Abstract
We isolated peptides that home to mouse dorsal root ganglion (DRG) from a phage library expressing random 7-mer peptides fused to a minor coat protein (pIII) of the M13 phage. An in vitro biopanning procedure yielded 113 phage plaques after five cycles of enrichment by incubation with isolated DRG neurons and two cycles of subtraction by exposure to irrelevant cell lines. Analyses of the sequences of this collection identified three peptide clones that occurred repeatedly during the biopanning procedure. Phage-antibody staining revealed that the three peptides bound to DRG neurons of different sizes. To determine if the peptides would recognize neuronal cells in vivo, we injected individual GST-peptide fusion proteins into the subarachnoid space of mice and observed the appearance of immunoreactive GST in the cytosol of DRG neurons with a similar size distribution as that observed in vitro, indicating that the GST-peptide fusion proteins were recognized and taken up by different DRG neurons in vivo. The identification of homing peptide sequences provides a powerful tool for future studies on DRG neuronal function in vitro and in vivo, and opens up the possibility of neuron-specific drug and gene delivery in the treatment of diseases affecting DRG neurons.
Keywords: DRG, phage display, neuropathic pain, biopanning, neuropathy
Phage display is a powerful technology to identify peptide sequence motifs that target a particular tissue or cell type in the body [2, 8, 22]. Coupling such peptides to drugs and genes would enable their targeted delivery to specific cells and tissues in vitro and in vivo [8, 17, 20, 25]. A commonly used platform is the combinatorial filamentous M13 phage library that displays short random peptides fused to a minor coat protein (pIII) [22], which can be used to isolate specific cell type-binding peptides by a procedure called biopanning [17]. In this study, we have applied the technology to identify peptide motifs that recognize, and are specifically taken up by, neurons in the dorsal root ganglion (DRG) in mice.
DRG neurons are potential target cells for the treatment of diseases of the peripheral sensory nervous system [21]. For instance, neuropathic pain is a common symptom in various disorders, including metabolic abnormalities, malignancies, physical injuries, toxins and poisons, and hereditary diseases [21], and is the cause of much morbidity and misery. Although various pharmaceutical agents, anesthetics, surgical operations, or procedures such as transcutaneous electrical nerve stimulation, have been used to treat the symptoms of neuropathic pain [15], such palliative treatments are mostly non-targeted and of limited efficacy [15]. DRG neurons are the primary afferent neurons and can be classified into two broad groups: large and small neurons. Large neurons are thought to be involved mainly in proprioception, while most small neurons are involved in nociception [26]. Although both neuronal populations have also been subclassified by biochemical and histological methods, such as lineage tracing or immunostaining of different markers (neurotransmitters, cell surface carbohydrates), much work is needed before the physiological functions of the different neuronal subtypes are clearly defined [3, 7, 13, 18], a deficiency that limits our understanding of various pathophysiological processes [13], as well as our ability to develop novel therapeuties for disease processes affecting the peripheral sensory nervous system [4].
In this study, we have isolated three peptides that recognize specific, defined sizes of DRG neurons. These peptides will be powerful tools for research into the structure and function of subpopulations of DRG neurons, and for developing new therapies for diseases involving specific subtypes of DRG neurons.
Adult C57BL/6 mice aged 8–12 weeks were used in all experiments, and were housed in an animal room with a 12-h light and 12-h dark cycle in an illumination-controlled facility. All experiments were conducted with the approval of the Research Center for Animal Life Science at Shiga University of Medical Science. A phage library expressing random 7-mer peptides fused to a minor coat protein (pIII) of the M13 phage, at a complexity of about 1.3 × 109 independent sequences, was purchased from New England BioLabs (The Ph.D.-C7C Phage display Peptide Library kit, Beverly, MA). Rabbit anti-fd bacteriophage antibody was purchased from Sigma Aldrich Corp. (St. Louis, MO). Donkey FITC-labeled anti-rabbit IgG was purchased from Chemicon (Temecula, CA). The three synthetic peptides, DRG1: SPGARAF, DRG2: DGPWRKM, DRG3: FGQKASS, were supplied from Yanaihara Institute (Shizuoka, Japan). Various cell lines; mouse neuroblastoma cells (Neuro-2a), human embryonic kidney epithelial cells (HEK-293), opossum kidney cells, were purchased from American Type Culture Collection (Manassas, VA) for use in a subtractive panning protocol. These cultured cells were maintained in DMEM medium with 10% fetal calf serum (FCS, Invitrogen, Gaithersburg, MD) with antibiotics at 37°C in 5% CO2.
Mouse DRG neurons were isolated from lumbar segments L1-L5 of C57BL/6 mice using an enzymatic procedure described previously [23]. The neurons were purified with 30% percoll (MP Biomedicals, Solon, OH), washed twice with Ham’s F12 medium supplemented with 10% fetal calf serum (Invitrogen), and then plated on poly-L-lysine (Sigma)-coated 10 cm dishes for in vitro biopanning or on 8 well Lab-Tek Chamber Slides (NUNC, Naperville, IL) for in vitro phage binding. The cells were allowed to attach at 37°C for 12 hours in 5% CO2.
The in vitro phage display library screening protocols described by Hong et al. [5] were employed with minor modifications. Briefly, after washing isolated DRG neurons three times with PBS, the culture medium was changed FCS-free DMEM medium. Then, 1 × 1010 plaque-forming units (pfu) of the M13 phage library were added to isolated DRG neurons (1 × 106/well), and incubated at 37°C in 5% CO2 for 10 min. After washing three times, the bound phages were recovered by grinding the cells with protease inhibitor in DMEM medium. Aliquots were diluted appropriately and infected E. coli ER2738, mixed with Agarose Top, and incubated in LB/IPTG/X-gal plates at 37°C overnight. After titrating plaque forming units, phages in the aliquot were further amplified by infecting E. coli with them in a shaker at 37°C for 4.5 h. Phages from the E. coli were purified, and 1 × 1010–11 pfu were added to isolated DRG neurons again. In all, we carried out five such rounds of screening with DRG neurons. To select more specific phages for DRG neurons, a subtractive panning protocol [16] against irrelevant cell lines (Neuro-2a, HEK-293, and opossum kidney cells from American Type Culture Collection, Manassas, VA) was performed twice. These cultured cells were maintained in DMEM medium with 10% fetal calf serum (FCS, Invitrogen, Gaithersburg, MD) with antibiotics at 37°C in 5% CO2. To avoid the selection of polystyrene and poly-L-lysine-specific peptides, a subtractive panning protocol against poly-L-lysine culture dishes was also performed twice [1].
In vitro phage binding to DRG neurons was carried out on cultured neurons in 8-well chamber slides following three washes with PBS to clear the cell surface (n =1 × 105 cells/well). Cells were incubated at 37°C for 1 h with either the three selected phages or control library phage at 2 × 109 pfu/well (1 × 1010 pfu/ml), with or without synthetic peptides (10−4 or 10−6 M; DRG1: SPGARAF, DRG2: DGPWRKM, DRG3: FGQKASS from Yanaihara Institute, Shizuoka, Japan), which had 7 amino-acid sequences homologous to the selected phages. Subsequently, unbounded phages and/or peptides were removed by washing the chamber slides three times with PBS. To detect phages bound to the cells, the cultured cells were fixed with 4% paraformaldehyde and incubated for 1 h with rabbit anti-fd antibody (diluted 1:1000 in PBS with 3% donkey serum, Sigma Aldrich Corp., St. Louis, MO), washed three times with PBS, and subsequently incubated with FITC-conjugated donkey anti-rabbit IgG diluted 1:1000 for 1 h (Chemicon, Temecula, CA). After three washes, the cells were treated with propidium iodine (PI) for nuclear staining, and then observed under a confocal laser-scanning microscope (LSM510, Carl Zeiss, Jena, Germany). For quantitative analysis, we photographed ten randomly selected fields in each well and measured diameters (long and short) of the cells positive for phage immunostaining and PI staining. The mean cell size was calculated as the mean of the long and short diameter, and cell-size differences were evaluated in different groups.
To evaluate their binding to DRG cells in vivo, GST fusion proteins of three selected peptides were synthesized using the pGEX4T-1 vector kit (Amersham, Piscataway, NJ) according to the manufacturer’s instructions. Briefly, oligonucleotides for C-DRG1-C, C-DRG2-C, and C-DRG3-C were synthesized cloned into the pGEX4T-1 vector, and transformed in E. coli. The synthesized GST-fusion proteins were pull down by Glutathione Sepharose 4B (Amersham), and purified. The purities and reactions to anti-GST antibodies (Amersham) were confirmed by gel-electrophoresis stained by Coomassie Blue and anti-GST immunoblotting.
Under intraperitoneal pentobarbiturate anesthesia (0.1 mg/g BW), 10 µl (500 µg/ml) of purified GST-fusion proteins (GST-DRG1, GST-DRG2, GST-DRG3, or GST alone) were injected into the subarachnoid space of 8-week-old female C57BL/6 mice (n = 3 each). After 1 h, the mice were sacrificed and transcardially perfused with cold saline followed by 4% paraformaldehyde, 0.5% glutaraldehyde and 0.3% picric acid in 0.1 M PBS (pH 7.4). The L5 DRGs were then isolated and cut sectioned (10 µm) for fluorescence immunohistochemistry. After blocking with 10% horse serum, the sections were incubated overnight at 4°C with goat anti-GST antibody (Amersham) diluted 1:1000 and rabbit anti-neurofilament L (NF-L, non-phosphorylated form, Chemicon, Temecula, CA) antibody diluted 1:100 in PBST. The sections were incubated for 1 h with Alexa Fluor 488-labeled anti-goat IgG and Alexa Fluor 555-labeled anti-rabbit IgG (Molecular Probes, Eugene, OR) at room temperature, mounted with VECTASHIELD mounting medium containing 4',6-diamidino-2-phenylindole (DAPI) (Vector Laboratories Inc., Burlingame, CA), and observed under a fluorescence microscope (Axioplan 2 imaging and Axiocam, Carl Zeiss, Jena, Germany). For quantitative analysis, we took pictures of randomly selected fields and measured diameters (long and short) of the cells positive for GST, NF-L and DAPI. The mean cell size was calculated as the mean of the long and short diameters, and cell-size differences were evaluated in the three different groups.
BLAST (http://www.ncbi. nlm.nih.gov/blast/) searchs of the mouse database with the phage peptide sequences were carried out to identify homologies with proteins of interest, including neuronal growth factors, cytokines, hormones, and cell adhesion molecules.
Data were analyzed by ANOVA for multiple comparisons and shown as mean ± SD. Significance was assigned at P<0.05.
We used an in vitro biopanning protocol, and isolated 113 phage plaques in five rounds of screening with DRG neurons and four rounds of negative selection, two against irrelevant cell lines, and two against poly-L-lysine-coated culture plates. Of the 113 plaques, five displayed an identical 7-amino acid sequence (DRG1), four shared another 7-mer sequence (DRG2), and three others displayed a third shared sequence (DRG3); 17 other sequences (DRG4 to DRG20) were each shared by two different plaques. The sequences of the other 67 clones were all different from one another (Table 1). We further analyzed the three phage clones, DRG1, DRG2 and DRG3, since these 3 clones were appeared at least three times by binding to DRG neurons in vitro; these phage clones were designated DRG-p1, DRG-p2 and DRG-p3, respectively.
Table 1.
Amino-acid sequence of DRG-specific 7-mer peptides and the numbers of phage plaques with identical amino-acid sequences.
| Name | Sequence | Counts | Name | Sequence | Counts | Name | Sequence | Counts |
|---|---|---|---|---|---|---|---|---|
| DRG1 | SPGARAF | 5 | DRG30 | HTTSSLY | 1 | DRG59 | HTGPFGL | 1 |
| DRG2 | DGPWRKM | 4 | DRG31 | MGQNLRF | 1 | DRG60 | LSTSSKK | 1 |
| DRG3 | FGQKASS | 3 | DRG32 | NLQLAPD | 1 | DRG61 | TPPSPRT | 1 |
| DRG4 | TGFQSGS | 2 | DRG33 | SSFRGAT | 1 | DRG62 | PALSHST | 1 |
| DRG5 | DSSRTRL | 2 | DRG34 | LHKSHLL | 1 | DRG63 | TPSWSKK | 1 |
| DRG6 | DFIRTQA | 2 | DRG35 | APPELRL | 1 | DRG64 | STPAVPP | 1 |
| DRG7 | LKHTNEA | 2 | DRG36 | HRTIASG | 1 | DRG65 | NLNAHHK | 1 |
| DRG8 | QGAHNNN | 2 | DRG37 | TESIGDK | 1 | DRG66 | QHQKQGY | 1 |
| DRG9 | NPHKAPN | 2 | DRG38 | APDETER | 1 | DRG67 | NKTTNIM | 1 |
| DRG10 | NPSLQAP | 2 | DRG39 | KGLPPGH | 1 | DRG68 | TSASLSS | 1 |
| DRG11 | PPWSSPK | 2 | DRG40 | PSGTPSY | 1 | DRG69 | RSSPPNT | 1 |
| DRG12 | AQSHNKL | 2 | DRG41 | SNRSPLM | 1 | DRG70 | SPPRPTG | 1 |
| DRG13 | LPTSKKM | 2 | DRG42 | TIGQSYR | 1 | DRG71 | VNTPERH | 1 |
| DRG14 | NHLKNPA | 2 | DRG43 | SPTEGTP | 1 | DRG72 | TPQYPKL | 1 |
| DRG15 | TFSIGEK | 2 | DRG44 | PLSGAPW | 1 | DRG73 | PTLLPHQ | 1 |
| DRG16 | QAIQNST | 2 | DRG45 | DAPTHMH | 1 | DRG74 | NNANYRL | 1 |
| DRG17 | HNTNAQH | 2 | DRG46 | TDFRSRV | 1 | DRG75 | GPHFHQS | 1 |
| DRG18 | TPSLPQT | 2 | DRG47 | LVLPPLA | 1 | DRG76 | PAMNSVK | 1 |
| DRG19 | NMPTQRS | 2 | DRG48 | SSSPARL | 1 | DRG77 | GTTPTST | 1 |
| DRG20 | PVRSPAV | 2 | DRG49 | TATNTRT | 1 | DRG78 | HNSTRGS | 1 |
| DRG21 | SSQAPQS | 1 | DRG50 | DGAGTWV | 1 | DRG79 | DDSGPLR | 1 |
| DRG22 | DAQKNMN | 1 | DRG51 | EKHLAPR | 1 | DRG80 | NMHPTAT | 1 |
| DRG23 | GLQLSQT | 1 | DRG52 | PLTPLGF | 1 | DRG81 | HQNWRHT | 1 |
| DRG24 | SASNTQY | 1 | DRG53 | MTPFMGS | 1 | DRG82 | PSTKYHS | 1 |
| DRG25 | EGHLVSQ | 1 | DRG54 | DSPGWPH | 1 | DRG83 | PLRLAHQ | 1 |
| DRG26 | SDPGNYM | 1 | DRG55 | GERHSLT | 1 | DRG84 | QMPGNNL | 1 |
| DRG27 | ALDNVPH | 1 | DRG56 | TTAVALR | 1 | DRG85 | LATPLRN | 1 |
| DRG28 | PTKQHAK | 1 | DRG57 | NGLHVQR | 1 | DRG86 | LNGLKAA | 1 |
| DRG29 | TELQRHN | 1 | DRG58 | TLSPRSA | 1 | DRG87 | TVSSHRA | 1 |
We analyzed the binding of the 3 different clones to isolated DRG neurons maintained in culture (Fig. 1). DRG-p1 bound to 38% of the DRG neurons (83/219), DRG-p2 to 43% (100/231), and DRG-p3 to 29% (69/235), while the control phage library bound to 8% (45/539) of the DRG neurons. The addition of peptides (10−4 M) with identical amino-acid sequences blocked the staining with anti-phage antibodies by 97% for DRG-p1, 94% for DRG-p2, and 94% for DRG-p3, while adding 10−6 M peptide blocked staining by 47% for DRG-p1, 54% for DRG-p2, and 53% for DRG-p3, as compared with similarly bound cultures stained with antibody alone. As each peptide was only capable of competing for the binding of the phage with an identical 7-amino acid sequence (data not shown), the phage-neuron binding was concluded to be sequence specific (Fig. 1B).
Figure 1.
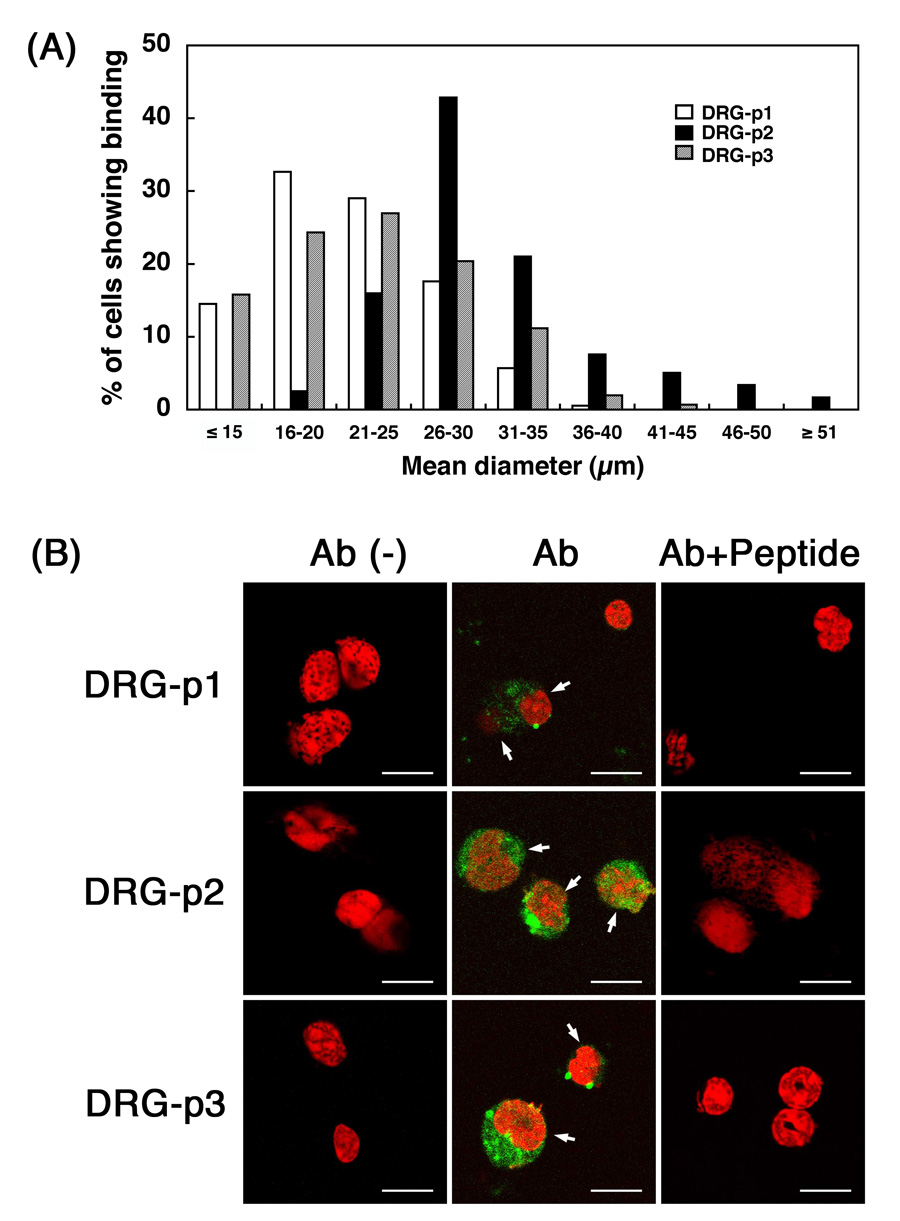
Binding of 3 different phages to cultured-DRG neurons. The cells were analyzed by a confocal laser-scanning microscopy. (A) Cell size distribution of the DRG neurons positive for DRG-p1, DRG-p2 or DRG-p3. (B) Bindings of the 3 different phage clones to mouse DRG neurons. Positive reactions stained with anti-fd antibody (Ab) are green. Ab (−) is without antibody and Ab + peptide is antibody plus peptides (10−4 M) corresponding to each phage. Nuclei were stained with PI (red). Bars = 20 µm.
We then analyzed the cell size distribution of the DRG neurons that bound to individual phage clones. Interestingly, DRG-p1 and DRG-p3 staining occurred mainly in small-sized neurons with a mean diameter of 22.0 ± 5.3 µm (for DRG-p1) and 23.2 ± 6.7 µm (for DRG-3p), while DRG-p2 staining occurred mainly in large-sized neurons with a mean diameter of 30.9 ± 7.0 µm (Fig. 1A). There was a significant difference between the mean diameter of the cells positive for DRG-p1 and that for DRG-p2 (p<0.001), and of the cells positive for DRG-p2 and for DRG-p3 (p<0.001), but no difference between the cells positive for DRG-p1 and DRG-p3.
To examine the DRG-targeting of peptides in vivo, we injected individual GST-peptide-fusion proteins (5 µg each) or GST alone into the subarachnoid space in C57BL/6 mice, isolated the DRGs, and used anti-GST immunostaining to detect the neuron-associated GST-peptide-fusion proteins (see Materials and Methods). Additionally, we double-immunostained the cells against anti-neurofilament L (NF-L, non-phosphorylated form) antibodies, and found that essentially all (99%) cells positive for GST-peptide-fusion proteins were also positive for neurofilament (Fig. 2B). In DRG cells from control mice injected with GST, no anti-GST staining was observed (Fig. 2B). Furthermore, 39% (368/949) of the NF-L-positive neurons were positive for GST-DRG1, 25% (214/848) for GST-DRG2, and 14% (147/1081) for GST-DRG3. Cell size analysis showed that GST-DRG1- and GST-DRG3-positive cells were mainly small-sized neurons, whereas GST-DRG2-positive cells were mainly large-sized DRG neurons (Fig. 2B). The mean diameter of the cells positive for GST-DRG1 was 21.6 ± 5.2 µm, GST-DRG2 33.5 ± 7.4 µm, and GST-DRG3 22.7 ± 6.6 µm. There was a significant difference in mean diameter between GST-DRG1-positive cells and GST-DRG2-positive cells (p<0.001), and between GST-DRG2-positive and GST-DRG3-positive cells (p<0.001), but no size difference between GST-DRG1-postive and GST-DRG3-positive cells. Therefore, the cell size specificity of the three different peptides in phages in vitro (Fig. 1A) was confirmed by the in vivo-binding experiment. Moreover, as most of the GST-positive immunoreactivity was detected in the cytoplasm of the DRG neurons (Fig. 2B), and as anti-GST antibodies used in this experiment were specific for recombinant GST-peptides fusion proteins synthesized in E. coli, and did not react with mouse endogenous GST, the bound GST-peptide-fusion proteins appear to have been internalized into the cytosol of the targeted neurons. In contrast, we examined the GST immunoreactivities in the central nervous system (brain and spinal cord), and obtained no specific staining (data not shown).
Figure 2.
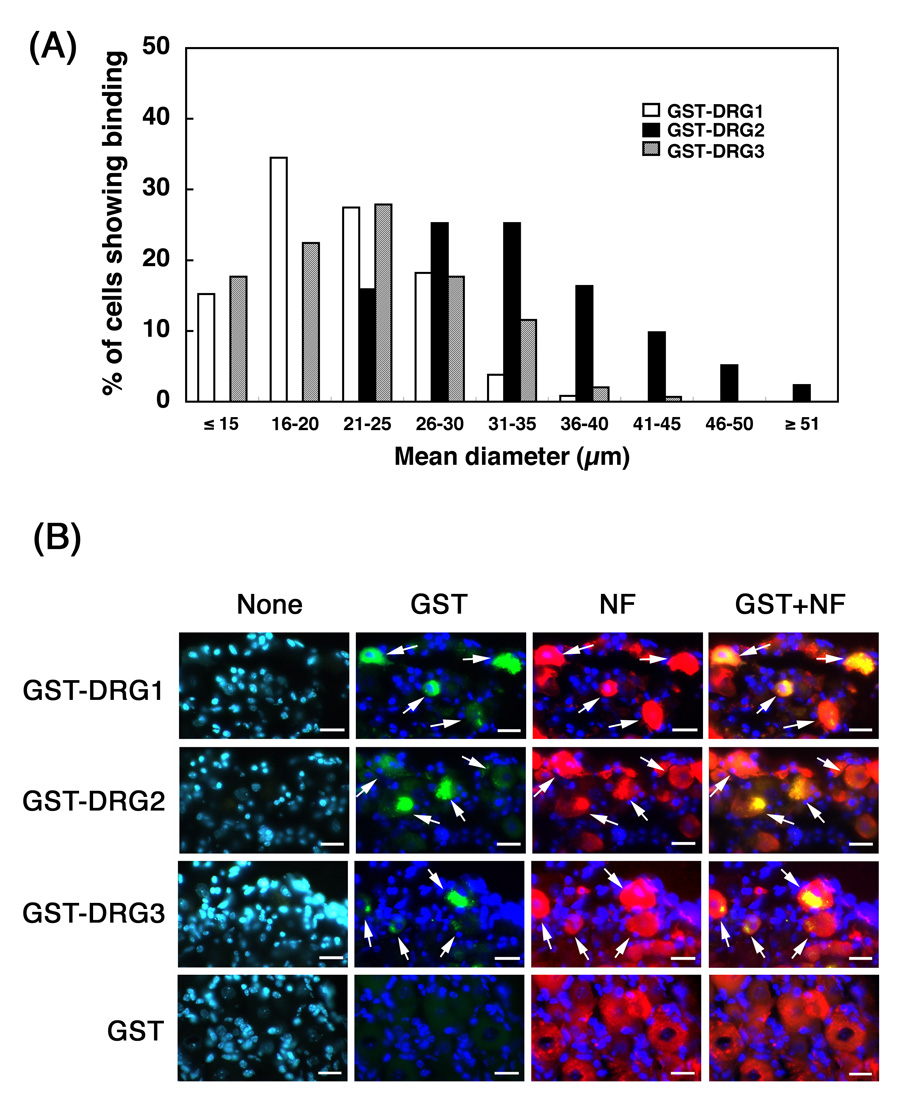
Fluorescence microscopy of in vivo binding of GST-peptide-fusion proteins to DRG neurons. (A) Cell size distribution of the DRG neurons positive for GST-DRG1, GST-DRG2 or GST-DRG3. (B) Double immunofluorescence staining of DRG-neurons with anti-GST (green) and anti-neurofilament L (NF-L, non-phosphorylated form, red). Nuclei were stained with DAPI (blue). None indicates no primary antibody. Arrows indicated the cells positive for both GST and NF-L. Bars = 20 µm.
We searched for possible endogenous candidates in the mouse protein database that might bind to the DRG, “receptors” for the three 7-amino acid peptides and found the same DRG1 7-amino-acid sequence in the extra cellular matrix protein TM14 (NP_077199.2) [24]. However, 6 out the 7 amino acids were located in the putative signal peptide and not in the putative binding domain in TM14. The mouse neuronal differentiation-related protein (NDRP, NP_001096649.1) has the same 7-amino acid sequence as that in DRG2 [6]. NDRP is expressed in the sensory neuronal system including in DRG neurons, though our current knowledge of its function is limited. We failed to find any peptide sequence in the database similar to that in DRG3.
We used the in vitro screening of a C7C peptide library displayed on M13 filamentous phages against isolated mouse DRG neurons to isolate phage-clones that encompassed three different 7-amino acid peptides that displayed specific binding for these neurons in vitro. Immunohistochemical analysis following the injection of GST-peptide fusion proteins in vivo demonstrated that the three peptides bind to DRG neurons with different sizes; furthermore, these fusion proteins were internalized into the cytoplasm of the targeted neurons.
The Ph.D.-C7C Phage display Peptide Library kit expresses random 7-amino acid peptides fused to the minor coat protein (pIII) of the M13 phage. By sequence analysis, only 113 phage plaques were represented in the bound peptide, consistent with a very efficient selection process (Table 1). The final binding studies allowed us to identify three phages (DRG1, DRG2, or DRG3) that occurred repeatedly. We believe that the efficient identification of these clones was the result of multiple rounds of positive and negative selection using the biopanning protocol [11]. We cannot exclude the possibility that some of the other 84 clones (Table 1) might represent true DRG-binding peptide-phages, but we decided to pursue the three best-represented clones at this time for practical reasons.
The three clones that we analyzed further showed interesting specificities to the targeted neurons: DRG-p1 and DRG-p3 to small-sized neurons and DRG-p2 to large-sized neurons. Moreover, the size specificity of target neurons for individual peptides was confirmed by in vivo binding experiments: GST-DRG1 and GST-DRG3 recognized small-sized neurons, and GST-DRG2 recognized large-sized neurons. Since both DRG1 and DRG3 recognized small sized neurons, two peptide ligands might bind to the same cell populations. Therefore, we performed double staining of isolated DRG neurons after the incubation with DRG-p1 and GST-DRG3 by antibodies against phage and GST, respectively. Double positive staining was obtained in 51% of DRG-p1-positive neurons, and was in 71% of GST-DRG3-positive neurons, respectively (data not shown). The result indicates that both DRG1 and DRG3 recognized different targeting molecules, but may bind to the same cell populations in specific subpopulations in small-sized neurons. Histologically, mammalian sensory DRG neurons have been classified into two major types: large-light and small-dark cells on the basis of their staining characteristics seen under light microscopy [9]. Clinically, peripheral sensory neuropathy (including neuronopathy) has been classified into two types based on fiber size: large fiber sensory neuropathy and small fiber sensory neuropathy [15, 21]. The symptom typically found in large fiber sensory neuropathy is an ataxic gait, and that in small fiber sensory neuropathy mainly pain [15, 21]. Neuronal size is directly related to axonal characteristics [9, 10, 21]. Thus, each targeting motif included in DRG1, DRG2, DRG3, and, potentially, in some of the other 84 peptides sequences, may be useful tools for pathophysiological analysis such as a cell-identification marker, or for the generation of gene-delivery constructs for sensory neuropathies [12, 14, 19]. Future studies will involve the isolation and identification of the DRG neuronal cell surface proteins that bind to the three peptides.
We also tried to determine the size of the targeted protein using the whole homogenate of DRG tissues from mice, and found some specific bands by Western blotting analysis (data not shown). Since those bands may be proteins targeted by the peptides, DRG1, DRG2, and DRG3, and should be clarified in the future studies.
In conclusion, we have used in vitro phage display technology to isolate and identify three different peptides that bind to DRG neurons in vitro and in vivo. We further showed that the peptides recognize neurons of two different sizes. While additional studies are needed to characterize the potential binding proteins and their receptors, the identification of three DRG neuron-specific peptides provides a powerful tool that will facilitate future research into the structural basis of neuronal cellular subpopulations and the generation of molecular delivery systems targeting different DRG neurons in vivo.
Acknowledgments
We thank M. Koike, H Urabe, Y. Katayama, K. Matsumura, T. Yamamoto, Y. Mori, R. Fujiwara, T. Watanabe, F. Kimura, and A. Yamaguchi for technical support. This work was supported by a Grant-in-Aid (#18390100 to H. Kojima and #18590934 to H. Yasuda) for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan, by the President’s Discretionary Fund from Shiga University of Medical Science (#1515503L to H. Kojima), and by USA National Institutes of Health grant (HL-51586 to L. Chan).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Adey NB, Mataragnon AH, Rider JE, Carter JM, Kay BK. Characterization of phage that bind plastic from phage-displayed random peptide libraries. Gene. 1995;156:27–31. doi: 10.1016/0378-1119(95)00058-e. [DOI] [PubMed] [Google Scholar]
- 2.Arap W, Kolonin MG, Trepel M, Lahdenranta J, Cardo-Vila M, Giordano RJ, Mintz PJ, Ardelt PU, Yao VJ, Vidal CI, Chen L, Flamm A, Valtanen H, Weavind LM, Hicks ME, Pollock RE, Botz GH, Bucana CD, Koivunen E, Cahill D, Troncoso P, Baggerly KA, Pentz RD, Do KA, Logothetis J, Pasqualini R. Steps toward mapping the human vasculature by phage display. Nat. Med. 2002;8:121–127. doi: 10.1038/nm0202-121. [DOI] [PubMed] [Google Scholar]
- 3.Dodd J, Jessell TM. Lactoseries carbohydrates specify subsets of dorsal root ganglion neurons projecting to the superficial dorsal horn of rat spinal cord. J. Neurosci. 1985;5:3278–3294. doi: 10.1523/JNEUROSCI.05-12-03278.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goss JR. The therapeutic potential of gene transfer for the treatment of peripheral neuropathies. Expert Rev. Mol. Med. 2007;9:1–20. doi: 10.1017/S1462399407000270. [DOI] [PubMed] [Google Scholar]
- 5.Hong FD, Clayman GL. Isolation of a peptide for targeted drug delivery into human head and neck solid tumors. Cancer Res. 2000;60:6551–6556. [PubMed] [Google Scholar]
- 6.Kato H, Chen S, Kiyama H, Ikeda K, Kimura N, Nakashima K, Taga T. Identification of a novel WD repeat-containing gene predominantly expressed in developing and regenerating neurons. J. Biochem. (Tokyo) 2000;128:923–932. doi: 10.1093/oxfordjournals.jbchem.a022843. [DOI] [PubMed] [Google Scholar]
- 7.Kusunoki S, Inoue K, Iwamori M, Nagai Y, Mannen T. Fucosylated glycoconjugates in human dorsal root ganglion cells with unmyelinated axons. Neurosci. Lett. 1991;126:159–162. doi: 10.1016/0304-3940(91)90543-3. [DOI] [PubMed] [Google Scholar]
- 8.Kolonin MG, Saha PK, Chan L, Pasqualini R, Arap W. Reversal of obesity by targeted ablation of adipose tissue. Nat. Med. 2004;10:625–632. doi: 10.1038/nm1048. [DOI] [PubMed] [Google Scholar]
- 9.Lawson SN. Phenotype and function of somatic primary afferent nociceptive neurones with C-, Adelta- or Aalpha/beta-fibres. Exp. Physiol. 2002;87:239–244. doi: 10.1113/eph8702350. [DOI] [PubMed] [Google Scholar]
- 10.Lawson SN, Waddell PJ. Soma neurofilament immunoreactivity is related to cell size and fibre conduction velocity in rat primary sensory neurons. J. Physiol. 1991;435:41–63. doi: 10.1113/jphysiol.1991.sp018497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liang S, Lin T, Ding J, Pan Y, Dang D, Guo C, Zhi M, Zhao P, Sun L, Hong L, Shi Y, Yao L, Liu J, Wu K, Fan D. Screening and identification of vascular-endothelial-cell-specific binding peptide in gastric cancer. J. Mol. Med. 2006;84:764–773. doi: 10.1007/s00109-006-0064-2. [DOI] [PubMed] [Google Scholar]
- 12.Liu JK, Teng Q, Garrity-Moses M, Federici T, Tanase D, Imperiale MJ, Boulis NM. A novel peptide defined through phage display for therapeutic protein and vector neuronal targeting. Neurobiol. Dis. 2005;19:407–418. doi: 10.1016/j.nbd.2005.01.022. [DOI] [PubMed] [Google Scholar]
- 13.Marmigere F, Ernfors P. Specification and connectivity of neuronal subtypes in the sensory lineage. Nat. Rev. Neurosci. 2007;8:114–127. doi: 10.1038/nrn2057. [DOI] [PubMed] [Google Scholar]
- 14.Mata M, Chattopadhyay M, Fink DJ. Gene therapy for the treatment of sensory neuropathy. Expert Opin. Biol. Ther. 2006;6:499–507. doi: 10.1517/14712598.6.5.499. [DOI] [PubMed] [Google Scholar]
- 15.Mendell JR, Sahenk Z. Clinical practice. Painful sensory neuropathy. N. Engl. J. Med. 2003;348:1243–1255. doi: 10.1056/NEJMcp022282. [DOI] [PubMed] [Google Scholar]
- 16.Nicklin SA, White SJ, Watkins SJ, Hawkins RE, Baker AH. Selective targeting of gene transfer to vascular endothelial cells by use of peptides isolated by phage display. Circulation. 2000;102:231–237. doi: 10.1161/01.cir.102.2.231. [DOI] [PubMed] [Google Scholar]
- 17.Petty NK, Evans TJ, Fineran PC, Salmond GP. Biotechnological exploitation of bacteriophage research. Trends Biotechnol. 2007;25:7–15. doi: 10.1016/j.tibtech.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 18.Price TJ, Flores CM. Critical evaluation of the colocalization between calcitonin gene-related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities, and isolectin B4 binding in primary afferent neurons of the rat and mouse. J. Pain. 2007;8:263–272. doi: 10.1016/j.jpain.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sah DW. Therapeutic potential of RNA interference for neurological disorders. Life Sci. 2006;79:1773–1780. doi: 10.1016/j.lfs.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Sergeeva A, Kolonin MG, Molldrem JJ, Pasqualini R, Arap W. Display technologies: application for the discovery of drug and gene delivery agents. Adv. Drug Deliv. Rev. 2006;58:1622–1654. doi: 10.1016/j.addr.2006.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sghirlanzoni A, Pareyson D, Lauria G. Sensory neuron diseases. Lancet Neurol. 2005;4:349–361. doi: 10.1016/S1474-4422(05)70096-X. [DOI] [PubMed] [Google Scholar]
- 22.Sidhu SS. Engineering M13 for phage display. Biomol. Eng. 2001;18:57–63. doi: 10.1016/s1389-0344(01)00087-9. [DOI] [PubMed] [Google Scholar]
- 23.Terashima T, Kojima H, Fujimiya M, Matsumura K, Oi J, Hara M, Kashiwagi A, Kimura H, Yasuda H, Chan L. The fusion of bone-marrow-derived proinsulin-expressing cells with nerve cells underlies diabetic neuropathy. Proc. Natl. Acad. Sci. U. S. A. 2005;102:12525–12530. doi: 10.1073/pnas.0505717102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Vega S, Iwamoto T, Nakamura T, Hozumi K, McKnight DA, Fisher LW, Fukumoto S, Yamada Y. TM14 is a new member of the fibulin family (fibulin-7) that interacts with extracellular matrix molecules and is active for cell binding. J. Biol. Chem. 2007 doi: 10.1074/jbc.M705847200. Epub ahead of print PMID: 17699513 [PubMed - as supplied by publisher] [DOI] [PubMed] [Google Scholar]
- 25.White SJ, Nicklin SA, Buning H, Brosnan MJ, Leike K, Papadakis ED, Hallek M, Baker AH. Targeted gene delivery to vascular tissue in vivo by tropism-modified adeno-associated virus vectors. Circulation. 2004;109:513–519. doi: 10.1161/01.CIR.0000109697.68832.5D. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Bao L. The development and modulation of nociceptive circuitry. Curr. Opin. Neurobiol. 2006;16:460–466. doi: 10.1016/j.conb.2006.06.002. [DOI] [PubMed] [Google Scholar]


