Abstract
Extracellular matrix protein adhesin A (EmaA) is a 202-kDa nonfimbrial adhesin, which mediates the adhesion of the oral pathogen Aggregatibacter actinomycetemcomitans to collagen. EmaA oligomers form surface antenna-like protrusions consisting of a long helical rod with an ellipsoidal ending. The functional analysis of in-frame emaA deletion mutants has located the collagen binding activity to the amino terminus of the protein corresponding to amino acids 70 to 386. The level of collagen binding of this deletion mutant was comparable to the emaA mutant strain. Transmission electron microscopy studies indicate that the first 330 amino acids of the mature protein form the ellipsoidal ending of the EmaA protrusions, where the activity resides. Amino acid substitution analysis within this sequence has identified a critical amino acid, which is essential for the formation of the ellipsoidal ending and for collagen binding activity.
The interaction with extracellular matrix (ECM) proteins of the host is an established virulence determinant in many bacterial pathogens (13). Bacteria express several surface proteinaceous structures for adhesion to ECM proteins. These structures are categorized as fimbrial and nonfimbrial adhesins and can be visualized in negatively stained images of bacteria by transmission electron microscopy (TEM) (12, 32, 40). Fimbrial adhesins are typically long structures with lengths of 0.3 to 1 μm (5) composed of multiple copies of homo- or heteropolymeric subunits (14). Nonfimbrial adhesins form smaller structures, up to 0.3 μm in length, which consist of a single protein monomer, or small homo-oligomers anchored to the surface of the bacteria (12, 13, 29).
Homo-oligomeric adhesins compose a subclass of the type V autotransporter secretion system, which include types Va, Vb, and Vc. Autotransporters are defined as proteins containing all of the molecular information required for translocation to the surface of the bacterium (15). The modular structure of an autotransporter protein is composed of three domains: an amino-terminal signal sequence, a passenger domain, and a carboxyl-terminal translocation unit (27). The signal sequence targets the protein to the inner membrane for further export into the periplasm via the sec-dependent system, followed by the cleavage of the signal peptide (11, 41). The translocation unit in the periplasm inserts into the outer membrane to form an integral membrane β-barrel pore and facilitates translocation of the passenger domain through the outer membrane (11, 27). In the type Vc class of autotransporters, a minimum of three monomers of the protein are incorporated into a quaternary structure in which the carboxyl termini autoaggregate to form a transmembrane pore for the translocation of the trimeric passenger domain (30, 36). The passenger domain displays the diverse functions of the autotransporters (27). Once on the surface, the passenger domain of the protein can remain with the translocation unit, be cleaved and released, or be cleaved but still remain in contact with the bacterial surface via noncovalent interactions (19, 20, 27, 35).
The oligomeric coiled-coil adhesin (Oca) proteins are classified as a type Vc secretion system. These proteins possess common structural characteristics, which include: a highly conserved neck domain, a stalk domain that varies in length with a high probability of coiled-coil formation, and a translocation unit with a coiled-coil segment, followed by four amphipathic transmembrane β-strands (4, 30). Cysteine residues are absent in the mature polypeptide but can be found in the signal sequence (30). Stable oligomer formation is important for the correct folding of these proteins to produce a functionally active structure (3). The monomer is not sufficient to form the active moiety. The members of this family also share functional characteristics, such as binding to epithelial cells and ECM proteins (e.g., collagen and fibronectin). YadA of Yersinia enterocolitica (30), UspA1 and UspA2 of Moraxella catarrhalis (1, 2, 18), Hia of Haemophilus influenzae (35), Vomps of Bartonella quintana (43), BadA of Bartonella henselae (29), and XadA of Xanthomonas oryzae (28) belong to the type Vc autotransporter class.
We have identified a high-molecular-mass protein (202 kDa, 1,965 amino acids), extracellular matrix adhesin A (EmaA), which is critical for the interaction of the oral pathogen Aggregatibacter (Actinobacillus) actinomycetemcomitans with collagen (21). EmaA is one of the largest members of the Oca family, compared to the YadA monomer of 44 kDa and the Hia monomer of 114 kDa (12, 17, 35). Three or four monomers of EmaA form antenna-like protrusions on the bacterial surface, which are composed of a long rod that terminates in an ellipsoidal structure with axes of approximately 3 by 5 nm (32). The long rod has a diameter of 4.1 nm and a minimum length of 150 nm (32). The EmaA structures are much larger than the YadA structures, which have an overall length of 23 nm and a globular ending of ∼5 nm (12).
In the present study, we have taken a molecular and structural approach to determine the region of the EmaA molecule required for collagen binding. In-frame deletion studies have located the collagen binding activity to amino acids 70 to 386, which correspond to the ellipsoidal head domain, as visualized by TEM. Within this sequence, we have identified a critical amino acid for the formation of the ellipsoidal structure and collagen binding.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in the present study are listed in Table 1. All A. actinomycetemcomitans strains were grown statically in Trypticase soy broth supplemented with 0.6% yeast extract (TSBYE; Becton Dickerson, Sparks, MD) in a humidified, 10% CO2 atmosphere at 37°C. The emaA mutant strain was grown as described above in the presence of 50 μg of spectinomycin/ml. Escherichia coli DH10B cells were grown in Luria-Bertani broth (USB Co., Cleveland, OH) with shaking at 37°C.
TABLE 1.
Strains and plasmids
| Strain or plasmid | Description | Source or reference |
|---|---|---|
| Strains | ||
| A. actinomycetemcomitans | ||
| Wild-type VT1169 | Spontaneous rifampin- and nalidixic acid-resistant strain of SUNY465 | 23 |
| emaA mutant | Insertion of an antibiotic cassette coding for resistance to spectinomycin into a unique HindIII site within the emaA sequence | 21 |
| E. coli DH10B | F−endA1 recA1 galU galK deoR nupG rpsL ΔlacX74 φ80lacZΔM15 araD139 Δ(ara leu)7697 mcrA Δ(mrr-hsdRMS-mcrBC) λ− | 9 |
| Plasmids | ||
| pKM1 | Derived from pDMG4 by replacing the spectinomycin adenyltransferase gene (aad9) with the aminoglycoside phosphotransferase gene (kan) | 32 |
| pKM9 | emaA sequence, including 500 bp upstream of the start methionine cloned into pKM1 | 32 |
| pKMSmaI208 | pKM9 with a novel SmaI site introduced 208 bp downstream of the start codon of emaA | This study |
| pKMSmaI433 | pKM9 with a novel SmaI site introduced 433 bp downstream of the start codon of emaA | This study |
| pKMSmaI556 | pKM9 with a novel SmaI site introduced 556 bp downstream of the start codon of emaA | This study |
| pKMSmaI619 | pKM9 with a novel SmaI site introduced 619 bp downstream of the start codon of emaA | This study |
| pKMSmaI1159 | pKM9 with a novel SmaI site introduced 1,159 bp downstream of the start codon of emaA | This study |
| pKMΔ70-386 | In-frame deletion of bp 208 to 1158 of the coding sequence of the emaA gene engineered using plasmids pKMSmaI208 and pKMSmaI1159 | This study |
| pKMΔ70-206 | In-frame deletion of bp 208 to 618 of the coding sequence of the emaA gene engineered using plasmids pKMSmaI208 and pKMSmaI619 | This study |
| pKMΔ207-386 | In-frame deletion of bp 619 to 1158 of the coding sequence of the emaA gene engineered using plasmids pKMSmaI619 and pKMSmaI1159 | This study |
| pKMΔ70-144 | In-frame deletion of bp 208 to 432 of the coding sequence of the emaA gene engineered using plasmids pKMSmaI208 and pKMSmaI433 | This study |
| pKMΔ145-185 | In-frame deletion of bp 433 to 555 of the coding sequence of the emaA gene engineered using plasmids pKMSmaI433 and pKMSmaI556 | This study |
| pKMΔ186-206 | In-frame deletion of bp 556 to 618 of the coding sequence of the emaA gene engineered using plasmids pKMSmaI556 and pKMSmaI619 | This study |
| pKM I163E | I163 was substituted with E163 on pKM9 | This study |
| pKM I165E | I165 was substituted with E165 on pKM9 | This study |
| pKM A164D | A164 was substituted with D164 on pKM9 | This study |
| pKM I163E-A164D | I163 and A164 were substituted with E163 and D164 on pKM9 | This study |
| pKM I163E-A164D-I165E | I163, A164, and I165 were substituted with E163, D164, and E165 on pKM9 | This study |
| pKM I163A-I165A | I163 and I165 were substituted with A163 and A165 on pKM9 | This study |
| pKM G162A | G162 was substituted with A162 on pKM9 | This study |
| pKM G162S | G162 was substituted with S162 on pKM9 | This study |
The shuttle plasmid pKM1 was used to construct pKM9, which contains the full-length emaA and 500 bp upstream of the start methionine (accession number AY344064), as previously described (32). Bacteria containing pKM9 and derivatives were propagated in TSBYE in the presence of either 35 or 50 μg of kanamycin/ml. All emaA mutations generated in the present study were based on pKM9.
Construction of in-frame deletion mutants of emaA.
In-frame deletion mutants were generated by using a strategy based on the QuikChange XL site-directed mutagenesis kit (Stratagene, La Jolla, CA). Unique SmaI restriction sites were introduced within the specific sequences of emaA (Table 2). The base pairs required for mutagenesis were minimized to make the site-directed mutagenesis easier to perform; therefore, we chose sequences that were similar to the SmaI cleavage site (CCCGGG). The modified plasmids were digested with SmaI, which generated two fragments, due to the presence of a unique SmaI site on the plasmid backbone of pKM1, upstream of the emaA gene, and separated on 0.8% agarose (Bio-Rad Laboratories, Hercules, CA) gels. The fragment containing the 5′ of the emaA gene, including the upstream promoter region and signal sequence, was excised from the gel and purified by using QIAquick gel extraction kit (Qiagen, Hilden, Germany). In a separate reaction, another construct engineered with a unique SmaI site, to generate the remainder of the emaA molecule and the plasmid backbone, was restricted with the enzyme and gel purified. The SmaI fragment containing the 5′ end of the emaA gene and the SmaI fragment containing the 3′ end of the emaA gene and the plasmid backbone were ligated and transformed into electrocompetent E. coli DH10B cells. Transformants were selected on LB agar plates containing 50 μg of kanamycin/ml. The plasmids containing the truncated emaA were purified by using a QIAprep spin miniprep kit (Qiagen), and the in-frame deletion was confirmed by sequencing using an ABI 3130×1 genetic analyzer (Applied Biosystems, Foster City, CA) in the DNA Analysis Core Facility, Vermont Cancer Center, at the University of Vermont.
TABLE 2.
Oligonucleotides used for the generation of SmaI sites for deletion analysis of EmaA
| Oligonucleotide | Sequence (5′-3′)a | Positionsb |
|---|---|---|
| SmaI208f | AACAATTCTGTGAAACCCGGGGGGGCAGAAGCCTCC | 832-867 |
| SmaI208r | GGAGGCTTCTGCCCCCCCGGGTTTCACAGAATTGTT | 832-867 |
| SmaI433f | TTATTCTAAAGCTACCCCCGGGCTTGCCATGGCCTTAGG | 1056-1094 |
| SmaI433r | CCTAAGGCCATGGCAAGCCCGGGGGTAGCTTTAGAATAA | 1056-1094 |
| SmaI556f | GTCAATCTGCAGCACCCGGGGATTATTCTACTG | 1181-1213 |
| SmaI556r | CAGTAGAATAATCCCCGGGTGCTGCAGATTGAC | 1181-1231 |
| SmaI619f | TCAATCAAGCTTCGCACCCGGGGCTTCTGCTAC | 1242-1274 |
| SmaI619r | GTAGCAGAAGCCCCGGGTGCGAAGCTTGATTGA | 1242-1274 |
| SmaI1159f | CATTGTCAACAGTGTCGATCCCGGGCAATCTAAATTTACTG | 1779-1819 |
| SmaI1159r | CAGTAAATTTAGATTGCCCGGGATCGACACTGTTGACAATG | 1779-1819 |
Generated SmaI sites are indicated in boldface.
That is, the positions of the oligonucleotides in the emaA sequence (accession number AY344064).
Substitution mutations were generated by using a QuikChange XL site-directed mutagenesis kit according to the manufacturer's protocols with the primers listed in Table 3. The plasmids were purified by using a QIAprep spin miniprep kit (Qiagen), and substitutions were confirmed by DNA sequencing.
TABLE 3.
Oligonucleotides used to generate substitution mutations in the DNA sequence coding for amino acids 162-GIAIG-166
| Oligonucleotide | Sequence (5′-3′)a | Positionsb |
|---|---|---|
| I163Ef | GAGCAGAAAAAAATGGCGGTGAGGCAATCGGTATAGCTTCCAG | 1106-1148 |
| I163Er | CTGGAAGCTATACCGATTGCCTCACCGCCATTTTTTTCTGCTC | 1106-1148 |
| I165Ef | GAAAAAAATGGCGGTATTGCAGAGGGTATAGCTTCCAGATCATCAG | 1111-1156 |
| I165Er | CTGATGATCTGGAAGCTATACCCTCTGCAATACCGCCATTTTTTTC | 1111-1156 |
| A164Df | GAGCAGAAAAAAATGGCGGTATTGATATCGGTATAGCTTCCAG | 1106-1148 |
| A164Dr | CTGGAAGCTATACCGATATCAATACCGCCATTTTTTTCTGCTC | 1106-1148 |
| I163E-A164Df | GCAGAAAAAAATGGCGGTGAGGACATCGGTATAGCTTCCA | 1108-1147 |
| I163E-A164Dr | TGGAAGCTATACCGATGTCCTCACCGCCATTTTTTTCTGC | 1108-1147 |
| I163E-A164D-I165Ef | CAGAAAAAAATGGCGGTGAGGACGAGGGTATAGCTTCCAGATC | 1109-1153 |
| I163E-A164D-I165Er | GATCTGGAAGCTATACCCTCGTCCTCACCGCCATTTTTTTCTG | 1109-1153 |
| I163A-I165Af | AGAAAAAAATGGCGGTGCTGCAGCCGGTATAGCTTCCA | 1110-1147 |
| I163A-I165Ar | TGGAAGCTATACCGGCTGCAGCACCGCCATTTTTTTCT | 1110-1147 |
| G162Af | GCAGAAAAAAATGGCGCTATTGCAATCGGTATAGCTTC | 1108-1145 |
| G162Ar | GAAGCTATACCGATTGCAATAGCGCCATTTTTTTCTGC | 1108-1145 |
| G162Sf | TTTCCAGAGCAGAAAAAAATGGCAGTATTGCAATCGGTATAG | 1100-1141 |
| G162Sr | CTATACCGATTGCAATACTGCCATTTTTTTCTGCTCTGGAAA | 1100-1141 |
Substituted codons are indicated in boldface.
That is, the positions of the oligonucleotides in emaA sequence (accession number AY344064).
Plasmids containing the modified emaA sequences were transformed into the emaA mutant strain of A. actinomycetemcomitans by electroporation as described previously (34). Transformants were selected on TSBYE plates containing 50 μg of kanamycin/ml. A 2-kb PCR product covering the mutation sequences was amplified by colony PCR and sequenced to confirm the transformants, using the forward primer 5′-CCCTTTCTACCACTACAGATATACC-3′ and the reverse primer 5′-GACTGCTAAATTCTTTCCTGCC-3′. Transformation of the emaA mutant with the replicating plasmid containing emaA and some of the mutant constructs resulted in an increase of the doubling time of the bacterium. To synchronize the cultures for RNA isolation, membrane preparation, and collagen binding assays, dilutions of the overnight bacterial cultures were varied to obtain comparable absorbance values.
Determination of collagen binding activity.
One bacterial colony was inoculated in 6 ml of TSBYE broth supplemented with 35 μg of kanamycin/ml for 16 h. Cultures were subsequently diluted into 10 ml of fresh TSBYE broth containing 35 μg of kanamycin/ml and incubated an additional 2 to 3 h until mid-log phase (optical density at 495 nm [OD495] = 0.2 to 0.4).
The binding of A. actinomycetemcomitans to human type V collagen (Sigma Chemical Co., St. Louis, MO) was detected by using a solid-phase binding assay (23). Type V collagen dissolved in 0.5 N acetic acid (Mallinckrodt Baker, Phillipsburg, NJ) was diluted to 10 μg/ml in sodium carbonate buffer (16 mM sodium carbonate [Sigma Chemical Co.] and 34 mM sodium bicarbonate [Mallinckrodt Baker]; pH 9.6), and 1 μg was added to wells of 96-well microtiter plates. Plates were incubated overnight at 4°C, the protein solution was removed, and the wells were washed with phosphate-buffered saline (PBS; 10 mM sodium phosphate [Fisher Scientific, Waltham, MA], 150 mM NaCl [Sigma Chemical Co.]; pH 7.4). The wells were incubated with a 0.5% solution of bovine serum albumin-PBS for 1 h to block any exposed plastic binding sites. Mid-log-phase bacteria (4 × 107 CFU) were added to the wells, and the plates were incubated for 1 h at 37°C in an atmosphere containing 10% CO2. Subsequently, the bacterial suspensions were removed, and the wells were washed with PBS. Bound bacteria were detected by using a purified immunoglobulin fraction of A. actinomycetemcomitans SUNY 465 polyclonal antiserum (22). The immune complexes were detected by using horseradish peroxidase-conjugated goat anti-rabbit immunoglobulin, and the enzymatic activity was determined by incubation with hydrogen peroxide in the presence of o-phenylenediamine (Sigma Chemical Co.). The reactions were stopped by the addition of 4 M sulfuric acid after 2.5 min, and the color development was quantified by measuring the absorbance at 490 nm. Each strain was tested in triplicate. The OD490 value of the negative control (emaA mutant strain transformed by pKM1) was subtracted from the mean OD490 value of the experimental sample and the data was represented as the percentage of binding of the complemented strain to collagen: (OD490(target) − OD490(pKM1))/(OD490(pKM9) − OD490(pKM1)) × 100%. The final results are presented as the mean ± the standard deviation of three independent experiments.
TEM.
Bacteria were grown as described above but with 50 μg of kanamycin/ml in TSBYE. A total of 5 × 109 CFU mid-logarithmic-phase A. actinomycetemcomitans were centrifuged at 4,500 × g at 4°C for 5 min and then resuspended in 100 μl of PBS on ice. Then, 5-μl portions of the bacterial suspensions were applied to carbon-coated 400-mesh copper grids. The grids were rinsed with PBS and negatively stained by washing them with a stream (a few drops) of 2% phosphotungstic acid (pH 7; Ted Pella, Redding, CA). The last drop of stain was left on the grid for 30 s. The excess liquid was wicked off, and the grids were fast air dried. Grids were observed on a Tecnai 12 electron microscope (FEI, Aachem, The Netherlands) equipped with an LaB6 cathode (Kimball International, Inc., Jasper, IN) running at 100 kV. Images were collected on a 14-μm 2,048 × 2,048-pixel charge-coupled device camera (TVIPS, Gauting, Germany) at 67,000 nominal magnification (32).
Isolation of membrane proteins.
Bacterial membranes were isolated according to the protocol of Nikaido (24). Briefly, 250 ml of mid-logarithmic-phase bacteria (OD495 = 0.2 to 0.4) was collected by centrifugation at 5,680 × g for 15 min and washed with PBS. The pellet was resuspended in 3 ml of 10 mM HEPES (pH 7.4) (USB) containing 1 mM phenylmethylsulfonyl fluoride (USB) and 120 μl (1×) of Complete protease inhibitor cocktail (Roche, Basel, Switzerland). Bacteria were disrupted by using a French press with a minicell at 18,000 lb/in2 (124,200 kPa). The intact bacteria and debris were collected by centrifugation at 7,650 × g for 10 min and then discarded. The supernatant was carefully removed and centrifuged at 100,000 × g for 30 min. The pellet was resuspended in 10 mM HEPES (pH 7.4) and centrifuged at 100,000 × g for 30 min. The membrane pellet was resuspended in 10 mM HEPES (pH 7.4).
Immunodot blot analysis.
Isolated bacterial membranes were solubilized in 2% sodium dodecyl sulfate. The protein concentration of the membrane samples was determined by using a BCA protein assay kit (Pierce, Rockford, IL) according to the manufacturer's protocol. The EmaA monomer is not consistently visualized in the 5 to 15% polyacrylamide-sodium dodecyl sulfate separating gel (data not shown). Most of the immunoreactive material remains associated within the 3% stacking gel. Therefore, we were unable to use immunoblots to determine the nature of the protein products generated in the present study. For quantification by immunodot blot, equal amounts of membrane proteins were denatured by boiling for 10 min and immobilized on nitrocellulose membranes (pore size, 0.2 μm; Schleicher & Schuell, Keene, NH) using a Bio-Dot microfiltration apparatus (Bio-Rad Laboratories). The nitrocellulose membrane was incubated with blocking solution (5% skim milk; Bio-Rad Laboratories) for 30 min and washed twice with PBST (PBS containing 0.1% Tween 20 [Fisher Scientific]). Then, 10 μg/ml of a purified monoclonal antibody (39) was applied to the membrane, followed by incubation at 4°C for 16 h. The nitrocellulose was washed with PBST three times, and the membrane was incubated with a dilution of horseradish peroxidase-conjugated goat anti-mouse immunoglobulins (Jackson Laboratories, Bar Harbor, ME) in PBST for 1 h. After three additional washes with PBST, the membrane was incubated with ECL substrate for 5 min (Amersham Life Sciences, Little Chalfont, Buckinghamshire, United Kingdom) and exposed to photographic film (XAR-5; Eastman Kodak, Rochester, NY). The intensities of the dots were quantitated by using ImageJ software.
Quantitative real-time PCR.
Total RNA was isolated from 5 × 108 CFU of mid-logarithmic-phase bacteria by using an RNeasy minikit (Qiagen) according to the manufacturer's protocol and treated with amplification grade DNase I (Invitrogen, Carlsbad, CA) to degrade any contaminating DNA. RNA was quantified spectrophotometrically, and equal amounts of RNA from the mutant and the full-length complemented strain were used as a template for reverse transcription (RT). cDNA was synthesized by using the SuperScript III first-strand synthesis system for RT-PCR (Invitrogen) according to the manufacturer's protocol.
emaA gene expression was quantitated by quantitative RT-PCR using an ABI Prism 7700 sequence detection system (Applied Biosystems) in the DNA Analysis Core Facility, Vermont Cancer Center, at the University of Vermont. The primers and the TaqMan fluorogenic probes (Sigma Chemical Co.) used are as follows: 16SrRNA (strain HK1651, oral pathogen sequence database of Los Alamos National Laboratory), sense primer 5′-GAACCTTACCTACTCTTGACATCC-3′, antisense primer 5′-GGACTTAACCCAACATTTCACAAC-3′ and probe 5′-6-FAM-CTGACGACAGCCATGCAGCACCTG-BHQ-1-3′ for 16SrRNA gene, sense primer 5′-GGTCAATAACGACGGTGTTACG-3′, antisense primer 5′-TTCCCTTTCGCAACGTTAGC-3′ and probe 5′-6-FAM-CGGTCCAAGCATGACAAGCCACGG-BHQ-1-3′ for emaA. The expression of emaA was normalized to the endogenous 16SrRNA gene for variation in RNA quantity and quality.
The relative quantification of target gene expression was done by using the comparative cycle threshold (CT) method (Applied Biosystems, User Bulletin No. 2: Relative Quantification of Gene Expression, 1997). The CT is defined as the cycle number at which the fluorescent signal generated by cleavage of the dual-labeled probe is first detectable. The relative target expression was given by the formula: 2−ΔΔCT, where ΔΔCT = ΔCT(target sample) − ΔCT(calibrator sample) with ΔCT = CTemaA − CT 16SrRNA. The emaA mutant transformed with pKM9 was chosen as the calibrator. The results are presented as the mean ± the standard deviation of three independent experiments.
RESULTS
All of the emaA constructs generated in this study contain the endogenous promoter sequence and encode the signal sequence, the coiled-coil domain, and the membrane anchor domain of EmaA. All plasmids were transformed into an emaA mutant strain as described previously (21). This strain does not form EmaA surface-associated structures and displays a defect in collagen binding (21, 32).
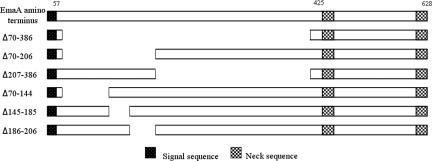
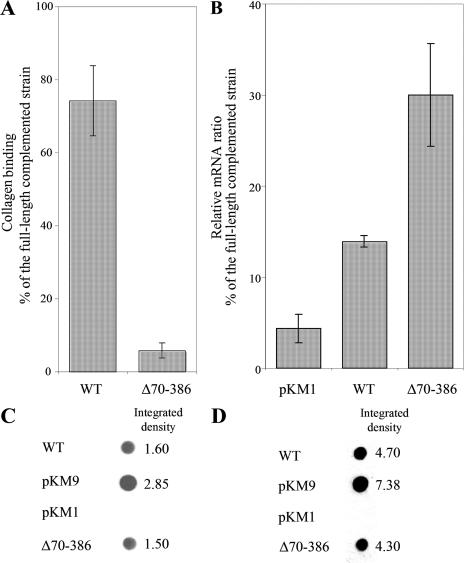
An initial study of an in-frame deletion construct of amino acids 70 to 386 (Fig. 1) indicated that this sequence was required for collagen binding (Fig. 2A). Bacteria transformed with this deletion construct did not complement the collagen binding activity of the emaA mutant compared to a strain transformed with a copy of the full-length emaA (pKM9) (Fig. 2A). Quantitative RT-PCR of these strains indicated that the transcription of emaA in the strain containing the Δ70-386 construct was ∼30% of the transcriptional activity of the strain transformed with the full-length emaA (Fig. 2B). This mRNA reduction may be due to a significant secondary structure alteration of the truncated mRNA (predicted by www.genebee.msu.su [data not shown]). However, the transcription of the Δ70-386 was significantly higher than that of the wild-type strain, which was ca. 14% of the complemented strain (Fig. 2B). Immunoblot analysis using a monoclonal antibody raised against a recombinant protein corresponding to the stalk domain (amino acids 631 to 1355) corroborated the transcriptional analysis. EmaA was present in higher concentration in both the whole-cell lysate and the membrane fraction of the complemented strain compared to the same fractions of the wild-type strain (Fig. 2C and D). In comparison, the amount of EmaA in the strain containing the Δ70-386 construct and the wild-type strain were equivalent (Fig. 2C and D). The EmaA immunoreactive protein was absent in the mutant strain containing the empty vector (Fig. 2C and D). The similarity of the expression levels of EmaA between the Δ70-386 construct and the wild-type strain indicated equivalent protein translation of the different EmaA molecules. Therefore, the collagen binding activity was addressed to amino acids 70 to 386.
FIG. 1.
Schematic diagram of the amino terminus (amino acids 1 to 628) of EmaA and the in-frame deletions of EmaA constructed in the present study.
FIG. 2.
(A) Collagen binding activity of the wild-type (WT) strain VT1169 and the emaA mutant strain complemented with pKMΔ70-386 (plasmid containing an in-frame deletion corresponding to amino acids 70 to 386) as measured by enzyme-linked immunosorbent assay (ELISA). The data are represented as the percentage of the binding of the emaA mutant strain complemented with the full-length sequence (pKM9). (B) Quantitative real-time PCR for emaA of RNA isolated from the wild type or the emaA mutant strains transformed with the empty plasmid (pKM1) or pKMΔ70-386. The results are expressed as ratios of the emaA RNA from the emaA mutant strain transformed with the full-length sequence (pKM9). (C) Immunodot blot of whole-cell lysates from 2 × 108 CFU of bacterial preparations. EmaA was detected by using a monoclonal antibody specific for amino acids 631 to 1355. The integrated density of each dot was quantified by using the software ImageJ. (D) Immunodot blot of 5 μg of membrane proteins from each strain.
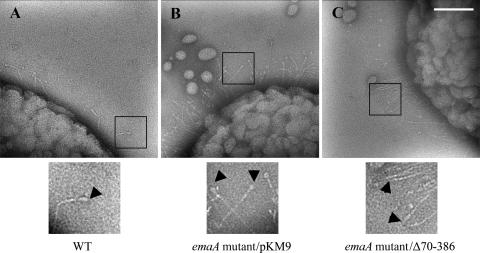
The strain transformed with the Δ70-386 plasmid was radically different in the EmaA appendages on the bacterial surface. Both the wild-type and the full-length complemented strains expressed the antenna-like protrusions (Fig. 3A and B). In the strain transformed with the Δ70-386 plasmid, rod-like structures were clearly visible on the surface of the bacterium. However, the ellipsoidal ending of the appendages observed on the wild-type strain were absent (Fig. 3C). These data indicate that amino acids 70 to 386 of the EmaA sequence form the ellipsoidal head of the antenna-like protrusions where the collagen activity resides.
FIG. 3.
Transmission electron micrographs of A. actinomycetemcomitans strains stained with 2% phosphotungstic acid (pH 7). (A) WT, wild type. (B) pKM9, emaA mutant strain complemented with the full-length sequence of emaA. (C) pKMΔ70-386, emaA mutant complemented with a plasmid containing an in-frame deletion corresponding to amino acids 70 to 386. The ellipsoidal endings (arrows) are visualized in both the wild-type strain and the emaA mutant transformed with pKM9, but absent in the emaA mutant complemented with the pKMΔ70-386 construct. The small oval-shaped particles in the micrographs corresponded to membrane vesicles secreted by both wild-type and mutant bacteria. Scale bar, 100 nm.
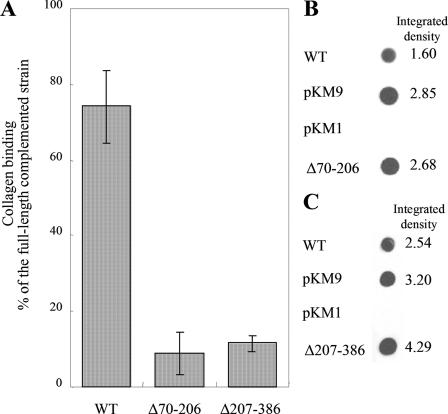
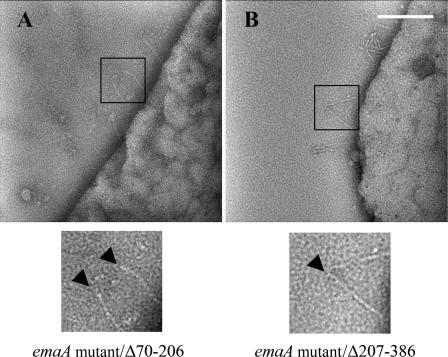
Plasmids containing emaA coding for proteins with deletions in amino acids 70 to 206 and amino acids 207 to 386 were generated to identify the minimal sequence required for binding activity (Fig. 1). Transformations of the emaA mutant with either the Δ70-206 or the Δ207-386 construct did not rescue collagen binding activity and showed a similar binding activity as the Δ70-386 construct (Fig. 4A). In addition, these strains formed similar appendages; the stalks of the appendages were present but the ellipsoidal endings were absent (Fig. 5). The relative amount of EmaA present in the whole-cell lysate of strains containing these two in trans deletion constructs was equivalent to the strain transformed with the full-length emaA sequence (Fig. 4B and C). The data suggest that the entire 70 to 386 amino acid region of the protein is required for the formation of the functional collagen binding domain.
FIG. 4.
(A) Adhesion of A. actinomycetemcomitans to type V collagen as measured by ELISA. WT, wild-type strain VT1169; pKMΔ70-206, emaA mutant complemented with a plasmid containing an in-frame deletion corresponding to amino acids 70 to 206; pKMΔ207-386, emaA mutant complemented with a plasmid containing an in-frame deletion corresponding to amino acids 207 to 386. (B) Immunodot blot of whole-cell lysate of 2 × 108 CFU from the emaA mutant strain complemented the empty plasmid (pKM1), the full-length sequence (pKM9), or pKMΔ70-206. (C) Immunodot blot of whole-cell lysate of 2 × 108 CFU from the emaA mutant strain complemented with pKM1, pKM9, or pKMΔ207-386.
FIG. 5.
Transmission electron micrographs of A. actinomycetemcomitans strains stained with 2% phosphotungstic acid (pH 7). (A) emaA mutant strain complemented with pKMΔ70-206 (a plasmid containing a deletion corresponding to amino acids 70 to 206). (B) emaA mutant strain complemented with pKMΔ207-386 (a plasmid containing a deletion corresponding to amino acids 207 to 386). The arrows indicate the ending of the EmaA appendages, showing that the ellipsoidal endings are absent in the deletion mutants. Scale bar, 100 nm.
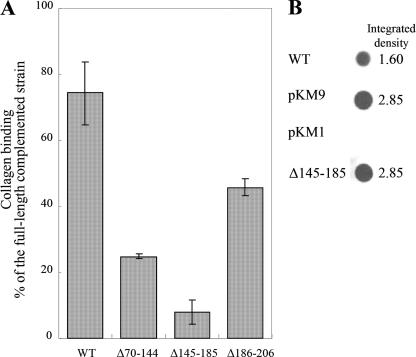
Nested deletion constructs (Δ70-144, Δ145-185, and Δ186-206) of the sequence spanning amino acids 70 to 206 were generated and analyzed for collagen binding activity to identify the critical amino acid(s) in this region of the protein. Strains transformed with plasmids containing the Δ70-144 and Δ186-206 constructs did not completely restore collagen binding (Fig. 6A). Strains containing the Δ70-144 construct rescued ca. 25% of the collagen binding activity, and the Δ186-206 construct restored 50% binding activity compared to the strain transformed with the full-length emaA (which is significantly higher than that of the Δ70-206 construct) (Fig. 4A and 6A). Interestingly, the strain transformed with the Δ145-185 plasmid did not rescue binding activity and displayed an activity similar to that of the strains containing the Δ70-206 and the Δ70-386 plasmids (Fig. 4A and 6A). Moreover, the Δ145-185 construct containing strain produced equivalent levels of protein in the whole-cell lysate compared to the complemented strain and 180% of the wild-type strain (Fig. 6B).
FIG. 6.
(A) Collagen binding activity of the wild-type (WT) strain VT1169 or the emaA mutant strain complemented with pKMΔ70-144, pKMΔ145-185, or pKMΔ186-206 (plasmids containing an in-frame deletion corresponding to amino acids 70 to 144, 145 to 185, or 186 to 206) measured by ELISA. The data are represented as a percentage binding relative to the emaA mutant strain complemented with the full-length sequence (pKM9). (B) Immunodot blot of the whole-cell lysates from 2 × 108 CFU of the wild-type (WT) strain VT1169 or the emaA mutant strain complemented with the empty plasmid (pKM1), the full-length sequence (pKM9), or pKMΔ145-185 (a plasmid containing a deletion corresponding to amino acids 145 to 185).
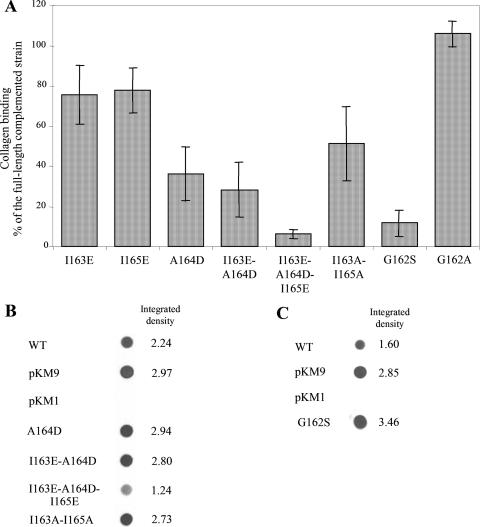
Sequence analysis of amino acids 70 to 386 has indicated the presence of repetitive pentameric sequences. The pentamers begin with either a serine or glycine, followed by three hydrophobic amino acids, and end with a conserved glycine residue. The repetitive nature of these pentamers suggests an important functional or structural role on the active collagen binding domain of EmaA. A pentameric sequence (162-GIAIG-166) is present within amino acids 145 to 185. The importance of these amino acids was tested by generating amino acid substitution mutations by site-directed mutagenesis. The hydrophobic amino acids 163-IAI-165 were individually substituted for charged amino acids with either glutamic acid (I163E and I165E) or aspartic acid (A164D). Substitution of either I163 or I165 with glutamic acid had little effect on the collagen binding activity compared to the complemented strain (Fig. 7A). This was in contrast to the substitution of amino acid 164 (A164D) in the prototypic sequence. This substitution reduced the binding activity of the strain to ∼60% compared to the full-length complemented strain (Fig. 7A). A double mutation of I163E-A164D showed similar reduction on collagen binding as the single substitution A164D (Fig. 7A). These strains (A164D and I163E-A164D) expressed equivalent concentrations of EmaA protein compared to the full-length complemented strain (Fig. 7B). Substitution of all three hydrophobic amino acids with charged amino acids resulted in a strain with a binding activity similar to the strain containing the Δ70-206 construct (Fig. 7A). Although the expression of EmaA was reduced ∼50% in this strain (Fig. 7B), the reduction in protein expression does not entirely account for the diminution in collagen binding activity. Individual substitution of I163 or I165 with alanine had no effect on collagen binding (data not shown). However, substituting both I163 and I165 with alanine resulted in ∼50% decrease in binding activity (Fig. 7A) without affecting EmaA expression (Fig. 7B). These studies suggest that the hydrophobicity of these amino acid side chains is required for the formation of the collagen binding domain of EmaA.
FIG. 7.
(A) Collagen binding activity of the emaA mutant strain complemented with the full-length sequence (pKM9), pKMI163E, pKMI165E, pKMA164D, pKMI163E-A164D, pKMI163E-A164D-I165E, pKMI163A-I165A, pKMG162S, or pKMG162A (plasmids containing substitution mutations corresponding to amino acids 162-GIAIG-166) as measured by ELISA. The data are represented as a percentage binding relative to the emaA mutant strain complemented with the full-length sequence (pKM9). (B) Immunodot blot of the whole-cell lysate of 2 × 108 CFU of the wild-type (WT) strain VT1169 or the emaA mutant strain complemented with the full-length sequence (pKM9), empty plasmid (pKM1), and plasmids corresponding to substitutions of amino acids in the 162-GIAIG-166 sequence. (C) Immunodot blot of the whole-cell lysate of 2 × 108 CFU of the wild-type (WT) strain VT1169 or the emaA mutant strain complemented with the full-length sequence (pKM9), empty plasmid (pKM1), or pKMG162S (a plasmid containing substitution of glycine with serine at position 162 the EmaA sequence).
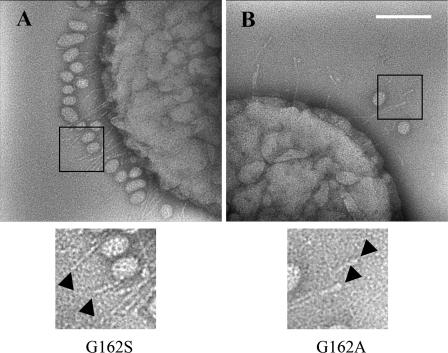
The importance of the first amino acid of this sequence, G162, was analyzed by substituting glycine for either serine or alanine. In 70% of these pentameric sequences, serine is the first amino acid in these sequences. Interestingly, replacement of G162 with serine abolished the binding activity of this strain compared to the strain containing the native sequence (Fig. 7A) but not synthesis of the EmaA protein (Fig. 7C). In contrast, the substitution of the G162 to A162 showed a binding activity similar to that of the full-length complemented strain (Fig. 7A). The loss of the restoration of collagen binding by a single amino acid substitution (G162S) was reminiscent of the strain containing the deletion of the entire head domain (Δ70-386). Visualization of the G162S substitution strain by TEM revealed antenna-like appendages on the surface of bacteria. However, the terminal ellipsoidal endings were not present (Fig. 8A). This was in stark contrast to the G162A substitution (Fig. 8B), which produced appendages similar to those visualized on the surface of the wild-type strain (Fig. 3A). These data suggest that G162 is a critical amino acid for the structural integrity of the ellipsoidal ending of EmaA and collagen binding.
FIG. 8.
Transmission electron micrographs of A. actinomycetemcomitans strains stained with 2% phosphotungstic acid (pH 7). (A) emaA mutant strain complemented with plasmid pKMG162S. (B) emaA mutant strain complemented with plasmid pKMG162A. The arrows indicate the ending of the EmaA appendages, showing that the ellipsoidal endings are present in the pKMG162A complemented strain but absent in the pKMG162S complemented strain. The small oval-shaped particles in the micrographs corresponded to membrane vesicles secreted by both wild-type and mutant bacteria. Scale bar, 100 nm.
DISCUSSION
A. actinomycetemcomitans, a gram-negative, facultative anaerobic bacterium, is widely distributed across human populations and is strongly associated with periodontal diseases (10, 33). This bacterium is found associated with or internalized in buccal and gingival epithelial cells, as well as connective tissue in the oral cavity (7, 23, 31). Colonization of the connective tissue may allow this opportunistic pathogen to cause other serious systemic infections, including endocarditis, pulmonary infections, and osteitis (26).
EmaA, a high-molecular-weight outer membrane protein, forms surface appendages associated with the binding of A. actinomycetemcomitans to collagen (32). The structures are assumed to arise from the assembly of three to four monomers of EmaA. Nucleation, by integration into the membrane of the hydrophobic carboxyl termini, forms a membrane pore, as suggested for other type Vc autotransporters (16, 30, 36). The remaining regions of the protein are threaded through the pore, by an as-yet-undefined mechanism, and the active adhesin is presented on the surface of the bacterium (11).
Strains of A. actinomycetemcomitans have been identified containing a 279-amino-acid in-frame deletion in the rod-like stalk domain of EmaA. This deletion does not affect collagen binding (39). Therefore, the collagen binding activity was predicted to be associated with the head domain of the protein. In the present study, we localized the active moiety of the protein to the amino terminus. This region of the monomers assembles to form the ellipsoidal ending of the antenna-like protrusions as visualized by TEM. The absence of the ellipsoidal ending and the presence of the rod-like structure on the surface of the bacterium in the strain containing the Δ70-386 suggest that this protein sequence is not required for oligomerization and translocation of the protein across the outer membrane. The formation of oligomers and protein translocation in a Y. enterocolitica strain expressing in-frame truncations in the amino terminus of YadA also strengthen the interpretation of our data (30).
One commonality in EmaA and YadA is the presence of repetitive sequences in the amino terminus. A conserved consensus sequence (NSVAIGXXS) is found in the YadA amino terminus, as well as in other Oca family members such as Vomps of Bartonella quintana and BadA of Bartonella henselae (29, 37, 43). These conserved sequences are not present in Hia (17). In YadA the eight consensus sequences are found on average every 14 amino acids and play a structural role in the formation of the adhesin (25). In contrast, a minimum of 10 homologous sequences are present in EmaA, which are not evenly distributed throughout the binding domain (Fig. 9). However, we cannot exclude the possibility that additional potential pentameric motifs are contained in amino acid sequences 139 to 158 and 272 to 355. The ten pentameric sequences present in EmaA are critical for the integrity of the ellipsoidal structure, possibly via oligomer formation.
FIG. 9.
Alignment of consensus pentameric sequences (indicated in boldface letters) of the EmaA amino-terminal sequence encompassing amino acids 70 to 386.
A subtle change in the ellipsoidal structure due to the deletion of the pentameric sequences 116-GIAIG-120 and 134-GIALG-139 or 190-STAIG-194 may account for the partial binding activity associated with these deletions. In contrast, the strain containing a deletion spanning the pentameric sequence (162-GIAIG-166) was devoid of any binding activity. The loss of activity indicates a critical role for this sequence in the formation of the ellipsoidal structure which is required for activity. Based on our alignment of the 10 pentameric sequences, the 162-GIAIG-166 pentamer differs in its nearest neighbor location compared to other pentameric sequences in the linear sequence. The 162-GIAIG-166 sequence is separated by 23 amino acids from the neighboring pentameric sequences (134-GIALG-138 and 190-STAIG-194) (Fig. 9). This differs from the 9 to 13 amino acids that separate the other pentameric sequences in this region of the binding domain. The additional number of intervening amino acids may be important for positioning or stabilizing the presentation of this critical sequence.
The pentamers are involved in stabilization of the YadA trimer and are located in the hydrophobic core of the molecule (25). In the YadA sequence (SVAIG), substitution of the middle three residues (VAI) with charged amino acids prevented collagen binding by breaking apart the essential trimer (25, 37). In the EmaA sequence (162-GIAIG-166), substitution of the corresponding hydrophobic amino acids (IAI) similarly destroyed the collage binding potential of EmaA (as indicated by the failure of the construct to complement the emaA mutant strain). Like the YadA residues, the hydrophobic character of the motif was fundamental to collagen binding. Interestingly, substitution of the individual residues had limited affect on the binding activity. However, the internal alanine residue appears to play a more important role in oligomerization of the head domain.
In the present study, G162 was found to be essential for formation of the ellipsoidal ending and thus collagen binding activity. The substitution of G162S eliminated the collagen binding potential of EmaA (Fig. 7), although serine is a common first amino acid of the majority the pentameric sequences of EmaA. Associated with the loss of activity was the absence of the ellipsoidal ending of the structure. The lack of the ellipsoidal endings at the tip of the EmaA appendages may be due to a higher degree of disorder in the structure and not due to proteolysis. Substitution of the hydrogen of the α carbon of glycine with a methyl group (alanine) had no effect on binding, and the appendages maintain the ellipsoidal endings. Therefore, we can conclude that the size and polarity of the side chain of G162 are crucial for maintaining the quaternary structure and activity of the ellipsoidal endings of the EmaA protrusions.
The sequences of EmaA and YadA show certain commonalities. However, EmaA differs in many regards from its YadA counterpart. The number of amino acids (a minimum of 316) required for the formation of the EmaA ellipsoidal structure is ∼3 times greater than the number of amino acids (i.e., 111) associated with the YadA collagen binding domain. In addition, the crystal structure of YadA indicates that the eight pentameric sequences, which are evenly distributed, form the turns and hydrophobic core of the novel left-handed parallel YadA β-roll and trimer (25). The variable length of amino acids between pentamers in the EmaA sequence may form loops similar to those formed between the β-strands of the epithelial binding domains of Hia (42). The Hia structure consists of groups of β-strands separated by variable-length sequences of either random coils or α helices (42). Our data suggest that the EmaA binding domain structure includes features from both YadA and Hia. Confirmation of this hypothesis awaits higher resolution structural data.
The structural differences in the binding domains for EmaA, YadA, and Hia may reflect the disparate substrates for these adhesins. EmaA interacts with collagen, but the loss of this gene does not affect bacteria binding to epithelial or endothelial cells (data not shown). In contrast, YadA, a multifunctional adhesin, is involved in binding to collagen and epithelial cells (6, 38), whereas Hia shows specificity for epithelial cell binding (17). However, the proteins utilize similar basic structural building blocks arranged differently to confer specificity of the organism and binding partner. Similar observations have been reported for the enzymes of the glycolytic pathway, where the building block is an αβα sandwich and the substrate specificity is achieved by varying the stoichiometry of the components (8). The conservation of structures with different activities among these bacteria may reflect the different physiological niches occupied by these pathogens.
Acknowledgments
We thank Gaoyan Tang for helpful discussions.
This study was supported by NIH-NIDCR grant RO1-DE13824.
Footnotes
Published ahead of print on 29 February 2008.
REFERENCES
- 1.Aebi, C., E. R. Lafontaine, L. D. Cope, J. L. Latimer, S. L. Lumbley, G. H. McCracken, Jr., and E. J. Hansen. 1998. Phenotypic effect of isogenic uspA1 and uspA2 mutations on Moraxella catarrhalis 035E. Infect. Immun. 663113-3119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aebi, C., I. Maciver, J. L. Latimer, L. D. Cope, M. K. Stevens, S. E. Thomas, G. H. McCracken, Jr., and E. J. Hansen. 1997. A protective epitope of Moraxella catarrhalis is encoded by two different genes. Infect. Immun. 654367-4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cotter, S. E., N. K. Surana, S. Grass, and J. W. St Geme III. 2006. Trimeric autotransporters require trimerization of the passenger domain for stability and adhesive activity. J. Bacteriol. 1885400-5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cotter, S. E., N. K. Surana, and J. W. St Geme III. 2005. Trimeric autotransporters: a distinct subfamily of autotransporter proteins. Trends Microbiol. 13199-205. [DOI] [PubMed] [Google Scholar]
- 5.Duguid, J. P., I. W. Smith, G. Dempster, and P. N. Edmunds. 1955. Non-flagellar filamentous appendages (fimbriae) and haemagglutinating activity in Bacterium coli. J. Pathol. Bacteriol. 70335-348. [DOI] [PubMed] [Google Scholar]
- 6.Emody, L., J. Heesemann, H. Wolf-Watz, M. Skurnik, G. Kapperud, P. O'Toole, and T. Wadstrom. 1989. Binding to collagen by Yersinia enterocolitica and Yersinia pseudotuberculosis: evidence for YopA-mediated and chromosomally encoded mechanisms. J. Bacteriol. 1716674-6679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fives-Taylor, P., D. Meyer, and K. Mintz. 1995. Characteristics of Actinobacillus actinomycetemcomitans invasion of and adhesion to cultured epithelial cells. Adv. Dent. Res. 955-62. [DOI] [PubMed] [Google Scholar]
- 8.Fothergill-Gilmore, L. A., and P. A. Michels. 1993. Evolution of glycolysis. Prog. Biophys. Mol. Biol. 59105-235. [DOI] [PubMed] [Google Scholar]
- 9.Grant, S. G., J. Jessee, F. R. Bloom, and D. Hanahan. 1990. Differential plasmid rescue from transgenic mouse DNAs into Escherichia coli methylation-restriction mutants. Proc. Natl. Acad. Sci. USA 874645-4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haubek, D., O. K. Ennibi, L. Abdellaoui, N. Benzarti, and S. Poulsen. 2002. Attachment loss in Moroccan early onset periodontitis patients and infection with the JP2-type of Actinobacillus actinomycetemcomitans. J. Clin. Periodontol 29657-660. [DOI] [PubMed] [Google Scholar]
- 11.Henderson, I. R., F. Navarro-Garcia, and J. P. Nataro. 1998. The great escape: structure and function of the autotransporter proteins. Trends Microbiol. 6370-378. [DOI] [PubMed] [Google Scholar]
- 12.Hoiczyk, E., A. Roggenkamp, M. Reichenbecher, A. Lupas, and J. Heesemann. 2000. Structure and sequence analysis of Yersinia YadA and Moraxella UspAs reveal a novel class of adhesins. EMBO J. 195989-5999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hultgren, S. J., S. Abraham, M. Caparon, P. Falk, J. W. St Geme III, and S. Normark. 1993. Pilus and nonpilus bacterial adhesins: assembly and function in cell recognition. Cell 73887-901. [DOI] [PubMed] [Google Scholar]
- 14.Hultgren, S. J., S. Normark, and S. N. Abraham. 1991. Chaperone-assisted assembly and molecular architecture of adhesive pili. Annu. Rev. Microbiol. 45383-415. [DOI] [PubMed] [Google Scholar]
- 15.Jose, J., F. Jahnig, and T. F. Meyer. 1995. Common structural features of IgA1 protease-like outer membrane protein autotransporters. Mol. Microbiol. 18378-380. [DOI] [PubMed] [Google Scholar]
- 16.Koretke, K. K., P. Szczesny, M. Gruber, and A. N. Lupas. 2006. Model structure of the prototypical non-fimbrial adhesin YadA of Yersinia enterocolitica. J. Struct. Biol. 155154-161. [DOI] [PubMed] [Google Scholar]
- 17.Laarmann, S., D. Cutter, T. Juehne, S. J. Barenkamp, and J. W. St Geme. 2002. The Haemophilus influenzae Hia autotransporter harbours two adhesive pockets that reside in the passenger domain and recognize the same host cell receptor. Mol. Microbiol. 46731-743. [DOI] [PubMed] [Google Scholar]
- 18.Lafontaine, E. R., L. D. Cope, C. Aebi, J. L. Latimer, G. H. McCracken, Jr., and E. J. Hansen. 2000. The UspA1 protein and a second type of UspA2 protein mediate adherence of Moraxella catarrhalis to human epithelial cells in vitro. J. Bacteriol. 1821364-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leininger, E., M. Roberts, J. G. Kenimer, I. G. Charles, N. Fairweather, P. Novotny, and M. J. Brennan. 1991. Pertactin, an Arg-Gly-Asp-containing Bordetella pertussis surface protein that promotes adherence of mammalian cells. Proc. Natl. Acad. Sci. USA 88345-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li, L. J., G. Dougan, P. Novotny, and I. G. Charles. 1991. P.70 pertactin, an outer-membrane protein from Bordetella parapertussis: cloning, nucleotide sequence and surface expression in Escherichia coli. Mol. Microbiol. 5409-417. [DOI] [PubMed] [Google Scholar]
- 21.Mintz, K. P. 2004. Identification of an extracellular matrix protein adhesin, EmaA, which mediates the adhesion of Actinobacillus actinomycetemcomitans to collagen. Microbiology 1502677-2688. [DOI] [PubMed] [Google Scholar]
- 22.Mintz, K. P., and P. M. Fives-Taylor. 1994. Adhesion of Actinobacillus actinomycetemcomitans to a human oral cell line. Infect. Immun. 623672-3678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mintz, K. P., and P. M. Fives-Taylor. 1999. Binding of the periodontal pathogen Actinobacillus actinomycetemcomitans to extracellular matrix proteins. Oral Microbiol. Immunol. 14109-116. [DOI] [PubMed] [Google Scholar]
- 24.Nikaido, H. 1994. Isolation of outer membranes. Methods Enzymol. 235225-234. [DOI] [PubMed] [Google Scholar]
- 25.Nummelin, H., M. C. Merckel, J. C. Leo, H. Lankinen, M. Skurnik, and A. Goldman. 2004. The Yersinia adhesin YadA collagen-binding domain structure is a novel left-handed parallel beta-roll. EMBO J. 23701-711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paju, S., P. Carlson, H. Jousimies-Somer, and S. Asikainen. 2000. Heterogeneity of Actinobacillus actinomycetemcomitans strains in various human infections and relationships between serotype, genotype, and antimicrobial susceptibility. J. Clin. Microbiol. 3879-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pohlner, J., R. Halter, K. Beyreuther, and T. F. Meyer. 1987. Gene structure and extracellular secretion of Neisseria gonorrhoeae IgA protease. Nature 325458-462. [DOI] [PubMed] [Google Scholar]
- 28.Ray, S. K., R. Rajeshwari, Y. Sharma, and R. V. Sonti. 2002. A high-molecular-weight outer membrane protein of Xanthomonas oryzae pv. oryzae exhibits similarity to non-fimbrial adhesins of animal pathogenic bacteria and is required for optimum virulence. Mol. Microbiol. 46637-647. [DOI] [PubMed] [Google Scholar]
- 29.Riess, T., S. G. Andersson, A. Lupas, M. Schaller, A. Schafer, P. Kyme, J. Martin, J. H. Walzlein, U. Ehehalt, H. Lindroos, M. Schirle, A. Nordheim, I. B. Autenrieth, and V. A. Kempf. 2004. Bartonella adhesin a mediates a proangiogenic host cell response. J. Exp. Med. 2001267-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roggenkamp, A., N. Ackermann, C. A. Jacobi, K. Truelzsch, H. Hoffmann, and J. Heesemann. 2003. Molecular analysis of transport and oligomerization of the Yersinia enterocolitica adhesin YadA. J. Bacteriol. 1853735-3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rudney, J. D., R. Chen, and G. J. Sedgewick. 2001. Intracellular Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in buccal epithelial cells collected from human subjects. Infect. Immun. 692700-2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruiz, T., C. Lenox, M. Radermacher, and K. P. Mintz. 2006. Novel surface structures are associated with the adhesion of Actinobacillus actinomycetemcomitans to collagen. Infect. Immun. 746163-6170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Slots, J., H. S. Reynolds, and R. J. Genco. 1980. Actinobacillus actinomycetemcomitans in human periodontal disease: a cross-sectional microbiological investigation. Infect. Immun. 291013-1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sreenivasan, P. K., D. J. LeBlanc, L. N. Lee, and P. Fives-Taylor. 1991. Transformation of Actinobacillus actinomycetemcomitans by electroporation, utilizing constructed shuttle plasmids. Infect. Immun. 594621-4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.St Geme, J. W., III, and D. Cutter. 2000. The Haemophilus influenzae Hia adhesin is an autotransporter protein that remains uncleaved at the C terminus and fully cell associated. J. Bacteriol. 1826005-6013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Surana, N. K., D. Cutter, S. J. Barenkamp, and J. W. St Geme III. 2004. The Haemophilus influenzae Hia autotransporter contains an unusually short trimeric translocator domain. J. Biol. Chem. 27914679-14685. [DOI] [PubMed] [Google Scholar]
- 37.Tahir, Y. E., P. Kuusela, and M. Skurnik. 2000. Functional mapping of the Yersinia enterocolitica adhesin YadA: identification of eight NSVAIG-S motifs in the amino-terminal half of the protein involved in collagen binding. Mol. Microbiol. 37192-206. [DOI] [PubMed] [Google Scholar]
- 38.Tamm, A., A. M. Tarkkanen, T. K. Korhonen, P. Kuusela, P. Toivanen, and M. Skurnik. 1993. Hydrophobic domains affect the collagen-binding specificity and surface polymerization as well as the virulence potential of the YadA protein of Yersinia enterocolitica. Mol. Microbiol. 10995-1011. [DOI] [PubMed] [Google Scholar]
- 39.Tang, G., T. Ruiz, R. Barrantes-Reynolds, and K. P. Mintz. 2007. Molecular heterogeneity of EmaA, an oligomeric autotransporter adhesin of Aggregatibacter (Actinobacillus) actinomycetemcomitans. Microbiology 1532447-2457. [DOI] [PubMed] [Google Scholar]
- 40.Thornley, M. J., and R. W. Horne. 1962. Electron microscope observations on the structure of fimbriae, with particular reference to Klebsiella strains, by the use of the negative staining technique. J. Gen. Microbiol. 2851-56. [DOI] [PubMed] [Google Scholar]
- 41.Wickner, W., A. J. Driessen, and F. U. Hartl. 1991. The enzymology of protein translocation across the Escherichia coli plasma membrane. Annu. Rev. Biochem. 60101-124. [DOI] [PubMed] [Google Scholar]
- 42.Yeo, H. J., S. E. Cotter, S. Laarmann, T. Juehne, J. W. St Geme III, and G. Waksman. 2004. Structural basis for host recognition by the Haemophilus influenzae Hia autotransporter. EMBO J. 231245-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang, P., B. B. Chomel, M. K. Schau, J. S. Goo, S. Droz, K. L. Kelminson, S. S. George, N. W. Lerche, and J. E. Koehler. 2004. A family of variably expressed outer-membrane proteins (Vomp) mediates adhesion and autoaggregation in Bartonella quintana. Proc. Natl. Acad. Sci. USA 10113630-13635. [DOI] [PMC free article] [PubMed] [Google Scholar]