectable range of the assay and we used these to normalize the S100 protein measurements. In these samples both S100A8/A9 heterodimer and S100A12 were much higher than in either COPD or healthy controls, similar to the non-normalized data seen in the raw S100 protein measurements. (Fig available as online supplement).
Figure 3. S100A8/A9 and S100A12 expression in the sputum.
S100A12 (open symbols) and S100A8/A9 (closed symbols) were measured in the sputum of COPD patients and healthy controls. Results are presented in log-scale, corrected for total protein with the median values for each subject group indicated.
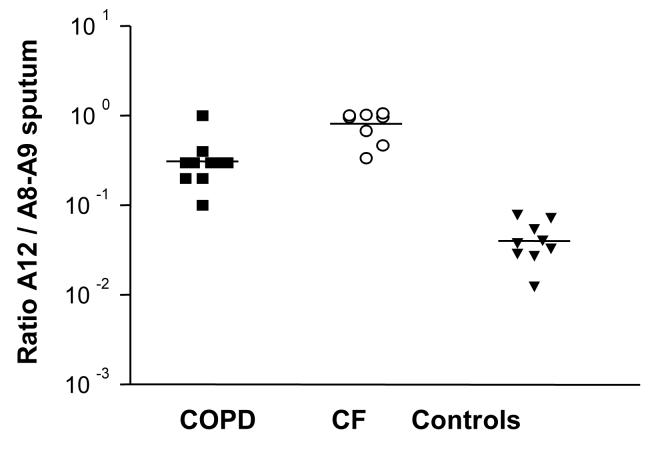
The ratio of S100A12 to S100 A8/A9 was similar in CF and COPD sputum with stable disease and tended to be higher than in healthy controls, indicating that S100A8/A9 and S100A12 increase in chronic lung inflammation with only a mild predominance of the S100A8/A9 complex (Fig. 4). This is in contrast to the difference in the ratio of S100A8/A9 to S100A12 between CF patients and healthy controls or ARDS patients; in latter two sample groups S100A12 expression predominates. It is conceivable that the change in ratio is related to the acute onset of ARDS and other underlying mechanisms of inflammation.
Figure 4. Ratio of calgranulin expression in the sputum.
The ratio of S100A12 to S100A8/A9 was determined in the sputum of COPD patients, CF patients and healthy volunteers. Results are presented in log-scale, with the median values for each subject group indicated.
We also measured an array of cytokines expressed in sputum samples during COPD, CF and normal controls. The following cytokines were analyzed: RANTES, IL-6, IL-8, IP-10, TNF-α and ICAM. There was no difference in cytokine concentrations when normalized to total protein in COPD versus CF patients but the number of samples available for this analysis was low (data not shown). Interestingly, only IL-10 was significantly different between COPD and control patients, with higher values in normal controls.
Discussion
The development of ARDS is a serious and common complication in sepsis patients and trauma patients. The diagnosis of ARDS in critically ill patients is linked to severely impaired lung function and increased mortality. ARDS is characterized by the increased secretion of cytokines in the airways and intense influx of neutrophils in the lung. Previous research has related differences in neutrophil activation to outcome in acute lung injury (31), suggesting that factors regulating the neutrophil influx could be predictors of outcome. The calgranulin heterodimer S100A8/A9 has potent chemoattractant capabilities and its expression is required to mediate the influx of neutrophils following an acute stimulation with endotoxin in mice (29). As S100A12 is not expressed in mice and to address how the expression of calgranulins differs in acute and chronic human lung disease (30), we investigated the possible role of calgranulins S100A8/A9 and S100A12 in the acute inflammatory response of ARDS. Expression levels of S100A12 and S100A8/A9 levels in ARDS patients were not dependent on whether the patients suffered a direct or indirect injury, implying that calgranulin expression may not be related to the severity of ARDS or the risk of mortality both of which differ based on the injury type (32-35).
Calgranulin expression, in particular the elevated levels of S100A12, however, could be an underlying mechanism for augmenting the inflammatory response associated with acute pulmonary infection. Recent studies found elevated S100A12 levels in the BALF of CF patients during acute exacerbations. These studies reported levels of up to 11,000 ng/ml (20), similar to the average S100A12 expression detected in this study and much lower than the level of S100A12 detected in ARDS patients.
A possible limitation in our study is the comparison of BALF obtained from pediatric patients with CF versus adults with ARDS, reflecting the main groups of patients affected with these diseases. Adults with CF generally can produce sputum and are sicker than children and thus BAL is not indicated and could be dangerous for study purposes. On the other had underlying etiologies and definition of ARDS is even more heterogeneous in pediatric patients (36) The age and size difference in these groups may affect BAL fluid volumes and concentration as smaller volumes for BALF are used in children than adults. We elected not to use normalization to urea, as concentration of urea in BALF can be affected by underlying disease and dwell time of the lavage fluid, especially in children (37, 38). More importantly, we also measured the ratio of S100A8/A9 to S100A12, which is not affected by dilution, and this ratio is decreased in CF compared to ARDS.
The different pattern in the expression of an inflammatory marker between CF and ARDS patients is unique to calgranulins, since a panel of cytokines measured in the study show similar expression levels in both diseases when normalized to total protein. This finding supports the notion that changes in the calgranulin ratio between CF and ARDS relate to differences in the mechanism of inflammation. Since CF is characterized by chronic neutrophil dominated inflammation (39, 40), the increased expression of S100A12 in ARDS suggests that S100A12 could be more important in the onset of neutrophil influx and is therefore less elevated in chronic inflammation. Another possibility is the difference between the systemic inflammation in ARDS whereas the inflammation in CF is mostly compartmentalized to the lung. Elevated levels of S100A12 have also been described in Kawasaki syndrome (41) and rheumatoid arthritis (42), two diseases associated with systemic inflammation. One could thus speculate that the increased concentration of S100A12 seen in ARDS is linked to the systemic inflammation e.g. IL-6 and subsequent higher expression in subepithelial cells with diffusion into the alveolar space.
Our comparison of calgranulin expression in the sputum of CF and COPD patients underscores the conclusion that the elevated levels of S100A12 are associated with acute inflammation. We compared calgranulin expression in CF with COPD patients who, despite a different underlying pathogenesis, share the chronic inflammation seen in CF patients. Both, COPD and CF patients, showed higher levels of S100A8/A9 expression than S100A12, indicating that S100A12 is associated with an acute infection, while an underlying chronic infection will primarily be mediated by S100A8/A9. In support of this conclusion, healthy controls show low levels of S100A12 in their sputum, while the sputum of CF patients at admission showed increased levels of S100A12 compared to CF sputum at discharge (data not shown). This is consistent with findings of Foell et al. who showed increase of S100A12 in CF with exacerbations, which subsequently declined with therapy (20).
In summary, calgranulins are important markers of neutrophil dominated infections. However, our data of significantly elevated S100A12 in ARDS compared to healthy subjects and increased levels of S100A12 in exacerbations of chronic lung diseases point towards differential regulation of S100A12 versus S100A8/A9 in acute versus chronic inflammation. Our findings contribute to the growing evidence of increased expression of S100A12 in acute inflammatory diseases, and contrary to CF or rheumatoid arthritis significant elevation occurs acutely in previously healthy persons.
Supplementary Material
Table 1.
Patient demographics
| ARDS BALF |
CF BALF |
Controls BALF |
CF sputum |
COPD sputum |
Controls sputum |
|
|---|---|---|---|---|---|---|
| N | 13 | 15 | 14 | 8 | 10 | 10 |
| Age avg. (yrs) |
42 | 6 | 24 | 16 | 58 | 26 |
| Male | 8 | 6 | 6 | 4 | 6 | 3 |
| Female | 5 | 9 | 8 | 4 | 4 | 7 |
Acknowledgements
We thank Drs. B. Rubin, P. Miller, and M. Schechter, as well as Diana C. Chemotti, Bonnie Grier, Judy Smith, Lauren Clarkson, Marie-Astrid Raquil, and Karen Vandal for help in collecting and analyzing the patient samples.
This study was funded in part through an American Heart Association Grant-in-Aid, a Research Award from the American Lung Association, and grants from the General Clinical Research Center (WFUHS; M01-RR07122) (EL), NIH-HL64226 (MCS, RDH), NIH-HL66559 (NEA, DBP) as well as a scholarship from the Fonds de la Recherche en Santé du Québec, and a grant from the Canadian Institutes of Health Research (PAT). M. Muhlebach is supported by the Mentored Clinical Research Scholars K12 Program. NIH/NCRR RR17667.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Passey RJ, Xu K, Hume DA, Geczy CL. S100A8: emerging functions and regulation. J Leukoc Biol. 1999;66(4):549–56. doi: 10.1002/jlb.66.4.549. [DOI] [PubMed] [Google Scholar]
- 2.Donato R. Intracellular and extracellular roles of S100 proteins. Microsc Res Tech. 2003;60(6):540–51. doi: 10.1002/jemt.10296. [DOI] [PubMed] [Google Scholar]
- 3.Ryckman C, Vandal K, Rouleau P, Talbot M, Tessier PA. Proinflammatory Activities of S100: Proteins S100A8, S100A9, and S100A8/A9 Induce Neutrophil Chemotaxis and Adhesion. J Immunol. 2003;170(6):3233–42. doi: 10.4049/jimmunol.170.6.3233. [DOI] [PubMed] [Google Scholar]
- 4.Schäfer BW, Heizman CW. The S100 Family of EF-Hand Calcium-Binding Proteins: Functions and Pathology. TIBS. 1996;21:134–40. doi: 10.1016/s0968-0004(96)80167-8. [DOI] [PubMed] [Google Scholar]
- 5.Rouleau P, Vandal K, Ryckman C, Poubelle PE, Boivin A, Talbot M, et al. The calcium-binding protein S100A12 induces neutrophil adhesion, migration, and release from bone marrow in mouse at concentrations similar to those found in human inflammatory arthritis. Clin Immunol. 2003;107(1):46–54. doi: 10.1016/s1521-6616(02)00043-8. [DOI] [PubMed] [Google Scholar]
- 6.Eue I, Pietz B, Storck J, Klempt M, Sorg C. Transendothelial migration of 27E10+ human monocytes. Int Immunol. 2000;12(11):1593–604. doi: 10.1093/intimm/12.11.1593. [DOI] [PubMed] [Google Scholar]
- 7.Yang Z, Tao T, Raftery MJ, Youssef P, Di Girolamo N, Geczy CL. Proinflammatory properties of the human S100 protein S100A12. J Leukoc Biol. 2001;69(6):986–94. [PubMed] [Google Scholar]
- 8.Nacken W, Roth J, Sorg C, Kerkhoff C. S100A9/S100A8: Myeloid representatives of the S100 protein family as prominent players in innate immunity. Microsc Res Tech. 2003;60(6):569–80. doi: 10.1002/jemt.10299. [DOI] [PubMed] [Google Scholar]
- 9.Hasegawa T, Kosaki A, Kimura T, Matsubara H, Mori Y, Okigaki M, et al. The regulation of EN-RAGE (S100A12) gene expression in human THP-1 macrophages. Atherosclerosis. 2003;171(2):211–8. doi: 10.1016/j.atherosclerosis.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 10.Bhatia M, Moochhala S. Role of inflammatory mediators in the pathophysiology of acute respiratory distress syndrome. J Pathol. 2004;202(2):145–56. doi: 10.1002/path.1491. [DOI] [PubMed] [Google Scholar]
- 11.Baker CC, Oppenheimer L, Stephens B, Lewis FR, Trunkey DD. Epidemiology of trauma deaths. Am J Surg. 1980;140(1):144–50. doi: 10.1016/0002-9610(80)90431-6. [DOI] [PubMed] [Google Scholar]
- 12.Fowler AA, Hamman RF, Zerbe GO, Benson KN, Hyers TM. Adult respiratory distress syndrome. Prognosis after onset. Am Rev Respir Dis. 1985;132(3):472–8. doi: 10.1164/arrd.1985.132.3.472. [DOI] [PubMed] [Google Scholar]
- 13.Fowler AA, Hamman RF, Good JT, Benson KN, Baird M, Eberle DJ, et al. Adult respiratory distress syndrome: risk with common predispositions. Ann Intern Med. 1983;98(5 Pt 1):593–7. doi: 10.7326/0003-4819-98-5-593. [DOI] [PubMed] [Google Scholar]
- 14.Weinacker AB, Vaszar LT. Acute respiratory distress syndrome: physiology and new management strategies. Annu Rev Med. 2001;52:221–37. doi: 10.1146/annurev.med.52.1.221. [DOI] [PubMed] [Google Scholar]
- 15.Tasaka S, Hasegawa N, Ishizaka A. Pharmacology of acute lung injury. Pulm Pharmacol Ther. 2002;15(2):83–95. doi: 10.1006/pupt.2001.0325. [DOI] [PubMed] [Google Scholar]
- 16.Shimabukuro DW, Sawa T, Gropper MA. Injury and repair in lung and airways. Crit Care Med. 2003;31(8 Suppl):S524–31. doi: 10.1097/01.CCM.0000081437.06466.B3. [DOI] [PubMed] [Google Scholar]
- 17.Chollet-Martin S, Jourdain B, Gibert C, Elbim C, Chastre J, Gougerot-Pocidalo MA. Interactions between neutrophils and cytokines in blood and alveolar spaces during ARDS. Am J Respir Crit Care Med. 1996;154(3 Pt 1):594–601. doi: 10.1164/ajrccm.154.3.8810592. [DOI] [PubMed] [Google Scholar]
- 18.Booke M, Van Aken H. Neutrophils in acute respiratory distress syndrome: upregulated, uninhibited, or even both. Crit Care Med. 2001;29(10):2031. doi: 10.1097/00003246-200110000-00035. [DOI] [PubMed] [Google Scholar]
- 19.Bruggen J, Tarcsay L, Cerletti N, Odink K, Rutishauser M, Hollander G, et al. The molecular nature of the cystic fibrosis antigen. Nature. 1988;331(6157):570. doi: 10.1038/331570a0. [DOI] [PubMed] [Google Scholar]
- 20.Foell D, Seeliger S, Vogl T, Koch HG, Maschek H, Harms E, et al. Expression of S100A12 (EN-RAGE) in cystic fibrosis. Thorax. 2003;58(7):613–7. doi: 10.1136/thorax.58.7.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bernard G, Artigas A, Brigham K, Carlet J, Falke K, Hudson L, et al. The American-European consensus conference on ARDS definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med. 1994;149:818–24. doi: 10.1164/ajrccm.149.3.7509706. [DOI] [PubMed] [Google Scholar]
- 22.Rosenstein BJ, Cutting GR. The diagnosis of cystic fibrosis: a consensus statement. Cystic Fibrosis Foundation Consensus Panel. J Pediatr. 1998;132:589–95. doi: 10.1016/s0022-3476(98)70344-0. [DOI] [PubMed] [Google Scholar]
- 23.Pauwels R, Buist A, Calverley P, Jenkins C, Hurd S, Committee GS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163(5):1047–8. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 24.Alexis NE, Lay JC, Almond M, Bromberg PA, Patel DD, Peden DB. Acute LPS inhalation in healthy volunteers induces dendritic cell maturation in vivo. J Allergy Clin Immunol. 2005;115(2):345–50. doi: 10.1016/j.jaci.2004.11.040. [DOI] [PubMed] [Google Scholar]
- 25.Bowton DL, Seeds MC, Fasano MB, Goldsmith B, Bass DA. Phospholipase A2 and arachidonate increase in bronchoalveolar lavage fluid after inhaled antigen challenge in asthmatics. Am J Respir Crit Care Med. 1997;155(2):421–5. doi: 10.1164/ajrccm.155.2.9032172. [DOI] [PubMed] [Google Scholar]
- 26.Muhlebach MS, Reed W, Noah TL. Quantitative cytokine gene expression in CF airway. Pediatr Pulmonol. 2004;37(5):393–9. doi: 10.1002/ppul.20010. [DOI] [PubMed] [Google Scholar]
- 27.Alexis NE, Peden DB. Blunting ariway eosinophilic inflammation results in a decreased airway neutrophil response to inhaled LPS in patients with atopic asthma: a role for CD14. J Allergy Clin Immunol. 2001;108:577–80. doi: 10.1067/mai.2001.118511. [DOI] [PubMed] [Google Scholar]
- 28.Ryckman C, McColl SR, Vandal K, de Medicis R, Lussier A, Poubelle PE, et al. Role of S100A8 and S100A9 in neutrophil recruitment in response to monosodium urate monohydrate crystals in the air-pouch model of acute gouty arthritis. Arthritis Rheum. 2003;48(8):2310–20. doi: 10.1002/art.11079. [DOI] [PubMed] [Google Scholar]
- 29.Vandal K, Rouleau P, Boivin A, Ryckman C, Talbot M, Tessier PA. Blockade of S100A8 and S100A9 suppresses neutrophil migration in response to lipopolysaccharide. J Immunol. 2003;171(5):2602–9. doi: 10.4049/jimmunol.171.5.2602. [DOI] [PubMed] [Google Scholar]
- 30.Foell D, Wittkowski H, Vogl T, Roth J. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol. 2007;81(1):28–37. doi: 10.1189/jlb.0306170. [DOI] [PubMed] [Google Scholar]
- 31.Yang KY, Arcaroli JJ, Abraham E. Early alterations in neutrophil activation are associated with outcome in acute lung injury. Am J Respir Crit Care Med. 2003;167(11):1567–74. doi: 10.1164/rccm.200207-664OC. [DOI] [PubMed] [Google Scholar]
- 32.Hudson L, Milberg J, Anardi D, Maunder R. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151:293–301. doi: 10.1164/ajrccm.151.2.7842182. [DOI] [PubMed] [Google Scholar]
- 33.Doyle RL, Szaflarski N, Modin GW, Wiener-Kronish JP, Matthay MA. Identification of patients with acute lung injury. Predictors of mortality. Am J Respir Crit Care Med. 1995;152(6 Pt 1):1818–24. doi: 10.1164/ajrccm.152.6.8520742. [DOI] [PubMed] [Google Scholar]
- 34.Pepe PE, Potkin RT, Reus DH, Hudson LD, Carrico CJ. Clinical predictors of the adult respiratory distress syndrome. Am J Surg. 1982;144(1):124–30. doi: 10.1016/0002-9610(82)90612-2. [DOI] [PubMed] [Google Scholar]
- 35.Sloane PJ, Gee MH, Gottlieb JE, Albertine KH, Peters SP, Burns JR, et al. A multicenter registry of patients with acute respiratory distress syndrome. Physiology and outcome. Am Rev Respir Dis. 1992;146(2):419–26. doi: 10.1164/ajrccm/146.2.419. [DOI] [PubMed] [Google Scholar]
- 36.Rubenfeld GD, Herridge MS. Epidemiology and Outcomes of Acute Lung Injury. Chest. 2007;131:554–562. doi: 10.1378/chest.06-1976. [DOI] [PubMed] [Google Scholar]
- 37.Griese M, Potz C, Dietrich P, Westerburg B. Calcium, potassium, urea and total protein are not reliable dilutional markers of bronchoalveolar small volume-lavages in ventilated preterm human neonates. Eur J Med Res. 1996;1(12):565–70. [PubMed] [Google Scholar]
- 38.Dargaville PA, South M, Vervaart P, McDougall PN. Validity of markers of dilution in small volume lung lavage. Am J Respir Crit Care Med. 1999;160(3):778–84. doi: 10.1164/ajrccm.160.3.9811049. [DOI] [PubMed] [Google Scholar]
- 39.Muhlebach MS, Stewart PW, Leigh MW, Noah TL. Quantitation of inflammatory responses to bacteria in young cystic fibrosis and control patients. Am J Respir Crit Care Med. 1999;160(1):186–91. doi: 10.1164/ajrccm.160.1.9808096. [DOI] [PubMed] [Google Scholar]
- 40.Muhlebach MS, Noah TL. Endotoxin activity and inflammatory markers in the airways of young patients with cystic fibrosis. Am J Respir Crit Care Med. 2002;165(7):911–5. doi: 10.1164/ajrccm.165.7.2107114. [DOI] [PubMed] [Google Scholar]
- 41.Foell D, Ichida F, Vogl T, Yu X, Chen R, Miyawaki T, et al. S100A12 (ENRAGE) in monitoring Kawasaki disease. Lancet. 2003;361(9365):1270–2. doi: 10.1016/S0140-6736(03)12986-8. [DOI] [PubMed] [Google Scholar]
- 42.Foell D, Kane D, Bresnihan B, Vogl T, Nacken W, Sorg C, et al. Expression of the pro-inflammatory protein S100A12 (EN-RAGE) in rheumatoid and psoriatic arthritis. Rheumatology (Oxford) 2003;42(11):1383–9. doi: 10.1093/rheumatology/keg385. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.