Abstract
Olfactory signals play a central role in the identification of a mating partner in rodents, and the behavioral response to these cues varies markedly between the sexes. As several other sexually dimorphic traits, this response is thought to differentiate as a result of exposure of the developing individual to gonadal steroids, but both the identity of the specific steroid signal and the neural structures targeted for differentiation on this particular case are largely unknown. The present review summarizes results obtained in our lab using genetic males affected by the testicular feminization syndrome (Tfm) as experimental model, and that led to the identification of a role for non-aromatized gonadal steroids acting through the androgen receptor (AR) in the differentiation of olfactory cues processing in mice. The existing literature about AR-mediated sexual differentiation of the CNS in animal models is discussed, along with potential targets for the action of non-aromatized gonadal steroids in either one of the subsystems that detect and process olfactory information in rodents.
Keywords: Sexual orientation, testicular feminization, androgen insensitivity syndrome, olfactory system, sexual differentiation, androgen receptor
Introduction
Many sexual dimorphisms in mammals are the result of the differential exposure to steroid hormones secreted by the gonads during development (Jost, 1972; Jost, 1978; Jost, et al., 1973; Morris, et al., 2004). Included in this general model are sexually dimorphic behaviors related to reproduction, as well as non-reproductive behaviors. This was first demonstrated empirically by the classic experiments conducted by Phoenix and colleagues (Phoenix, et al., 1959), in which administration of elevated doses of testosterone to pregnant guinea pigs induced the display of typically masculine stereotyped behavior in their female offspring, as well as a reduced ability to exhibit lordosis later in life. Conversely, males castrated immediately after birth and therefore deprived of the source of differentiating steroids exhibited reduced masculine behavior and increased levels of sexual receptivity. These initial findings were later extended to other mammalian species, with the studies yielding similar results (Baum, et al., 1990; Beach, et al., 1969; Grady, et al., 1965; Whalen, 1964; Whalen and Edwards, 1966).
The endogenous steroid signal responsible for this differentiation was initially assumed to be testosterone, since this is the main steroid secreted by the testes (George, et al., 1978; Wilson and Lasnitzki, 1971). However, exogenous administration of estradiol to gonadectomized individuals during the perinatal period readily mimicked the effects of testosterone in the differentiation of reproductive behaviors (Feder and Whalen, 1964; Levine and Mullins, 1964). This initially puzzling observation was explained by the characterization of the estrogen synthase (aromatase) enzyme in the central nervous system, responsible for catalyzing the conversion of testosterone to estradiol (Ryan, et al., 1972). This led to the formulation of the “aromatization hypothesis”, which states that gonadal testosterone exerts its differentiating effects on the central nervous system of males by neural conversion to estradiol in areas expressing aromatase, and that this locally-synthesized estradiol binds to estrogen receptors present in the same areas as a first step in the organization of those circuits towards a masculine phenotype (Baum, 2003; Naftolin, 1994). The distribution pattern of aromatase expression in the brain (Lauber and Lichtensteiger, 1994; Roselli and Resko, 1993), the variation in the levels of expression during the lifespan with a peak matching the perinatal period in males (MacLusky, et al., 1985; Tobet, et al., 1985) and the disruptive effect that both aromatase inhibitors and anti-estrogens have in the masculinization of reproductive behavior (McEwen, et al., 1977; Vega Matuszczyk and Larsson, 1995; Vreeburg, et al., 1977) are all in good agreement with this hypothesis.
But despite the bulk of empirical support for this mechanism, the androgen receptor (AR) is also abundantly expressed in the same hypothalamic areas that are thought to undergo this estrogen-mediated differentiation (Attardi and Ohno, 1976; Fox, 1975; Kato, 1976; McAbee and DonCarlos, 1998; Vito, et al., 1979; Simerly, et al., 1990) and also exhibits some sexual dimorphism in its expression (Shah, et al., 2004). AR function is essential for the sexual differentiation of the male genitalia (Goldstein and Wilson, 1975) and the sexually dimorphic groups of motoneurons in the spinal cord that inervate the muscles attached to the penis and that are collectively known as the spinal nucleus of the bulbocavernosus (SNB) (Breedlove and Arnold, 1983a; Breedlove and Arnold, 1983b; Breedlove, et al., 1982; Freeman, et al., 1996) and dorsolateral nucleus (DLN) (Grisham, et al., 1992). Furthermore, some recent studies have uncovered that androgen action influences the morphology of several sexually dimorphic structures in the rodent brain, such as the posterodorsal medial amygdala, the suprachiasmatic nucleus (Morris, et al., 2005), the arcuate nucleus (Ciofi, et al., 2007), the ventromedial hypothalamus (Dugger, et al., 2007), the anteroventral periventricular nucleus (Lund, et al., 2000), the locus coeruleus (Garcia-Falgueras, et al., 2005), the bed nucleus of the accessory olfactory tract (Collado, et al., 1992) and the posteromedial bed nucleus of the stria terminalis (Durazzo, et al., 2007). This raises in turn the question of whether its interaction with non-aromatized ligands plays a role on the process of differentiation of sex-specific behavioral patterns (Sato, et al., 2004).
The initial studies on the role of gonadal steroids in the differentiation of the central nervous system focused specifically on mating behavior, but we now know that the range of behavioral traits affected by a differential exposure to these compounds is much wider (Casto, et al., 2003; Juarez, et al., 1998; Motelica-Heino, et al., 1993; Smith, et al., 1998). In particular, the ability to identify and exhibit a preference for an apropriate mating partner has also been studied. These behaviors collective refered to as “social preferences” are necessary for successful reproduction (Lumia, et al., 1987; Murphy and Schneider, 1970) and vary dramatically according to the sex of the individual. Perhaps because of the particular ecological niche that they occupy, rodent species rely heavily on olfactory cues derived from conspecifics for this particular function (Johnston, 1998). Therefore, neural circuits associated with the detection and processing of olfactory signals are good targets for sexual differentiation. Like many other terrestrial vertebrates, rodents exhibit two paralell olfactory systems that differ from each other both morphologically and functionally. Known as the main olfactory system (MOS) and the accesory olfactory system (AOS), they are associated with the main olfactory epithelium of the nasal cavity and the vomeronasal organ, respectively (Raisman, 1972; Scalia and Winans, 1975). In addition to olfactory receptors located in two different anatomical structures, the MOS and AOS also have segregated projection pathways to morphologically distinct areas of the olfactory bulbs, and from there to adjacent terminal fields in the limbic system (Halpern, 1987). Of these two systems, the accesory or vomeronasal system has traditionally been associated with the detection of pheromonal cues used for intra-species chemical communication and regulation of reproductive function (Powers and Winans, 1975; Wysocki, et al., 1982; Lepri and Wysocki, 1987). The VNS projects to the hypothalamus, including the medial preoptic area (Dong, et al., 2001; Gu, et al., 2003; Simerly and Swanson, 1988). Sex differences in the VNS are pronounced: The vomeronasal organ itself exhibits a higher overall and neuroepithelial volume, as well as a higher number of bipolar neurons in males compared to females (Segovia and Guillamon, 1982). In turn, the accesory olfactory bulb (AOB), which receives direct projections from the vomeronasal receptor neurons (Barber and Raisman, 1974), is larger in males than in females in terms of total volume, individual volume of each layer and several other morphometric variables (Caminero, et al., 1991; Segovia, et al., 1984; Valencia, et al., 1986). Finally, sexual dimorphisms have been described in areas that receive either direct or indirect projections from the AOB, including the bed nucleus of the accesory olfactory tract (BAOT) (Collado, et al., 1990), the medial division and encapsulated region of the bed nucleus of the stria terminalis (BNST) (del Abril, et al., 1987; Guillamon, et al., 1988; Hines, et al., 1992), posteromedial cortical and medial nuclei of the amygdala (Hines et al., 1992; Vinader-Caerols, et al., 1998) and medial preoptic area (Gorski, et al., 1978). All of these regions have been described to express AR in several rodent species, including rats (Sar and Stumpf, 1977; Handa, et al., 1986; Handa, et al., 1987; Sar, et al., 1990; Simerly et al., 1990; Menard and Harlan, 1993; Clancy, et al., 1992), mice (Lu, et al., 1998; Apostolinas, et al., 1999; Shah et al., 2004) and hamsters (Chen and Tu, 1992; Meek, et al., 1997). One of the studies performed in rats (Simerly et al., 1990) describes moderate expression of AR mRNA in both the main and accesory olfactory bulbs, as well as in the anterior olfactory nucleus. These data suggest that androgens secreted by the gonads during development may act directly through the AR to differentiate one or more components of the rodent olfactory pathway, which in turn could translate into a sexually dimorphic behavioral response.
Animals affected by the Tfm mutation as experimental models
To study the role of the androgen receptor in the sexual differentiation of brain circuits, we have adopted as our experimental model male mice carrying the testicular feminization mutation (Tfm). This syndrome was first described in humans (Morris, 1953) and later in mice (Lyon and Hawkes, 1970), rats (Stanley, et al., 1973), cattle (Nes, 1966) and chimpanzees (Eil, 1980). In all cases, the individuals affected are insensitive to androgens, and as a consequence the genetic males exhibit feminine external genitalia (Bardin, et al., 1973). These individuals present instead a blind vagina, a clitoris, developed mammary glands and a feminine number and location of nipples, with small testes present in an inguinal or abdominal position (Shapiro, et al., 1980; Stanley et al., 1973).
The Tfm syndrome is caused by several different naturally occurring mutations in the AR gene, whose common denominator is that they render the receptor non-functional (McPhaul, 2002a). Since the AR gene is located on the X chromosome, and males carrying a mutant allele are sterile, female homozygotes for the mutation have never been found in nature. The severity of androgen insensitivity is variable and directly related to extent of the disruption of receptor function (Lee and Chang, 2003; McPhaul, 2002b). Analysis of the mutation on the AR locus of the Tfm rat in the Stanley-Gumbreck strain showed that the disruption is caused by a single amino acid substitution on a position that is highly conserved within the nuclear receptor family (Yarbrough, et al., 1990). In contrast, the mutant sequence in the Tfm mouse presents a single-base deletion in the N-terminal region that causes a shift in the reading frame, generates a premature stop codon and thus causes an early termination of transcription and the production of a truncated receptor (Charest, et al., 1991; Gaspar, et al., 1991; He, et al., 1990). In both cases, androgen binding in brain tissue from Tfm animals has been shown to be only 10-20% compared with WT individuals, indicating that there is an important reduction in the number of functional receptors (Attardi, et al., 1976; Fox, 1975; MacLusky, et al., 1988). However, the small number of functional ARs present in Tfm rats appear to be indistinguishable from those of their WT counterparts (Wieland, et al., 1978). In contrast, the mutant protein in Tfm mice is considerably smaller, confirming what was predicted from the particular characteristics of the mutation and suggesting that in this case there is a qualitative deficit in addition to the quantitative deficit exhibited by both species (Young, et al., 1989). The biochemical evidence thus shows that Tfm rats retain some residual degree of responsiveness to androgens conferred by the few functional androgen receptors present.
Male Tfm rats have been described as completely asexual (Shapiro, et al., 1976), exhibiting neither mounting behavior when injected with testosterone nor lordosis when administered estradiol in combination with progesterone. However, only a year later, Beach and Buehler (Beach and Buehler, 1977) reported that almost all the mutant, gonadally intact subjects in their study exhibited mounting, with some individuals even exhibiting a characteristic behavioral pattern associated with ejaculation. Another report in Tfm rats (Shapiro et al., 1980) also stated that they of exhibited male-like mating behavior when treated with supraphysiological doses of gonadal steroids, and suggested that brain masculinization is independent of androgen action via the AR. The data on the ability of Tfm rats to exhibit feminine behavior has been much more consistent, with all the studies reporting that the lordotic coefficient was never significantly different from that of WT males, regardless of the hormone treatment (Beach and Buehler, 1977; Olsen, 1979; Olsen and Whalen, 1981; Shapiro et al., 1976). The relevance of the behavioral masculinization in Tfm rats has nevertheless been challenged (Bardin and Catterall, 1981) based on the data (discussed above) suggesting that Tfm rats may not be completely unresponsive to androgens. On the other hand, the AR signal appears to be entirely suppressed in mice, making them a more robust experimental model to study the role of androgen-mediated sexual differentiation of brain circuits.
AR-mediated differentiation of sexually dimorphic traits in mice
The first report of reproductive behavior in Tfm mice was included in the original work describing the syndrome on this species (Lyon and Hawkes, 1970). They were described as asexual, based entirely on anecdotic observations from gonadally-intact individuals, without any specific quantitative data. Four years later, Ohno and colleagues (Ohno, et al., 1974) reported that many of their Tfm subjects were capable of exhibiting male copulatory behavior, although in this study the Tfm males were heterozygotes obtained from a cross with a strain carrying the sex-reversed mutation, casting doubts on their complete unresponsiveness to androgens. A more detailed analysis was undertaken by Olsen (Olsen, 1993), who examined Tfm mice with intact gonads as well as gonadectomized and treated with testosterone, dihydrotestosterone or estradiol. The results confirmed that gonadally intact Tfm mice do not show masculine behavior spontaneously. However, hormone replacement was effective in eliciting some degree of masculine behavior in these animals, with 37% of the Tfm mice displaying mounting and thrusting after estrogen stimulation. Unfortunately, no data on specific behavioral patterns were reported in the study, and therefore it is not possible to assess whether the behavior exhibited by the androgen-insensitive individuals differed from their littermates with a functional AR.
When we tested Tfm subjects for masculine coital behavior after gonadectomy and estrogen replacement, we did not find significant differences between WT male littermates, both in terms of percentage of individuals exhibiting the behavior and in the values of the specific variables displayed during the sexual behavior tests. Moreover, WT female littermates exhibited similar levels of masculine behavior towards the stimulus animals (Bodo and Rissman, 2007). We concluded from this study that mice subjected to gonadectomy and estradiol treatment did not display any sex differences in the ability to mount exhibit pelvic thrusts with a sexually receptive female, however, some features of the temporal patterning of the behavior are dimorphic. This observation has been reported by other groups studying laboratory mice (see Table 1). Moreover, these behaviors do not require the activation of the AR for its differentiation at any point during development. An androgen receptor knockout mouse has been developed (Sato et al., 2004) and these mice have been reported to have deficiencies in their male sexual behavior. Interestingly, when 17β–estradiol was used as the activational hormone of choice in the sexual behavior test, a partial rescue of the mating phenotype was achieved, a result that is similar to what Olsen had previously reported using Tfm mice (Olsen, 1993). However, since the percentage of individuals exhibiting the behavior was approximately 50% compared with that of the WT group, the authors interpreted their results as evidence of an involvement of AR in the differentiation of the trait. A possible explanation for the discrepancy between our results and theirs may be differences in the doses of estradiol used. The estradiol-releasing pellet used by Sato and colleagues yield lower levels of estradiol in plasma (50–60 pg/ml)(Granholm, et al., 2002) compared to those attained with the implants used in our study (80-90 pg/ml), (Wersinger, et al., 1997). It may be necessary to use supraphysiological plasma levels of estradiol in order to elicit a high enough concentration in the brain to stimulate male sexual behavior. Confirmation of this hypothesis requires dose-response studies in AR knockouts and Tfm mice.
Table 1.
Original reports describing the occurrence of high levels of masculine mating behavior (mounting with pelvic thrusting) in female rats and mice.
| Reference | Species | Comparison with male group in the same study |
|---|---|---|
| (Beach, 1942) | Rat | no |
| (Beach and Rasquin, 1942) | Rat | no |
| (Whalen, et al., 1969) | Rat | no |
| (Pfaff, 1970) | Rat | yes |
| (Edwards and Burge, 1971) | Mouse | yes |
| (Coniglio and Clemens, 1972) | Rat | no |
| (Sodersten, 1972) | Rat | no |
| (Baum, et al., 1974) | Rat | no |
| (Emery and Sachs, 1975) | Rat | no |
| (Gladue, 1984) | Rat | no |
| (de Jonge, et al., 1986) | Rat | no |
| (Boehm and Aron, 1988) | Rat | no |
| (Oboh, et al., 1995) | Rat | yes |
| (Wersinger et al., 1997) | Mouse | yes |
| (Fang and Clemens, 1999a) | Rat | no |
| (Fang and Clemens, 1999b) | Rat | no |
| (Afonso and Pfaus, 2006) | Rat | no |
| (Bodo and Rissman, 2007) | Mouse | yes |
| (Jyotika, et al., 2007) | Mouse | yes |
In contrast with mating, when we tested our subjects for their olfactory preferences, males exhibited a clear preference for female-soiled bedding, whereas females preferred to spend more time investigating male odors (Bodo and Rissman, 2007). Interestingly, castrated and E2-treated Tfm males exhibited a female-like preference, spending more time sniffing male-soiled bedding (Fig 1). In addition, when a group of identically treated WT males were offered their own soiled bedding versus bedding soiled by a different male, they showed a clear preference to investigate the latter stimulus, indicating that the comparatively small amount of time spent investigating male-soiled bedding during regular tests by this group cannot be attributed to simple habituation to a familiar odor (Bodo and Rissman, unpublished results).
Fig. 1.
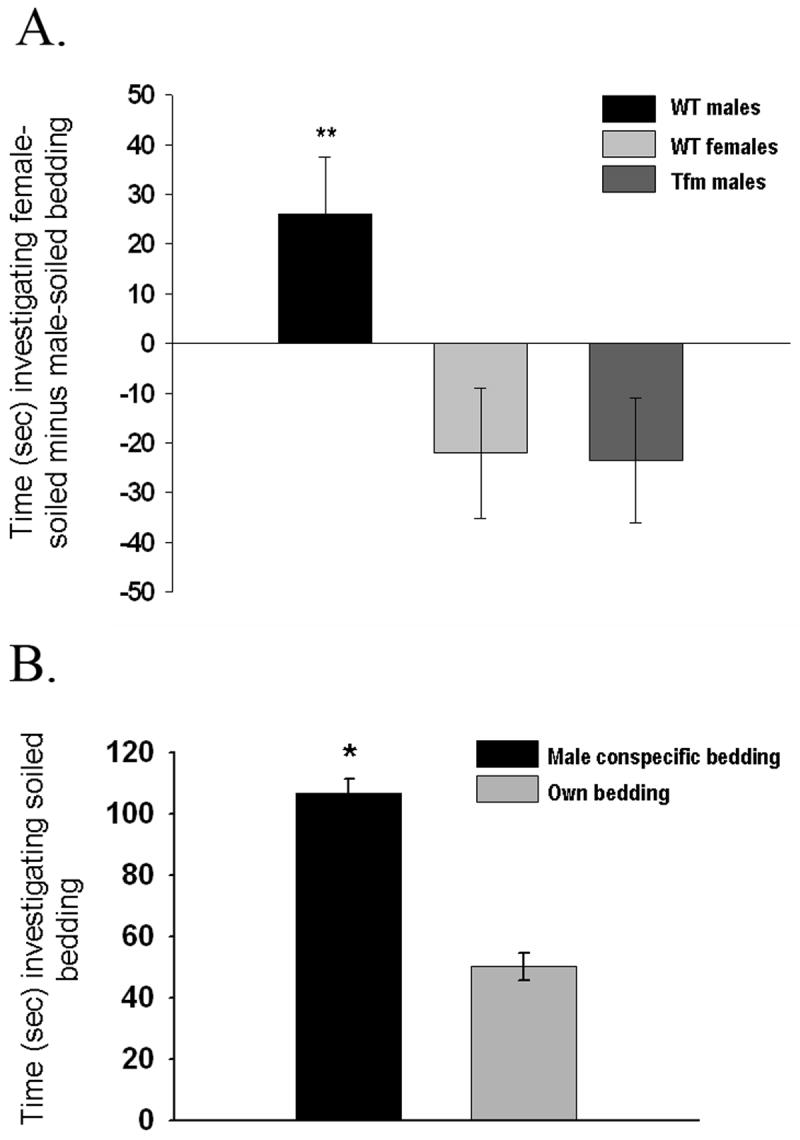
(A) Mean (± SEM) time spent sniffing soiled bedding from hormone-primed females minus time spent sniffing male-soiled bedding during the olfactory preference test. When time spent sniffing clean bedding was compared between the groups, no significant differences were found. WT males n=12, WT females n=9, Tfm males n=10. *Significantly different from the other two groups (p<0.01). Originally reported in (Bodo and Rissman, 2007)
(B) Mean (± SEM) time spent by gonadectimized, E2-treated adult males (n=4) sniffing soiled bedding from an unfamiliar male versus own bedding (p<0.001)
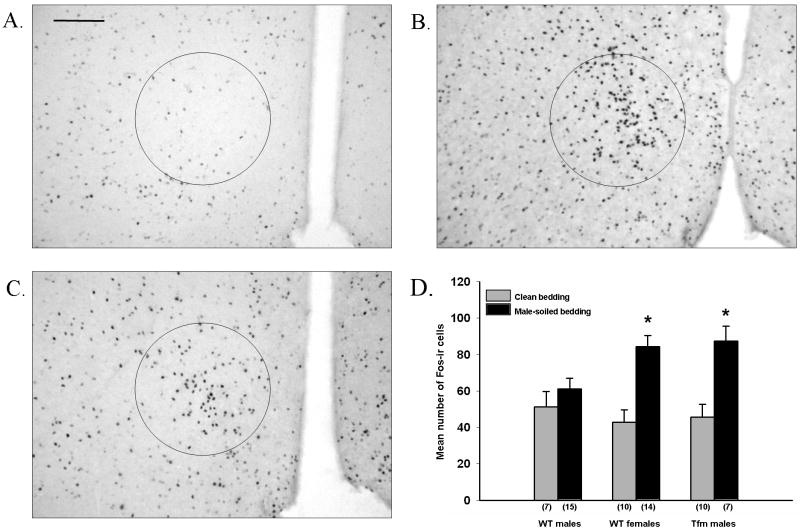
It has been previously shown in both rats (Bakker, et al., 1996) and mice (Halem, et al., 1999) that chemosignals present in male-soiled bedding are capable of eliciting neuronal activation in several brain areas in females, including the bed nucleus of the stria terminalis (BNST), medial preoptic area (mPOA) and medial amygdala (MeA), while males are unresponsive to the same chemical stimulus. We replicated these original results using gonadectomized, E2-implanted subjects, and found that two of the three areas (BNST and mPOA) exhibited increased numbers of c-fos-expressing neurons in WT females and Tfm males, but not in WT males (Fig 2). Finally, when actual partner preference was tested experimentally using a Y maze, Tfm males again failed to exhibit a clear preference to spend time in the vicinity of a receptive female as opposed to a male. The behavior of females was the same, whereas a preference for females was clearly marked in their WT male littermates. As in the other studies described all mice were gonadectomized and treated with E2 to activate the behavior.
Fig. 2.
(A-C) Representative photomicrographs of c-Fos-ir neurons in the MPOA of Wild-type males (A), Wild-type females (B) and Tfm males (C) after exposure to male-soiled bedding. The circle indicates the area sampled on each section. 3v, third ventricle. Scale bar: 100 μm. (D) Mean (± SEM) number of c-Fos-ir cells present in the MPOA of mice after exposure to either clean or male-soiled bedding. The number of individuals in each group is shown in parentheses below the bars *Significantly different from subjects of the same experimental group exposed to clean bedding (P < 0.05). Originally reported in (Bodo and Rissman, 2007)
In order to confirm the hypothesis that AR is involved in the normal differentiation of these traits, we administered dihydrotestosterone to WT females on postnatal day one (PN1) and then tested their preference for soiled bedding after they reached sexual maturity, as well as their pattern of neural activation in response to male-derived olfactory cues. Again all mice were gonadectomized and treated with E2, and the results were in agreement with the hypothesis that AR mediates sexual differentiation of this set of traits. DHT-injected females exhibited a marked preference for female-soiled bedding and a masculinized pattern of fos immunoreactivity in the same areas where we had previously characterized an abnormal response in our Tfm subjects (Bodo & Rissman, unpublished results).
General Conclusions
Considered together, the results described above show that the normal differentiation of the neural circuitry involved in recognizing and processing olfactory signals derived from conspecifics in mice is controlled by non-aromatized steroids interacting with the androgen receptor, and that this process takes place during the early neonatal period. Moreover, the differentiation of these traits is a prerequisite in male mice to making an appropriate choice of partner according to their genetic sex.
It is difficult with the data available so far to speculate on which specific component of the neural pathway that process the olfactory signal is targeted for differentiation by androgens in the male. The accessory (vomeronasal) olfactory system is a good candidate, since it is specialized in recognizing non-volatile cues used for chemical communication that are likely to be present in soiled bedding (Keverne, 2004). Moreover, the two hypothalamic areas where an abnormal c-Fos response was identified in Tfm individuals are central projections of the vomeronasal system. Genetic ablation of the TRPc2 ion channel, expressed specifically in the vomeronasal organ and required for transduction of the pheromonal signals, led to a gender-blind phenotype, with males initiating courtship with both females and other males, and failing to display territorial aggression towards the latter (Leypold, et al., 2002; Stowers, et al., 2002). There is a wealth of evidence, so far restricted mainly to the rat, pointing to specific sexual dimorphisms in several neural structures belonging to the VNS, and it has been shown empirically that gonadal steroids play a key role in the establishment of these differences during development. For instance, sex differences in the vomeronasal organ itself are abolished in males castrated immediately after birth or when females received exogenous androgens during the same developmental period (Segovia and Guillamon, 1982). These experimental manipulations had a similar effect on the morphology of the AOB (Segovia et al., 1984; Segovia, et al., 1986; Valencia et al., 1986), BAOT (Collado, et al., 1998; Collado, et al., 1993), BNST (Chung, et al., 2000; del Abril et al., 1987; Guillamon et al., 1988), MeA (Mizukami, et al., 1983) and sexually dimorphic nucleus of the POA (Rhees, et al., 1990a; Rhees, et al., 1990b). It is tempting to speculate that one or more of these morphological dimorphisms may underlie the differential response to olfactory cues determined by the organizational action of gonadal steroids. Already some evidence has been advanced on this direction, with one study linking reversal of the sex-specific morphology of the AOB as a result of experimental manipulation, with the expression of maternal behavior in male rats (Segovia, et al., 1996).
But it is important to remember that the original dual olfactory hypothesis, which postulated segregated, non-overlapping patterns of projections for the two olfactory systems, has been revised in recent years, with at least one study showing convergence of both pathway at the level of individual neurons in the hamster amygdala (Licht and Meredith, 1987). Moreover, recent studies have called into question the exclusive role of the vomeronasal system in the detection of socially relevant olfactory cues. Schaefer and colleagues (Schaefer, et al., 2002) showed that glomerular activation in the male olfactory bulb is sensitive to subtle variations in the MHC odortype of the individual providing the stimulus, a trait known to be relevant for mate choice in rodents (Penn and Potts, 1998). Removal of the vomeronasal organ, on the other hand, does not seem to affect the ability of mice to discriminate between these odortypes (Wysocki, et al., 2004). 2-Heptanone, a well-known mouse pheromone, elicits responses in both the main and accessory olfactory bulbs (Xu, et al., 2005). In addition, genetic manipulations to selectively disrupt the function of the main olfactory epithelium, result in the loss of olfactory investigation and discrimination between different types of urine, as well as deficits in mating and aggressive behavior (Ma, et al., 2002; Mandiyan, et al., 2005). Similar results have been recently reported using intranasal irrigation of zinc sulfate (Keller, et al., 2006a; Keller, et al., 2006b), whereas surgical removal of the VNO did not affect olfactory discrimination, at least in females (Keller, et al., 2006c). Additional data has been collected in other species pointing to the same direction, including the opossum (Shapiro, et al., 1996), the pig (Dorries, et al., 1997) and the ferret (Kelliher, et al., 1998). Taken together, these data strongly argue for an important role of the main olfactory system in the detection of chemical cues known to be important for intraspecies communication.
In order to discriminate between these two olfactory systems, it would be useful to collect additional information on morphological and/or biochemical dimorphisms that may exist in the olfactory pathways of other mammalian species, since at present this has only been studied with detail in rats (reviewed in Guillamon and Segovia, 1997). Furthermore, it is necessary to establish more firmly the functional significance of these dimorphisms, particularly since some studies have failed to find a link with dimorphic patterns of neural activation or behavioral responses to olfactory stimuli (Bressler and Baum, 1996; Paredes, et al., 1998). Finally, there is still a great gap in our knowledge of steroid receptor expression in neural structures during development, since the organizational actions of gonadal steroids are exerted in the immature brain whose characteristics may vary from those exhibited by adult animals. There is evidence suggesting that the levels of expression of steroid receptors may be considerable higher during development, and they may be present in certain areas that do not express them during adulthood (Karolczak and Beyer, 1998; Raab, et al., 1999). Such information may provide the key to explain, for instance, the sexual dimorphisms identified at the level of overall morphology (Segovia and Guillamon, 1982), pheromone receptors expression (Alekseyenko, et al., 2006) and functional response (Halem et al., 1999; Halem, et al., 2001) in the rodent VNO, even though this structure does not appear to express any receptor for gonadal steroids in the adult (Alekseyenko et al., 2006).
The available data are sufficient to postulate that non-aromatized steroids provide a signal for the differentiation of at least certain aspects of the olfactory processing system in male mice. Additional evidence of a direct involvement of the AR in the differentiation of play behavior (Casto et al., 2003), coital behavior (Brand and Slob, 1991; Casto et al., 2003; Clemens, et al., 1978), sexual receptivity (Gladue and Clemens, 1978) and even HPA axis function (Seale, et al., 2005) has been advanced, as well as in the remodeling of sexually dimorphic brain nuclei (Dugger et al., 2007; Durazzo et al., 2007; Goto, et al., 2005) and aromatase activity (Beyer and Hutchison, 1997). The picture that emerges is thus one of aromatized and non-aromatized compounds acting via their specific receptors and complementing each other in the overall process of gonadal steroids-mediated brain differentiation.
It is yet unclear why two different signals instead of a single one have to be used to achieve this result, and that is without taking into account gonad-independent mechanisms of brain differentiation that also appear to be acting in parallel, as increasing evidence collected from mammals seems to indicate (Arnold, et al., 2003; Gatewood, et al., 2006). Finally, and perhaps linked with this latter issue, there is the question of why there is so much variation in the relative contribution of each specific signaling pathway (via ER or AR), even between closely related species. In rats, the model in which sexual differentiation of the brain by steroids has been studied more extensively, convincing evidence has been presented supporting a role of aromatized steroids in the differentiation of partner preference in males (Brand, et al., 1991; Vega Matuszczyk and Larsson, 1995) as well as in some of the morphological dimorphisms characterized in the olfactory pathway in this species (Collado et al., 1993; Perez-Laso, et al., 1997). On the other hand, the differentiation of coital behavior seems to be at least partially AR-dependent in this case (Brand and Slob, 1991; Clemens et al., 1978). If it is so easy to switch the identity of the differentiating signal for a given trait in a relatively brief period of evolutionary divergence, why are both of them still functional among the different taxa? To find an answer to this will require studying these processes in a much wider range of species. Hopefully a phylogenetic approach will reveal a logical pattern behind what appears as random variation based on our present state of knowledge.
Acknowledgments
The author would like to thank Dr. Emilie Rissman for her useful comments on the manuscript. The work described from the Rissman Lab was supported by NIH grant R01 MH057759.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afonso VM, Pfaus JG. Hormonal and experiential control of female-male mounting in the female rat. Horm Behav. 2006;49(1):30–7. doi: 10.1016/j.yhbeh.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Alekseyenko OV, Baum MJ, Cherry JA. Sex and gonadal steroid modulation of pheromone receptor gene expression in the mouse vomeronasal organ. Neuroscience. 2006;140(4):1349–57. doi: 10.1016/j.neuroscience.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Apostolinas S, Rajendren G, Dobrjansky A, Gibson MJ. Androgen receptor immunoreactivity in specific neural regions in normal and hypogonadal male mice: effect of androgens. Brain Res. 1999;817(12):19–24. doi: 10.1016/s0006-8993(98)01180-9. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Rissman EF, De Vries GJ. Two perspectives on the origin of sex differences in the brain. Ann N Y Acad Sci. 2003;1007:176–88. doi: 10.1196/annals.1286.018. [DOI] [PubMed] [Google Scholar]
- Attardi B, Geller LN, Ohno S. Androgen and estrogen receptors in brain cytosol from male, female, and testicular feminized (tfm/y hermaphrodite) mice. Endocrinology. 1976;98(4):864–74. doi: 10.1210/endo-98-4-864. [DOI] [PubMed] [Google Scholar]
- Attardi B, Ohno S. Androgen and estrogen receptors in the developing mouse brain. Endocrinology. 1976;99(5):1279–90. doi: 10.1210/endo-99-5-1279. [DOI] [PubMed] [Google Scholar]
- Bakker J, Baum MJ, Slob AK. Neonatal inhibition of brain estrogen synthesis alters adult neural Fos responses to mating and pheromonal stimulation in the male rat. Neuroscience. 1996;74(1):251–60. doi: 10.1016/0306-4522(96)00096-6. [DOI] [PubMed] [Google Scholar]
- Barber PC, Raisman G. An autoradiographic investigation of the projection of the vomeronasal organ to the accessory olfactory bulb in the mouse. Brain Res. 1974;81(1):21–30. doi: 10.1016/0006-8993(74)90476-4. [DOI] [PubMed] [Google Scholar]
- Bardin CW, Bullock LP, Sherins RJ, Mowszowicz I, Blackburn WR. Androgen metabolism and mechanism of action in male pseudohermaphroditism: a study of testicular feminization. Recent Prog Horm Res. 1973;29:65–109. doi: 10.1016/b978-0-12-571129-6.50006-3. [DOI] [PubMed] [Google Scholar]
- Bardin CW, Catterall JF. Testosterone: a major determinant of extragenital sexual dimorphism. Science. 1981;211(4488):1285–94. doi: 10.1126/science.7010603. [DOI] [PubMed] [Google Scholar]
- Baum MJ. Activational and organizational effects of estradiol on male behavioral neuroendocrine function. Scand J Psychol. 2003;44(3):213–20. doi: 10.1111/1467-9450.00338. [DOI] [PubMed] [Google Scholar]
- Baum MJ, Erskine MS, Kornberg E, Weaver CE. Prenatal and neonatal testosterone exposure interact to affect differentiation of sexual behavior and partner preference in female ferrets. Behav Neurosci. 1990;104(1):183–98. doi: 10.1037//0735-7044.104.1.183. [DOI] [PubMed] [Google Scholar]
- Baum MJ, Sodersten P, Vreeburg JT. Mounting and receptive behavior in the ovariectomized female rat: influence of estradiol, dihydrotestosterone, and genital anesthetization. Horm Behav. 1974;5(2):175–90. doi: 10.1016/0018-506x(74)90042-7. [DOI] [PubMed] [Google Scholar]
- Beach FA. Execution of complete masculine copulatory behavior pattern by sexually receptive female rats. J Genet Psychol. 1942;60:137–142. [Google Scholar]
- Beach FA, Buehler MG. Male rats with inherited insensitivity to androgen show reduced sexual behavior. Endocrinology. 1977;100(1):197–200. doi: 10.1210/endo-100-1-197. [DOI] [PubMed] [Google Scholar]
- Beach FA, Noble RG, Orndoff RK. Effects of perinatal androgen treatment on responses of male rats to gonadal hormones in adulthood. J Comp Physiol Psychol. 1969;68(4):490–7. doi: 10.1037/h0027658. [DOI] [PubMed] [Google Scholar]
- Beach FA, Rasquin P. Masculine copulatory behavior in intact and castrated female rats. Endocrinology. 1942;31:393–409. [Google Scholar]
- Beyer C, Hutchison JB. Androgens stimulate the morphological maturation of embryonic hypothalamic aromatase-immunoreactive neurons in the mouse. Brain Res Dev Brain Res. 1997;98(1):74–81. doi: 10.1016/s0165-3806(96)00170-8. [DOI] [PubMed] [Google Scholar]
- Bodo C, Rissman EF. Androgen receptor is essential for sexual differentiation of responses to olfactory cues in mice. Eur J Neurosci. 2007 doi: 10.1111/j.1460-9568.2007.05484.x. in press. [DOI] [PubMed] [Google Scholar]
- Boehm N, Aron C. Facilitory effects of olfactory cues emitted by estrous females on mounting behavior in the female rat. Physiol Behav. 1988;43(5):669–71. doi: 10.1016/0031-9384(88)90224-7. [DOI] [PubMed] [Google Scholar]
- Brand T, Kroonen J, Mos J, Slob AK. Adult partner preference and sexual behavior of male rats affected by perinatal endocrine manipulations. Horm Behav. 1991;25(3):323–41. doi: 10.1016/0018-506x(91)90005-3. [DOI] [PubMed] [Google Scholar]
- Brand T, Slob AK. Perinatal flutamide and mounting and lordosis behavior in adult female Wistar and Sprague-Dawley rats. Behav Brain Res. 1991;44(1):43–51. doi: 10.1016/s0166-4328(05)80238-4. [DOI] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Hormonal control of a developing neuromuscular system. I. Complete Demasculinization of the male rat spinal nucleus of the bulbocavernosus using the anti-androgen flutamide. J Neurosci. 1983a;3(2):417–23. doi: 10.1523/JNEUROSCI.03-02-00417.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breedlove SM, Arnold AP. Hormonal control of a developing neuromuscular system. II. Sensitive periods for the androgen-induced masculinization of the rat spinal nucleus of the bulbocavernosus. J Neurosci. 1983b;3(2):424–32. doi: 10.1523/JNEUROSCI.03-02-00424.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breedlove SM, Jacobson CD, Gorski RA, Arnold AP. Masculinization of the female rat spinal cord following a single neonatal injection of testosterone propionate but not estradiol benzoate. Brain Res. 1982;237(1):173–81. doi: 10.1016/0006-8993(82)90565-0. [DOI] [PubMed] [Google Scholar]
- Bressler SC, Baum MJ. Sex comparison of neuronal Fos immunoreactivity in the rat vomeronasal projection circuit after chemosensory stimulation. Neuroscience. 1996;71(4):1063–72. doi: 10.1016/0306-4522(95)00493-9. [DOI] [PubMed] [Google Scholar]
- Caminero AA, Segovia S, Guillamon A. Sexual dimorphism in accessory olfactory bulb mitral cells: a quantitative Golgi study. Neuroscience. 1991;45(3):663–70. doi: 10.1016/0306-4522(91)90279-w. [DOI] [PubMed] [Google Scholar]
- Casto JM, Ward OB, Bartke A. Play, copulation, anatomy, and testosterone in gonadally intact male rats prenatally exposed to flutamide. Physiol Behav. 2003;79(45):633–41. doi: 10.1016/s0031-9384(03)00120-3. [DOI] [PubMed] [Google Scholar]
- Charest NJ, Zhou ZX, Lubahn DB, Olsen KL, Wilson EM, French FS. A frameshift mutation destabilizes androgen receptor messenger RNA in the Tfm mouse. Mol Endocrinol. 1991;5(4):573–81. doi: 10.1210/mend-5-4-573. [DOI] [PubMed] [Google Scholar]
- Chen TJ, Tu WW. Sex differences in estrogen and androgen receptors in hamster brain. Life Sci. 1992;50(21):1639–47. doi: 10.1016/0024-3205(92)90450-4. [DOI] [PubMed] [Google Scholar]
- Chung WC, Swaab DF, De Vries GJ. Apoptosis during sexual differentiation of the bed nucleus of the stria terminalis in the rat brain. J Neurobiol. 2000;43(3):234–43. [PubMed] [Google Scholar]
- Ciofi P, Lapirot OC, Tramu G. An androgen-dependent sexual dimorphism visible at puberty in the rat hypothalamus. Neuroscience. 2007;146(2):630–42. doi: 10.1016/j.neuroscience.2007.02.028. [DOI] [PubMed] [Google Scholar]
- Clancy AN, Bonsall RW, Michael RP. Immunohistochemical labeling of androgen receptors in the brain of rat and monkey. Life Sci. 1992;50(6):409–17. doi: 10.1016/0024-3205(92)90375-y. [DOI] [PubMed] [Google Scholar]
- Clemens LG, Gladue BA, Coniglio LP. Prenatal endogenous androgenic influences on masculine sexual behavior and genital morphology in male and female rats. Horm Behav. 1978;10(1):40–53. doi: 10.1016/0018-506x(78)90023-5. [DOI] [PubMed] [Google Scholar]
- Collado P, Guillamon A, Valencia A, Segovia S. Sexual dimorphism in the bed nucleus of the accessory olfactory tract in the rat. Brain Res Dev Brain Res. 1990;56(2):263–8. doi: 10.1016/0165-3806(90)90091-c. [DOI] [PubMed] [Google Scholar]
- Collado P, Segovia S, Cales JM, Perez Laso C, Rodriquez Zafra M, Guillamon A, Valencia A. Female's DHT controls sex differences in the rat bed nucleus of the accessory olfactory tract. Neuroreport. 1992;3(4):327–9. doi: 10.1097/00001756-199204000-00009. [DOI] [PubMed] [Google Scholar]
- Collado P, Segovia S, Guillamon A. Development of sex differences in the bed nucleus of the accessory olfactory tract in the rat. Brain Res Dev Brain Res. 1998;109(1):99–108. doi: 10.1016/s0165-3806(98)00068-6. [DOI] [PubMed] [Google Scholar]
- Collado P, Valencia A, Del Abril A, Rodriguez-Zafra M, Perez-Laso C, Segovia S, Guillamon A. Effects of estradiol on the development of sexual dimorphism in the bed nucleus of the accessory olfactory tract in the rat. Brain Res Dev Brain Res. 1993;75(2):285–7. doi: 10.1016/0165-3806(93)90033-7. [DOI] [PubMed] [Google Scholar]
- Coniglio L, Clemens LG. Stimulus and experiential factors controlling mounting behavior in the female rat. Physiol Behav. 1972;9(2):263–7. doi: 10.1016/0031-9384(72)90247-8. [DOI] [PubMed] [Google Scholar]
- de Jonge FH, Burger J, Van de Poll NE. Variable mounting levels in the female rat: the influence of experience and acute effects of testosterone. Behav Brain Res. 1986;20(1):39–46. doi: 10.1016/0166-4328(86)90099-9. [DOI] [PubMed] [Google Scholar]
- del Abril A, Segovia S, Guillamon A. The bed nucleus of the stria terminalis in the rat: regional sex differences controlled by gonadal steroids early after birth. Brain Res. 1987;429(2):295–300. doi: 10.1016/0165-3806(87)90110-6. [DOI] [PubMed] [Google Scholar]
- Dong HW, Petrovich GD, Swanson LW. Topography of projections from amygdala to bed nuclei of the stria terminalis. Brain Res Brain Res Rev. 2001;38(12):192–246. doi: 10.1016/s0165-0173(01)00079-0. [DOI] [PubMed] [Google Scholar]
- Dorries KM, Adkins-Regan E, Halpern BP. Sensitivity and behavioral responses to the pheromone androstenone are not mediated by the vomeronasal organ in domestic pigs. Brain Behav Evol. 1997;49(1):53–62. doi: 10.1159/000112981. [DOI] [PubMed] [Google Scholar]
- Dugger BN, Morris JA, Jordan CL, Breedlove SM. Androgen receptors are required for full masculinization of the ventromedial hypothalamus (VMH) in rats. Horm Behav. 2007;51(2):195–201. doi: 10.1016/j.yhbeh.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durazzo A, Morris JA, Breedlove SM, Jordan CL. Effects of the testicular feminization mutation (tfm) of the androgen receptor gene on BSTMPM volume and morphology in rats. Neurosci Lett. 2007;419(2):168–71. doi: 10.1016/j.neulet.2007.04.033. [DOI] [PubMed] [Google Scholar]
- Edwards DA, Burge KG. Early androgen treatment and male and female sexual behavior in mice. Horm Behav. 1971;2:49–58. [Google Scholar]
- Eil C, Merriam GR, Bowen J, Ebert J, Tabor E, White B, Douglass EC, Loriaux DL. Testicular feminization in the chimpanzee. Clinical Research. 1980;28:624. Abstracts. [Google Scholar]
- Emery DE, Sachs BD. Ejaculatory pattern in female rats without androgen treatment. Science. 1975;190(4213):484–6. doi: 10.1126/science.1174387. [DOI] [PubMed] [Google Scholar]
- Fang J, Clemens LG. Contextual determinants of female-female mounting in laboratory rats. Anim Behav. 1999a;57(3):545–555. doi: 10.1006/anbe.1998.1025. [DOI] [PubMed] [Google Scholar]
- Fang J, Clemens LG. Vaginocervical stimulation inhibits female-female mounting in laboratory rats. Physiol Behav. 1999b;67(1):75–9. doi: 10.1016/s0031-9384(99)00046-3. [DOI] [PubMed] [Google Scholar]
- Feder HH, Whalen RE. Feminine Behavior in Neonatally Castrated and Estrogen-Treated Male Rats. Science. 1964;147:306–7. doi: 10.1126/science.147.3655.306. [DOI] [PubMed] [Google Scholar]
- Fox TO. Androgen- and estrogen-binding macromolecules in developing mouse brain: biochemical and genetic evidence. Proc Natl Acad Sci U S A. 1975;72(11):4303–7. doi: 10.1073/pnas.72.11.4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman LM, Watson NV, Breedlove SM. Androgen spares androgen-insensitive motoneurons from apoptosis in the spinal nucleus of the bulbocavernosus in rats. Horm Behav. 1996;30(4):424–33. doi: 10.1006/hbeh.1996.0047. [DOI] [PubMed] [Google Scholar]
- Garcia-Falgueras A, Pinos H, Collado P, Pasaro E, Fernandez R, Jordan CL, Segovia S, Guillamon A. The role of the androgen receptor in CNS masculinization. Brain Res. 2005;1035(1):13–23. doi: 10.1016/j.brainres.2004.11.060. [DOI] [PubMed] [Google Scholar]
- Gaspar ML, Meo T, Bourgarel P, Guenet JL, Tosi M. A single base deletion in the Tfm androgen receptor gene creates a short-lived messenger RNA that directs internal translation initiation. Proc Natl Acad Sci U S A. 1991;88(19):8606–10. doi: 10.1073/pnas.88.19.8606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatewood JD, Wills A, Shetty S, Xu J, Arnold AP, Burgoyne PS, Rissman EF. Sex chromosome complement and gonadal sex influence aggressive and parental behaviors in mice. J Neurosci. 2006;26(8):2335–42. doi: 10.1523/JNEUROSCI.3743-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George FW, Catt KJ, Neaves WB, Wilson JD. Studies on the regulation of testosterone synthesis in the fetal rabbit testis. Endocrinology. 1978;102(3):665–73. doi: 10.1210/endo-102-3-665. [DOI] [PubMed] [Google Scholar]
- Gladue BA. Dihydrotestosterone stimulates mounting behavior but not lordosis in female rats. Physiol Behav. 1984;33(1):49–53. doi: 10.1016/0031-9384(84)90012-x. [DOI] [PubMed] [Google Scholar]
- Gladue BA, Clemens LG. Androgenic influences on feminine sexual behavior in male and female rats: defeminization blocked by prenatal antiandrogen treatment. Endocrinology. 1978;103(5):1702–9. doi: 10.1210/endo-103-5-1702. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Wilson JD. Genetic and hormonal control of male sexual differentiation. J Cell Physiol. 1975;85(2 Pt 2) 1:365–77. doi: 10.1002/jcp.1040850405. [DOI] [PubMed] [Google Scholar]
- Gorski RA, Gordon JH, Shryne JE, Southam AM. Evidence for a morphological sex difference within the medial preoptic area of the rat brain. Brain Res. 1978;148(2):333–46. doi: 10.1016/0006-8993(78)90723-0. [DOI] [PubMed] [Google Scholar]
- Goto K, Koizumi K, Ohta Y, Hashi M, Fujii Y, Ohbo N, Saika O, Suzuki H, Saito K, Suzuki K. Evaluation of general behavior, memory, learning performance, and brain sexual differentiation in F1 offspring males of rats treated with flutamide during late gestation. J Toxicol Sci. 2005;30(3):249–59. doi: 10.2131/jts.30.249. [DOI] [PubMed] [Google Scholar]
- Grady KL, Phoenix CH, Young WC. Role of the Developing Rat Testis in Differentiation of the Neural Tissues Mediating Mating Behavior. J Comp Physiol Psychol. 1965;59:176–82. doi: 10.1037/h0021824. [DOI] [PubMed] [Google Scholar]
- Granholm AC, Ford KA, Hyde LA, Bimonte HA, Hunter CL, Nelson M, Albeck D, Sanders LA, Mufson EJ, Crnic LS. Estrogen restores cognition and cholinergic phenotype in an animal model of Down syndrome. Physiol Behav. 2002;77(23):371–85. doi: 10.1016/s0031-9384(02)00884-3. [DOI] [PubMed] [Google Scholar]
- Grisham W, Casto JM, Kashon ML, Ward IL, Ward OB. Prenatal flutamide alters sexually dimorphic nuclei in the spinal cord of male rats. Brain Res. 1992;578(12):69–74. doi: 10.1016/0006-8993(92)90231-w. [DOI] [PubMed] [Google Scholar]
- Gu G, Cornea A, Simerly RB. Sexual differentiation of projections from the principal nucleus of the bed nuclei of the stria terminalis. J Comp Neurol. 2003;460(4):542–62. doi: 10.1002/cne.10677. [DOI] [PubMed] [Google Scholar]
- Guillamon A, Segovia S. Sex differences in the vomeronasal system. Brain Res Bull. 1997;44(4):377–82. doi: 10.1016/s0361-9230(97)00217-7. [DOI] [PubMed] [Google Scholar]
- Guillamon A, Segovia S, del Abril A. Early effects of gonadal steroids on the neuron number in the medial posterior region and the lateral division of the bed nucleus of the stria terminalis in the rat. Brain Res Dev Brain Res. 1988;44(2):281–90. doi: 10.1016/0165-3806(88)90226-x. [DOI] [PubMed] [Google Scholar]
- Halem HA, Baum MJ, Cherry JA. Sex difference and steroid modulation of pheromone-induced immediate early genes in the two zones of the mouse accessory olfactory system. J Neurosci. 2001;21(7):2474–80. doi: 10.1523/JNEUROSCI.21-07-02474.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halem HA, Cherry JA, Baum MJ. Vomeronasal neuroepithelium and forebrain Fos responses to male pheromones in male and female mice. J Neurobiol. 1999;39(2):249–63. [PubMed] [Google Scholar]
- Halpern M. The organization and function of the vomeronasal system. Annu Rev Neurosci. 1987;10:325–62. doi: 10.1146/annurev.ne.10.030187.001545. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Reid DL, Resko JA. Androgen receptors in brain and pituitary of female rats: cyclic changes and comparisons with the male. Biol Reprod. 1986;34(2):293–303. doi: 10.1095/biolreprod34.2.293. [DOI] [PubMed] [Google Scholar]
- Handa RJ, Roselli CE, Horton L, Resko JA. The quantitative distribution of cytosolic androgen receptors in microdissected areas of the male rat brain: effects of estrogen treatment. Endocrinology. 1987;121(1):233–40. doi: 10.1210/endo-121-1-233. [DOI] [PubMed] [Google Scholar]
- He WW, Young CY, Tindall DJ. The molecular basis of the mouse testicular feminization (Tfm) mutation: a frameshift mutation. 72nd Annual Meeting of the Endocrine Society; 1990. p. 240. [Google Scholar]
- Hines M, Allen LS, Gorski RA. Sex differences in subregions of the medial nucleus of the amygdala and the bed nucleus of the stria terminalis of the rat. Brain Res. 1992;579(2):321–6. doi: 10.1016/0006-8993(92)90068-k. [DOI] [PubMed] [Google Scholar]
- Johnston RE. Pheromones, the vomeronasal system, and communication. From hormonal responses to individual recognition. Ann N Y Acad Sci. 1998;855:333–48. doi: 10.1111/j.1749-6632.1998.tb10592.x. [DOI] [PubMed] [Google Scholar]
- Jost A. A new look at the mechanisms controlling sex differentiation in mammals. Johns Hopkins Med J. 1972;130(1):38–53. [PubMed] [Google Scholar]
- Jost A. Basic sexual trends in the development of vertebrates. Ciba Found Symp. 1978;(62):5–18. doi: 10.1002/9780470720448.ch2. [DOI] [PubMed] [Google Scholar]
- Jost A, Vigier B, Prepin J, Perchellet JP. Studies on sex differentiation in mammals. Recent Prog Horm Res. 1973;29:1–41. doi: 10.1016/b978-0-12-571129-6.50004-x. [DOI] [PubMed] [Google Scholar]
- Juarez J, del Rio-Portilla I, Corsi-Cabrera M. Effects of prenatal testosterone on sex and age differences in behavior elicited by stimulus pups in the rat. Dev Psychobiol. 1998;32(2):121–9. doi: 10.1002/(sici)1098-2302(199803)32:2<121::aid-dev5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Jyotika J, McCutcheon J, Laroche J, Blaustein JD, Forger NG. Deletion of the Bax gene disrupts sexual behavior and modestly impairs motor function in mice. Dev Neurobiol. 2007 doi: 10.1002/dneu.20525. [DOI] [PubMed] [Google Scholar]
- Karolczak M, Beyer C. Developmental sex differences in estrogen receptor-beta mRNA expression in the mouse hypothalamus/preoptic region. Neuroendocrinology. 1998;68(4):229–34. doi: 10.1159/000054370. [DOI] [PubMed] [Google Scholar]
- Kato J. Cytosol and nuclear receptors for 5alpha-dihydrotestosterone and testosterone in the hypothalamus and hypophysis, and testosterone receptors isolated from neonatal female rat hypothalamus. J Steroid Biochem. 1976;7(1112):1179–87. doi: 10.1016/0022-4731(76)90053-4. [DOI] [PubMed] [Google Scholar]
- Keller M, Douhard Q, Baum MJ, Bakker J. Destruction of the main olfactory epithelium reduces female sexual behavior and olfactory investigation in female mice. Chem Senses. 2006a;31(4):315–23. doi: 10.1093/chemse/bjj035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Douhard Q, Baum MJ, Bakker J. Sexual experience does not compensate for the disruptive effects of zinc sulfate--lesioning of the main olfactory epithelium on sexual behavior in male mice. Chem Senses. 2006b;31(8):753–62. doi: 10.1093/chemse/bjl018. [DOI] [PubMed] [Google Scholar]
- Keller M, Pierman S, Douhard Q, Baum MJ, Bakker J. The vomeronasal organ is required for the expression of lordosis behaviour, but not sex discrimination in female mice. Eur J Neurosci. 2006c;23(2):521–30. doi: 10.1111/j.1460-9568.2005.04589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher KR, Chang YM, Wersinger SR, Baum MJ. Sex difference and testosterone modulation of pheromone-induced NeuronalFos in the Ferret's main olfactory bulb and hypothalamus. Biol Reprod. 1998;59(6):1454–63. doi: 10.1095/biolreprod59.6.1454. [DOI] [PubMed] [Google Scholar]
- Keverne EB. Importance of olfactory and vomeronasal systems for male sexual function. Physiol Behav. 2004;83(2):177–87. doi: 10.1016/j.physbeh.2004.08.013. [DOI] [PubMed] [Google Scholar]
- Lauber ME, Lichtensteiger W. Pre- and postnatal ontogeny of aromatase cytochrome P450 messenger ribonucleic acid expression in the male rat brain studied by in situ hybridization. Endocrinology. 1994;135(4):1661–8. doi: 10.1210/endo.135.4.7925130. [DOI] [PubMed] [Google Scholar]
- Lee DK, Chang C. Endocrine mechanisms of disease: Expression and degradation of androgen receptor: mechanism and clinical implication. J Clin Endocrinol Metab. 2003;88(9):4043–54. doi: 10.1210/jc.2003-030261. [DOI] [PubMed] [Google Scholar]
- Lepri JJ, Wysocki CJ. Removal of the vomeronasal organ disrupts the activation of reproduction in female voles. Physiol Behav. 1987;40(3):349–55. doi: 10.1016/0031-9384(87)90058-8. [DOI] [PubMed] [Google Scholar]
- Levine S, Mullins R., Jr Estrogen Administered Neonatally Affects Adult Sexual Behavior in Male and Female Rats. Science. 1964;144:185–7. doi: 10.1126/science.144.3615.185. [DOI] [PubMed] [Google Scholar]
- Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, Axel R. Altered sexual and social behaviors in trp2 mutant mice. Proc Natl Acad Sci U S A. 2002;99(9):6376–81. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licht G, Meredith M. Convergence of main and accessory olfactory pathways onto single neurons in the hamster amygdala. Exp Brain Res. 1987;69(1):7–18. doi: 10.1007/BF00247024. [DOI] [PubMed] [Google Scholar]
- Lu SF, McKenna SE, Cologer-Clifford A, Nau EA, Simon NG. Androgen receptor in mouse brain: sex differences and similarities in autoregulation. Endocrinology. 1998;139(4):1594–601. doi: 10.1210/endo.139.4.5863. [DOI] [PubMed] [Google Scholar]
- Lumia AR, Zebrowski AF, McGinnis MY. Olfactory bulb removal decreases androgen receptor binding in amygdala and hypothalamus and disrupts masculine sexual behavior. Brain Res. 1987;404(12):121–6. doi: 10.1016/0006-8993(87)91362-x. [DOI] [PubMed] [Google Scholar]
- Lund TD, Salyer DL, Fleming DE, Lephart ED. Pre- or postnatal testosterone and flutamide effects on sexually dimorphic nuclei of the rat hypothalamus. Brain Res Dev Brain Res. 2000;120(2):261–6. doi: 10.1016/s0165-3806(00)00013-4. [DOI] [PubMed] [Google Scholar]
- Lyon MF, Hawkes SG. X-linked gene for testicular feminization in the mouse. Nature. 1970;227(5264):1217–9. doi: 10.1038/2271217a0. [DOI] [PubMed] [Google Scholar]
- Ma D, Allen ND, Van Bergen YC, Jones CM, Baum MJ, Keverne EB, Brennan PA. Selective ablation of olfactory receptor neurons without functional impairment of vomeronasal receptor neurons in OMP-ntr transgenic mice. Eur J Neurosci. 2002;16(12):2317–23. doi: 10.1046/j.1460-9568.2002.02303.x. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Luine VN, Gerlach JL, Fischette C, Naftolin F, McEwen BS. The role of androgen receptors in sexual differentiation of the brain: Effects of the testicular feminization (Tfm) gene on androgen metabolism, binding, and action in the mouse. Psychobiology. 1988;16:381–397. [Google Scholar]
- MacLusky NJ, Philip A, Hurlburt C, Naftolin F. Estrogen formation in the developing rat brain: sex differences in aromatase activity during early postnatal life. Psychoneuroendocrinology. 1985;10(3):355–61. doi: 10.1016/0306-4530(85)90013-7. [DOI] [PubMed] [Google Scholar]
- Mandiyan VS, Coats JK, Shah NM. Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nat Neurosci. 2005;8(12):1660–2. doi: 10.1038/nn1589. [DOI] [PubMed] [Google Scholar]
- McAbee MD, DonCarlos LL. Ontogeny of region-specific sex differences in androgen receptor messenger ribonucleic acid expression in the rat forebrain. Endocrinology. 1998;139(4):1738–45. doi: 10.1210/endo.139.4.5940. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Lieberburg I, Chaptal C, Krey LC. Aromatization: important for sexual differentiation of the neonatal rat brain. Horm Behav. 1977;9(3):249–63. doi: 10.1016/0018-506x(77)90060-5. [DOI] [PubMed] [Google Scholar]
- McPhaul MJ. Androgen receptor mutations and androgen insensitivity. Mol Cell Endocrinol. 2002a;198(12):61–7. doi: 10.1016/s0303-7207(02)00369-6. [DOI] [PubMed] [Google Scholar]
- McPhaul MJ. Molecular defects of the androgen receptor. Recent Prog Horm Res. 2002b;57:181–94. doi: 10.1210/rp.57.1.181. [DOI] [PubMed] [Google Scholar]
- Meek LR, Romeo RD, Novak CM, Sisk CL. Actions of testosterone in prepubertal and postpubertal male hamsters: dissociation of effects on reproductive behavior and brain androgen receptor immunoreactivity. Horm Behav. 1997;31(1):75–88. doi: 10.1006/hbeh.1997.1371. [DOI] [PubMed] [Google Scholar]
- Menard CS, Harlan RE. Up-regulation of androgen receptor immunoreactivity in the rat brain by androgenic-anabolic steroids. Brain Res. 1993;622(12):226–36. doi: 10.1016/0006-8993(93)90823-6. [DOI] [PubMed] [Google Scholar]
- Mizukami S, Nishizuka M, Arai Y. Sexual difference in nuclear volume and its ontogeny in the rat amygdala. Exp Neurol. 1983;79(2):569–75. doi: 10.1016/0014-4886(83)90235-2. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci. 2004;7(10):1034–9. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Dugger BN, Breedlove SM. Partial demasculinization of several brain regions in adult male (XY) rats with a dysfunctional androgen receptor gene. J Comp Neurol. 2005;487(2):217–26. doi: 10.1002/cne.20558. [DOI] [PubMed] [Google Scholar]
- Morris JM. The syndrome of testicular feminization in male pseudohermaphrodites. Am J Obstet Gynecol. 1953;65(6):1192–1211. doi: 10.1016/0002-9378(53)90359-7. [DOI] [PubMed] [Google Scholar]
- Motelica-Heino I, Edwards DA, Roffi J. Intermale aggression in mice: does hour of castration after birth influence adult behavior? Physiol Behav. 1993;53(5):1017–9. doi: 10.1016/0031-9384(93)90284-m. [DOI] [PubMed] [Google Scholar]
- Murphy MR, Schneider GE. Olfactory bulb removal eliminates mating behavior in the male golden hamster. Science. 1970;167(916):302–4. doi: 10.1126/science.167.3916.302. [DOI] [PubMed] [Google Scholar]
- Naftolin F. Brain aromatization of androgens. J Reprod Med. 1994;39(4):257–61. [PubMed] [Google Scholar]
- Nes N. Testikulaer feminisering hos storfe. Norwegian Veter Med. 1966;18:19–29. [Google Scholar]
- Oboh AM, Paredes RG, Baum MJ. A sex comparison of increments in FOS immunoreactivity in forebrain neurons of gonadectomized, testosterone-treated rats after mounting an estrous female. Neurobiol Learn Mem. 1995;63(1):66–73. doi: 10.1006/nlme.1995.1006. [DOI] [PubMed] [Google Scholar]
- Ohno S, Geller LN, Lai EV. TfM mutation and masculinization versus feminization of the mouse central nervous system. Cell. 1974;3(3):235–42. doi: 10.1016/0092-8674(74)90137-8. [DOI] [PubMed] [Google Scholar]
- Olsen KL. Androgen-insensitive rats are defeminised by their testes. Nature. 1979;279(5710):238–9. doi: 10.1038/279238a0. [DOI] [PubMed] [Google Scholar]
- Olsen KL. Sex and the Mutant Mouse: Strategies for Understanding the Sexual Differentiation of the Brain. In: M H, et al., editors. The Development of Sex Differences and Similarities in Behavior. Netherlands: 1993. pp. 255–278. [Google Scholar]
- Olsen KL, Whalen RE. Hormonal control of the development of sexual behavior in androgen-insensitive (tfm) rats. Physiol Behav. 1981;27(5):883–6. doi: 10.1016/0031-9384(81)90058-5. [DOI] [PubMed] [Google Scholar]
- Paredes RG, Lopez ME, Baum MJ. Testosterone augments neuronal Fos responses to estrous odors throughout the vomeronasal projection pathway of gonadectomized male and female rats. Horm Behav. 1998;33(1):48–57. doi: 10.1006/hbeh.1998.1435. [DOI] [PubMed] [Google Scholar]
- Penn D, Potts W. How do major histocompatibility complex genes influence odor and mating preferences? Adv Immunol. 1998;69:411–36. doi: 10.1016/s0065-2776(08)60612-4. [DOI] [PubMed] [Google Scholar]
- Perez-Laso C, Segovia S, Collado P, Rodriguez-Zafra M, Del Abril A, Guillamon A. Estradiol masculinizes the number of accessory olfactory bulb mitral cells in the rat. Brain Res Bull. 1997;42(3):227–30. doi: 10.1016/s0361-9230(96)00260-2. [DOI] [PubMed] [Google Scholar]
- Pfaff D. Nature of sex hormone effects on rat sex behavior: specificity of effects and individual patterns of response. J Comp Physiol Psychol. 1970;73(3):349–58. doi: 10.1037/h0030242. [DOI] [PubMed] [Google Scholar]
- Phoenix CH, Goy RW, Gerall AA, Young WC. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology. 1959;65:369–82. doi: 10.1210/endo-65-3-369. [DOI] [PubMed] [Google Scholar]
- Powers JB, Winans SS. Vomeronasal organ: critical role in mediating sexual behavior of the male hamster. Science. 1975;187(4180):961–3. doi: 10.1126/science.1145182. [DOI] [PubMed] [Google Scholar]
- Raab H, Karolczak M, Reisert I, Beyer C. Ontogenetic expression and splicing of estrogen receptor-alpha and beta mRNA in the rat midbrain. Neurosci Lett. 1999;275(1):21–4. doi: 10.1016/s0304-3940(99)00723-5. [DOI] [PubMed] [Google Scholar]
- Raisman G. An experimental study of the projection of the amygdala to the accessory olfactory bulb and its relationship to the concept of a dual olfactory system. Exp Brain Res. 1972;14(4):395–408. doi: 10.1007/BF00235035. [DOI] [PubMed] [Google Scholar]
- Rhees RW, Shryne JE, Gorski RA. Onset of the hormone-sensitive perinatal period for sexual differentiation of the sexually dimorphic nucleus of the preoptic area in female rats. J Neurobiol. 1990a;21(5):781–6. doi: 10.1002/neu.480210511. [DOI] [PubMed] [Google Scholar]
- Rhees RW, Shryne JE, Gorski RA. Termination of the hormone-sensitive period for differentiation of the sexually dimorphic nucleus of the preoptic area in male and female rats. Brain Res Dev Brain Res. 1990b;52(12):17–23. doi: 10.1016/0165-3806(90)90217-m. [DOI] [PubMed] [Google Scholar]
- Roselli CE, Resko JA. Aromatase activity in the rat brain: hormonal regulation and sex differences. J Steroid Biochem Mol Biol. 1993;44(46):499–508. doi: 10.1016/0960-0760(93)90254-t. [DOI] [PubMed] [Google Scholar]
- Ryan KJ, Naftolin F, Reddy V, Flores F, Petro Z. Estrogen formation in the brain. Am J Obstet Gynecol. 1972;114(4):454–60. doi: 10.1016/0002-9378(72)90204-9. [DOI] [PubMed] [Google Scholar]
- Sar M, Lubahn DB, French FS, Wilson EM. Immunohistochemical localization of the androgen receptor in rat and human tissues. Endocrinology. 1990;127(6):3180–6. doi: 10.1210/endo-127-6-3180. [DOI] [PubMed] [Google Scholar]
- Sar M, Stumpf WE. Distribution of androgen target cells in rat forebrain and pituitary after [3H]-dihydrotestosterone administration. J Steroid Biochem. 1977;8(11):1131–5. doi: 10.1016/0022-4731(77)90063-2. [DOI] [PubMed] [Google Scholar]
- Sato T, Matsumoto T, Kawano H, Watanabe T, Uematsu Y, Sekine K, Fukuda T, Aihara K, Krust A, Yamada T, Nakamichi Y, Yamamoto Y, Nakamura T, Yoshimura K, Yoshizawa T, Metzger D, Chambon P, Kato S. Brain masculinization requires androgen receptor function. Proc Natl Acad Sci U S A. 2004;101(6):1673–8. doi: 10.1073/pnas.0305303101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scalia F, Winans SS. The differential projections of the olfactory bulb and accessory olfactory bulb in mammals. J Comp Neurol. 1975;161(1):31–55. doi: 10.1002/cne.901610105. [DOI] [PubMed] [Google Scholar]
- Schaefer ML, Yamazaki K, Osada K, Restrepo D, Beauchamp GK. Olfactory fingerprints for major histocompatibility complex-determined body odors II: relationship among odor maps, genetics, odor composition, and behavior. J Neurosci. 2002;22(21):9513–21. doi: 10.1523/JNEUROSCI.22-21-09513.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale JV, Wood SA, Atkinson HC, Lightman SL, Harbuz MS. Organizational role for testosterone and estrogen on adult hypothalamic-pituitary-adrenal axis activity in the male rat. Endocrinology. 2005;146(4):1973–82. doi: 10.1210/en.2004-1201. [DOI] [PubMed] [Google Scholar]
- Segovia S, del Cerro MC, Ortega E, Perez-Laso C, Rodriguez-Zafra C, Izquierdo MA, Guillamon A. Role of GABAA receptors in the organization of brain and behavioural sex differences. Neuroreport. 1996;7(1517):2553–7. doi: 10.1097/00001756-199611040-00030. [DOI] [PubMed] [Google Scholar]
- Segovia S, Guillamon A. Effects of sex steroids on the development of the vomeronasal organ in the rat. Brain Res. 1982;281(2):209–12. doi: 10.1016/0165-3806(82)90160-2. [DOI] [PubMed] [Google Scholar]
- Segovia S, Orensanz LM, Valencia A, Guillamon A. Effects of sex steroids on the development of the accessory olfactory bulb in the rat: a volumetric study. Brain Res. 1984;318(2):312–4. doi: 10.1016/0165-3806(84)90036-1. [DOI] [PubMed] [Google Scholar]
- Segovia S, Valencia A, Cales JM, Guillamon A. Effects of sex steroids on the development of two granule cell subpopulations in the rat accessory olfactory bulb. Brain Res. 1986;395(2):283–6. doi: 10.1016/s0006-8993(86)80209-8. [DOI] [PubMed] [Google Scholar]
- Shah NM, Pisapia DJ, Maniatis S, Mendelsohn MM, Nemes A, Axel R. Visualizing sexual dimorphism in the brain. Neuron. 2004;43(3):313–9. doi: 10.1016/j.neuron.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Shapiro BH, Goldman AS, Steinbeck HF, Neumann F. Is feminine differentiation of the brain hormonally determined? Experientia. 1976;32(5):650–1. doi: 10.1007/BF01990214. [DOI] [PubMed] [Google Scholar]
- Shapiro BH, Levine DC, Adler NT. The testicular feminized rat: a naturally occurring model of androgen independent brain masculinization. Science. 1980;209(4454):418–20. doi: 10.1126/science.7384816. [DOI] [PubMed] [Google Scholar]
- Shapiro LS, Roland RM, Li CS, Halpern M. Vomeronasal system involvement in response to conspecific odors in adult male opossums, Monodelphis domestica. Behav Brain Res. 1996;77(12):101–13. doi: 10.1016/0166-4328(95)00206-5. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294(1):76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- Simerly RB, Swanson LW. Projections of the medial preoptic nucleus: a Phaseolus vulgaris leucoagglutinin anterograde tract-tracing study in the rat. J Comp Neurol. 1988;270(2):209–42. doi: 10.1002/cne.902700205. [DOI] [PubMed] [Google Scholar]
- Smith LK, Forgie ML, Pellis SM. Mechanisms underlying the absence of the pubertal shift in the playful defense of female rats. Dev Psychobiol. 1998;33(2):147–56. [PubMed] [Google Scholar]
- Sodersten P. Mounting behavior in the female rat during the estrous cycle, after ovariectomy, and after estrogen or testosterone administration. Horm Behav. 1972;3(4):307–320. [Google Scholar]
- Stanley AJ, Gumbreck LG, Allison JE, Easley RB. Male pseudohermaphroditism in the laboratory Norway rat. Recent Prog Horm Res. 1973;29:43–64. doi: 10.1016/b978-0-12-571129-6.50005-1. [DOI] [PubMed] [Google Scholar]
- Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295(5559):1493–500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- Tobet SA, Baum MJ, Tang HB, Shim JH, Canick JA. Aromatase activity in the perinatal rat forebrain: effects of age, sex and intrauterine position. Brain Res. 1985;355(2):171–8. doi: 10.1016/0165-3806(85)90038-0. [DOI] [PubMed] [Google Scholar]
- Valencia A, Segovia S, Guillamon A. Effects of sex steroids on the development of the accessory olfactory bulb mitral cells in the rat. Brain Res. 1986;389(12):287–90. doi: 10.1016/0165-3806(86)90197-5. [DOI] [PubMed] [Google Scholar]
- Vega Matuszczyk JV, Larsson K. Sexual preference and feminine and masculine sexual behavior of male rats prenatally exposed to antiandrogen or antiestrogen. Horm Behav. 1995;29(2):191–206. doi: 10.1006/hbeh.1995.1014. [DOI] [PubMed] [Google Scholar]
- Vinader-Caerols C, Collado P, Segovia S, Guillamon A. Sex differences in the posteromedial cortical nucleus of the amygdala in the rat. Neuroreport. 1998;9(11):2653–6. doi: 10.1097/00001756-199808030-00042. [DOI] [PubMed] [Google Scholar]
- Vito CC, Wieland SJ, Fox TO. Androgen receptors exist throughout the ‘critical period’ of brain sexual differentiation. Nature. 1979;282(5736):308–10. doi: 10.1038/282308a0. [DOI] [PubMed] [Google Scholar]
- Vreeburg JT, van der Vaart PD, van der Schoot P. Prevention of central defeminization but not masculinization in male rats by inhibition neonatally of oestrogen biosynthesis. J Endocrinol. 1977;74(3):375–82. doi: 10.1677/joe.0.0740375. [DOI] [PubMed] [Google Scholar]
- Wersinger SR, Sannen K, Villalba C, Lubahn DB, Rissman EF, De Vries GJ. Masculine sexual behavior is disrupted in male and female mice lacking a functional estrogen receptor alpha gene. Horm Behav. 1997;32(3):176–83. doi: 10.1006/hbeh.1997.1419. [DOI] [PubMed] [Google Scholar]
- Whalen RE. Hormone-Induced Changes in the Organization of Sexual Behavior in the Male Rat. J Comp Physiol Psychol. 1964;57:175–82. doi: 10.1037/h0041247. [DOI] [PubMed] [Google Scholar]
- Whalen RE, Edwards DA. Sexual reversibility in neonatally castrated male rats. J Comp Physiol Psychol. 1966;62(2):307–10. doi: 10.1037/h0023696. [DOI] [PubMed] [Google Scholar]
- Whalen RE, Edwards DA, Luttge WG, Robertson RT. Early androgen treatment and male sexual behavior in female rats. Physiol Behav. 1969;4(1):33–39. [Google Scholar]
- Wieland SJ, Fox TO, Savakis C. DNA-binding of androgen and estrogen receptors from mouse brain: behavior of residual androgen receptor from Tfm mutant. Brain Res. 1978;140(1):159–64. doi: 10.1016/0006-8993(78)90246-9. [DOI] [PubMed] [Google Scholar]
- Wilson JD, Lasnitzki I. Dihydrotestosterone formation in fetal tissues of the rabbit and rat. Endocrinology. 1971;89(3):659–68. doi: 10.1210/endo-89-3-659. [DOI] [PubMed] [Google Scholar]
- Wysocki CJ, Nyby J, Whitney G, Beauchamp GK, Katz Y. The vomeronasal organ: primary role in mouse chemosensory gender recognition. Physiol Behav. 1982;29(2):315–27. doi: 10.1016/0031-9384(82)90021-x. [DOI] [PubMed] [Google Scholar]
- Wysocki CJ, Yamazaki K, Curran M, Wysocki LM, Beauchamp GK. Mice (Mus musculus) lacking a vomeronasal organ can discriminate MHC-determined odortypes. Horm Behav. 2004;46(3):241–6. doi: 10.1016/j.yhbeh.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Xu F, Schaefer M, Kida I, Schafer J, Liu N, Rothman DL, Hyder F, Restrepo D, Shepherd GM. Simultaneous activation of mouse main and accessory olfactory bulbs by odors or pheromones. J Comp Neurol. 2005;489(4):491–500. doi: 10.1002/cne.20652. [DOI] [PubMed] [Google Scholar]
- Yarbrough WG, Quarmby VE, Simental JA, Joseph DR, Sar M, Lubahn DB, Olsen KL, French FS, Wilson EM. A single base mutation in the androgen receptor gene causes androgen insensitivity in the testicular feminized rat. J Biol Chem. 1990;265(15):8893–900. [PubMed] [Google Scholar]
- Young CY, Johnson MP, Prescott JL, Tindall DJ. The androgen receptor of the testicular-feminized (Tfm) mutant mouse is smaller than the wild-type receptor. Endocrinology. 1989;124(2):771–5. doi: 10.1210/endo-124-2-771. [DOI] [PubMed] [Google Scholar]