Abstract
Polycystine radiolaria are among few protistan groups that possess a comprehensive fossil record available for study by micropaleontologists. The Polycystinea and the Acantharea, whose skeletons do not become fossilized, were once members of the class “Radiolaria” (“Radiolaria” sensu lato: Polycystinea, Phaeodarea, and Acantharea) originally proposed by Haeckel but are now included in the superclass Actinopoda. Phylogenetic relationships within this superclass remain largely enigmatic. We investigated the evolutionary relationship of the Acantharea and the Polycystinea to other protists using phylogenetic analyses of 16S-like ribosomal RNA (rRNA) coding regions. We circumvented the need to culture these organisms by collecting and maintaining reproductive stages that contain many copies of their genomic DNA. This strategy facilitated extraction of genomic DNA and its purification from symbiont and prey DNA. Phylogenetic trees inferred from comparisons of 16S-like coding regions do not support a shared history between the Acantharea and the Polycystinea. However, the monophyly of the Acantharea and the separate monophyly of the Polycystinea (Spumellarida) are well supported by our molecular-based trees. The acantharian lineage branches among crown organisms whereas the polycystine lineage diverges before the radiation of the crown groups. We conclude that the Actinopoda does not represent a monophyletic evolutionary assemblage and recommend that this taxonomic designation be discarded.
The superclass Actinopoda (1) includes protists with specialized microtubule-stiffened pseudopodia called “axopodia.” Current taxonomic schemes of the superclass Actinopoda include the Acantharea, Polycystinea, and Phaeodarea and most members of the Heliozoea (except the pedinellids and heliomonads) (2, 3).
One of the major distinctions between the Acantharea and the Polycystinea is the composition, architecture, and symmetry of the skeleton, when present. Polycystines have a rich fossil record due to the presence of skeletal material comprised of silica, which is secreted by many members of this class and which becomes preserved in marine sediments. Polycystine skeletons exhibit a range of morphologies from simple spicules to more elaborate latticed shells possessing radial spines. All acantharia form skeletons comprised of monocrystals of strontium sulfate (SrSO4), which come together at the center of the cell in a symmetrical fashion known as Müller’s Law (4). Acantharian skeletons, however, are susceptible to dissolution and are therefore absent from the fossil record. In all phaeodaria, polycystines, and members of the acantharian order Arthracanthida, the central capsule or capsular wall divides the cell into an intracapsular region that includes the nucleus, mitochondria, golgi, and other major cellular machinery from the extracapsular region that contains the axopodial network of the cell. Phaeodarian and polycystine radiolaria possess pores in their central capsules whereas acantharia do not. Despite these differences, the common use of radial symmetry in cell body plan and shell architecture often results in a similar general appearance among members of the Acantharea and the polycystine order Spumellarida.
Today, the term “radiolaria” is generally an informal taxonomic descriptor for members of the Polycystinea (Spumellarida and Nassellarida) and the Phaeodarea, even though Ernst Haeckel (5) used the term to include members of the class Acantharea. Of the 4417 species of organisms described from collections of the Challenger Expedition, 3508 were new species of Radiolaria (5, 6). In his classification of the “Radiolaria,” Haeckel included four legions: the Acantharia, the Spumellaria, the Nassellaria, and the Phaeodaria. This classification was later modified (7–10) to exclude the Acantharia from the Spumellaria, Nassellaria, and Phaeodaria. Despite later taxonomic revision, many of Haeckel’s original descriptions of the Challenger Radiolaria persist today. Modern systematists place acantharia in a class distinct from polycystines and phaeodaria but generally agree that these classes are members of the Actinopoda (2, 3, 11–13).
The presence of axopodia and a central capsule are the predominant shared characters of the Acantharea and the Polycystinea. Most similarities occur between the Acantharea and the Polycystinea belonging to the order Spumellarida. Most acantharia are polynucleated, but the presence of a single nucleus in the acantharian genus Haliommatidium is a feature shared with the polycystines, which usually have only one nucleus. Similarities also occur between the “gelatinous pellicle” of some acantharia and that of certain Sphaerellarina, a suborder in the Spumellarida (14, 15). Both classes use SrSO4. Members of the Acantharea build entire skeletons of SrSO4 whereas adult vegetative colonial spumellaria and swarmer cells of reproductive spumellaria contain individual crystals of SrSO4 (2, 16–18).
The molecular diversity of the superclass Actinopoda is unexplored. We sequenced the 16S-like rRNA genes of representatives of the Acantharea and Polycystinea to test the hypothesis that the Acantharea and the Polycystinea share a common evolutionary history. According to phenotypic similarities, the taxa chosen for this study represent the most closely related classes in the Actinopoda. Therefore, this study addresses the appropriateness of the higher taxon designation Actinopoda and rekindles the debate over the best definition of “Radiolaria.”
MATERIALS AND METHODS
Sample Collection.
Individual specimens were collected by divers using glass or polycarbonate jars. Specimens were maintained in 0.22 μm of filtered Sargasso Sea water in glass culture tubes with brine shrimp (Artemia salina) as food. All acantharia were collected off of the southwestern coast of Bermuda in September 1994. Acantharian samples included Haliommatidium sp. (BBSR 235: Order: Symphyacanthida, Family: Pseudolithidae) and Chaunacanthid 218 (BBSR 218: Order: Chaunacanthida). Polycystine radiolarian specimens included one solitary and three colonial forms, all from the order Spumellarida. The solitary spumellarian Thalassicolla nucleata (BBS 3: Family: Thalassicollidae) was collected in May 1992, colonial spumellarian Collosphaera globularis-huxleyi (BBSR 173: Family: Collosphaeridae) in May 1994, and colonial spumellaria Sphaerozoum punctatum (CR4: Family: Sphaerozoidae) and Collozoum serpentinum (CR16: Family: Sphaerozoidae) in May 1995. Specimens used for in situ hybridizations were collected in September and October of 1995.
DNA Extraction, Amplification, Cloning, and Sequencing.
A natural amplification of DNA occurs before swarmer cell release from reproductive acantharia and spumellaria. Many species of acantharia and spumellaria either consume or expel endocytoplasmic symbiotic algae just before swarmer cell formation. We harvested specimens at this point of the life cycle to enrich for sarcodine DNA and to reduce the potential of amplifying nontarget DNA.
Individual central capsules or cysts were rinsed in 0.22 μm of filtered sea water and transferred to lysis or PCR buffer solution. Central capsules from T. nucleata specimens were lysed and genomic DNA was extracted and precipitated according to standard protocols (19). Central capsules or individual acantharian cysts from taxa other than T. nucleata were rinsed as above, placed in modified 1X PCR buffer [50 mM KCl/10 mM Tris, pH 8.3/2 mM MgCl2/0.001% gelatin/1.0% Nonidet P-40 (Sigma)], and lysed at 95°C for 10 minutes.
Thalassicolla nucleata, C. globularis-huxleyi, and Haliommatidium sp. 16S-like rDNAs were PCR-amplified using eukaryotic primers for nuclear 16S-like rRNA coding regions (20). Templates were cloned into either M13 or the plasmid vector pCRII (Invitrogen). Colonial spumellarian or acantharian specific probes described below were used as primers in combination with Medlin primers to amplify the remaining samples that were then sequenced directly. Double-stranded sequencing of the rRNA coding regions was accomplished using either Sequenase Ver. 2.0 (United States Biochemical) and existing rDNA primers (20, 21) or using a Li-Cor 4000L automated sequencer and Sequitherm cycle sequencing protocols developed by Li-Cor (Lincoln, NE) (22).
In Situ Hybridizations.
In situ whole cell hybridizations using oligonucleotide probes complementary to the presumptive sarcodine 16S-like rRNA verified that the sequence data were derived from the sarcodine DNA and not from contaminating algal symbionts or prey material. We designed probes that would specifically hybridize with colonial radiolaria or acantharia but no other eukaryotes in the Ribosomal Database Project (23). The biotin-labeled probes designed for acantharian samples were A497bio, 5′-TCATTCCAATCAACTCAC-3′ and A899bio, 5′-TCGTCATACAAAGGTCCA-3′. The colonial spumellarian probes were R906bio, 5′-AACGATAAAATACTAATA-3′ and R1451bio, 5′-TATTGTAGCCCGTGCGCT-3′. Eukaryote-specific, 5′-biotinylated probes that hybridize with all known eukaryotic 16S-like rRNAs served as positive controls. These were EUK502Rbio; 5′-ACCAGACTTGCCCTCC-3′ (24) and EUK1209Rbio; 5′-GGGCATCACAGACCTG-3′ (25).
In situ hybridizations were carried out using both fluorescence and colorimetric detection methods. Fluorescence in situ hybridizations on acantharia were carried out as described (26) using biotinylated probes and detection with fluorescein isothiocyanate (FITC)–avidin solution (20 μg/ml in 100 mM NaHCO3-buffered saline, pH 8.2). Acantharia were fixed for 1 h at 4°C in 1X Histochoice fixative (Amresco, Euclid, OH) diluted in 0.22 μm of filtered Sargasso Sea water. Individuals were then transferred to gel-subbed slides, overlaid with 0.05% agarose, and allowed to dry overnight. Probe was added to a final concentration of 5 ng/μl. Probe experiments consisted of a negative control (incubation in fluorescein-labeled avidin with no probe added), a positive control (biotinylated EUK 1209Rbio added), and two separate acantharian-specific probe experiments using A497bio and A899bio oligonucleotides, respectively. Hybridizations were carried out at 42°C for 6–8 h and washed at 45°C. Cells were mounted in Citifluor immersion oil (Citifluor, Kent, U.K.) and viewed on a Zeiss Axiophot equipped for epifluorescence microscopy.
We used colorimetry-based in situ hybridizations for the colonial spumellarian samples to avoid severe autofluorescence. Hybridizations were carried out on colonial spumellarian samples using the GIBCO/BRL In Situ Hybridization and Detection System with the following modifications for use with rRNA and larger sarcodines. Colonies were preserved in 1X Histochoice with 95% ethanol added in a ratio of 4:1 for 1 h at 4°C, transferred to 70% ethanol, and held overnight at 4°C. Aliquots of preserved central capsules from a single colony were placed on silanated glass slides (Midwest Scientific; St. Louis, MO) and allowed to air dry. Slides were then baked at 65°C for 1 hour to remove endogenous alkaline phosphatase activity. Hybridizations were carried out in 50 μl-capacity Probe-Clip “Press-to-Seal” incubation chambers and holders (Midwest Scientific, St. Louis). Four probe experiments were carried out using central capsules from the same colony: a negative control incubation (streptavidin-alkaline phosphatase conjugate with no probe added), a negative probe control (A899bio acantharian probe added), a positive probe control (EUK 502bio and EUK 1209bio added), and a colonial spumellarian probe experiment (R906bio and R1451bio added). The final concentration of probes in all experiments was 1 ng/μl.
Hybridizations were conducted according to the manufacturer’s instructions for “DNA Detection” with the above modifications and the omission of any steps specifically required for DNA targets. Slides were hybridized for 8 h, and probe detection was carried out according to manufacturer’s protocol with levamisole (Sigma) added at 200 μg/ml upon addition of alkaline phosphatase conjugate to remove endogenous alkaline phosphatase activity. Developed slides were permanently mounted in Crystal/Mount (Biomedia, Foster City, CA) and observed on a Zeiss standard microscope equipped with phase microscopy.
Phylogenetic Analysis.
The 16S-like rRNA sequences of acantharian and spumellarian samples were aligned against a subset of sequences representing a range of eukaryotic evolutionary lineages maintained by the Ribosomal Database Project (23). Initially, we aligned our sarcodine sequences with the subset of eukaryotic 16S-like rRNA sequences in the Ribosomal Database Project according to conservation of primary structure. The alignment was refined by juxtaposing regions that define evolutionarily conserved secondary structures. Phylogenetic analyses were based on 1369 positions considered to be in unambiguous alignment. Structural similarities were converted to evolutionary distances using the Jukes Cantor (27) correction, and a phylogeny was inferred by the method of Olsen (28). One hundred bootstrap replicates were conducted, and a consensus tree was obtained using phylip Ver. 3.5 (29). Phylogenetic trees were also inferred by the maximum likelihood method using the program fastdnaml (30) using a generalized two-parameter model of evolution (31) and the maximum parsimony method using the program paup, Ver. 3.1.1 (32). The maximum parsimony tree was obtained from a consensus of 100 bootstrap replications that were conducted using a heuristic search option with random addition sequence, 10 replicates, and the tree bisection–reconnection algorithm. Identical phylogenetic analyses were performed with Phreatamoeba balamuthi removed from the data set to determine stability of the relative branching order of the acantharia and the polycystine radiolaria.
RESULTS
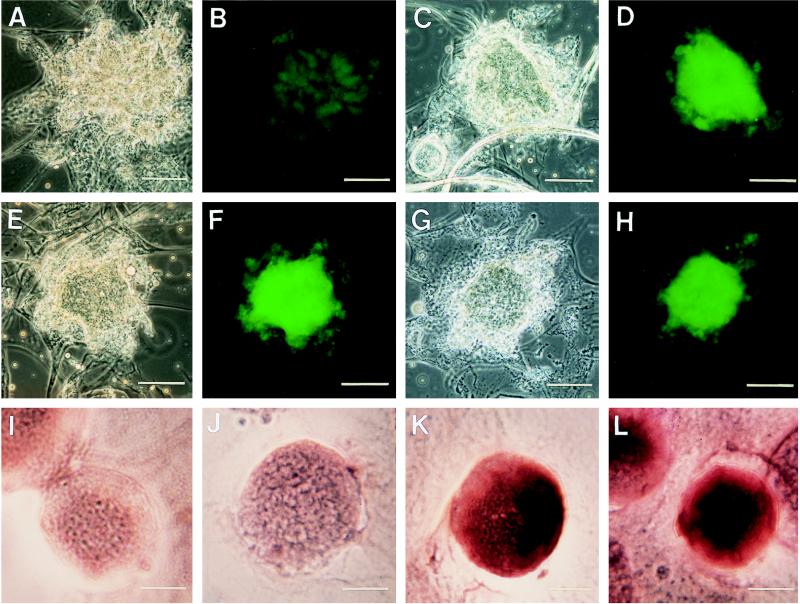
In situ hybridization experiments confirmed the origin of the acantharian and spumellarian sequences (Fig. 1). Acantharian-specific probes specifically hybridized to the acantharia (Fig. 1, F and H) but not to colonial spumellaria (Fig. 1J). Likewise, colonial spumellarian probes specifically hybridized with colonial spumellaria (Fig. 1L).
Figure 1.
In situ hybridization of Histochoice-preserved specimens using oligodeoxynucleotide probes complementary to the 16S-like (small subunit) rRNA sequences of acantharia (A–H) and colonial spumellaria (I–L). For both acantharian and colonial spumellarian cells, probes conjugated to biotin were detected by either FITC–avidin or streptavidin–alkaline phosphatase-conjugated secondary labels. For the acantharian cells, hybridization was detected by epifluorescence microscopy with settings specific for FITC excitation (B, D, F, and H). Colonial spumellarian cells were viewed using phase contrast microscopy, and hybridizations were detected colorimetrically using the localized, purple precipitate of the enzymatic reaction of alkaline phosphatase on nitroblue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate substrates. (A–H) Four different acantharian cells of the same species with corresponding transmitted light and epifluorescence photomicrographs of the same cell. Epifluorescence photomicrographs were taken with an integral camera system using a FITC filter set consisting of a 450- to 490-nm band-pass excitation filter, a 510-nm long-pass dichroic mirror, and a 515- to 565-nm band-pass emission filter. Fuji 100 ASA Provia color slide film was used for fluorescence pictures. Transmitted light photomicrographs of samples were taken with an Olympus OM4-T camera using Kodak 160 speed Tungsten film. All exposure times for a set of samples (i.e., negative control, positive control, taxon-specific probes) were kept constant so that the relative intensity was indicative of probe binding. (Bars = 75 μm for A–H.) (A and B) The negative control to which only FITC–avidin was added. Note the minimal background fluorescence of the cell under epifluorescence (B). (C and D) The positive control treatment to which a eukaryotic-specific probe designed to target all eukaryotes (EUK 1209R) was added. (E–H) Probing with two different acantharian probes (F, A497; H, A899). (I–L) Hybridization results for single individuals within the same spumellarian colony. (I) The negative control to which only the streptavidin-alkaline phosphatase-conjugated secondary label was added. (J) A negative probe control to which an acantharian probe was added. (K) The results of the positive control hybridization with eukaryote probes (EUK502R and EUK1209R). (L) Hybridization with colonial spumellarian probes (R906 and R1451). (Bars = 35 μm for I–L.)
The % G + C content of the 16S-like rRNA gene for Haliommatidium sp. and Chaunacanthid 218 was 44% and 45%, respectively, which was similar to many of the other taxa used in the analyses. However, spumellarian % G + C content values (35–38%) were similar to that of Entamoeba gingivalis (34%) and were low relative to typical eukaryotic values that are usually close to 50%. The lengths of 16S-like rRNA coding regions in base pairs for the spumellaria ranged from 1770 to 1798, which are typical of other eukaryotes.
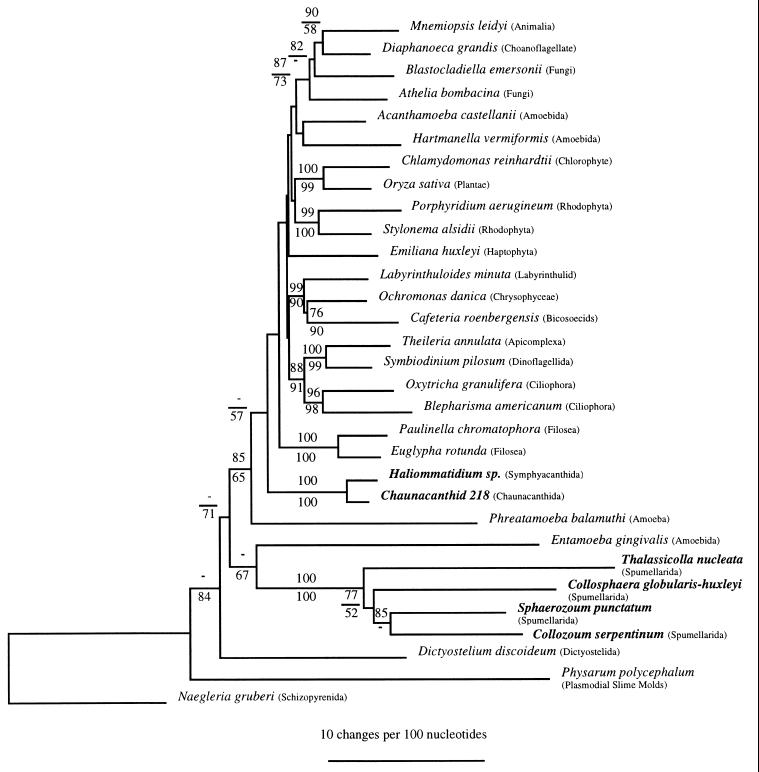
The phylogenetic trees inferred by the distance matrix, maximum parsimony (Fig. 2), and maximum likelihood (data not shown) methods clearly rejected a common ancestry between the acantharia and the spumellaria. Numbers at nodes represent bootstrap values as a percentage of 100 resamplings of the data set. Only bootstrap values >50% (which we interpret as relative measures of confidence for specific topological features of the tree) are shown. Both the distance and parsimony trees placed the spumellaria branching as a diverging lineage below the “crown” group (33), those taxa representing major eukaryotic assemblages simultaneously radiating from the node labeled with a bootstrap value of 85/65. Both methods revealed a poorly resolved branching point for the acantharia (Haliommatidium sp. and Chaunacanthid 218) among the crown radiation. The relative positions of the acantharia and the polycystine radiolaria were not affected by removal of Phreatamoeba balamuthi (data not shown). Removal of Phreatamoeba balamuthi from the data set resulted in higher bootstrap support values for the node leading to the crown (89/98) with acantharia branching among crown groups and spumellaria branching before the crown groups.
Figure 2.
The inferred phylogeny for the acantharia and the spumellaria. A distance tree is shown. Bootstrap values for distance analyses are given above the line whereas maximum parsimony values are below. A dash indicates that the bootstrap value for that node was below 50% in the method used for phylogeny reconstruction. The bar insert corresponds to 10 changes per 100 nt positions.
A low bootstrap value of 67% was obtained for the branching of the spumellaria with Entamoeba gingivalis in the parsimony analysis, but this support was not observed in the distance analysis nor the topology of the maximum likelihood analysis (data not shown). No other potential immediate common ancestors for the spumellaria were indicated by these data. The position of the spumellaria relative to other groups branching below the crown varied between the distance and parsimony analyses. Therefore, the exact branching order of the spumellaria also remains unresolved at this time. The monophyly of the acantharia and the separate monophyly of the spumellaria, however, were well supported (100% in all cases).
The branching patterns within the spumellaria in both distance and parsimony analyses showed the solitary spumellarian T. nucleata branches before the colonial spumellaria. Although the bootstrap support for this node was only 52% in the parsimony analysis, a higher bootstrap value (77%) was obtained with distance methods.
DISCUSSION
The relative positions of the acantharia and the spumellaria in molecular phylogenetic trees indicate that the presence of axopodia, a capsule membrane, and the ability to metabolize SrSO4 should be reconsidered as reliable phylogenetic markers for these taxonomic groups. Our molecular study of acantharian and spumellarian phylogeny confirms that axopodia have evolved more than once and most likely represent convergent structures created in response to similar ecological constraints through evolutionary time (34–38). An independent evolution of axopodia within the chromistan Pedinellea of the Heliozoea already has been suggested on morphological grounds (2). Given the results of this study, retention of the superclass Actinopoda seems inappropriate, as does the adoption of the new phylum Radiozoa, which has been described as a modern-day Radiolaria sensu lato (2, 3).
The presence of a central capsule and the ability to secrete SrSO4 are two synapomorphies for the Radiozoa (2). However, the central capsule found in spumellaria and that which exists in one order of the Acantharea (the Arthracanthida) differ (15, 37). The acantharian central capsule is nonperforated and of a different cellular origin than that found in polycystines and phaeodaria. Furthermore, the presence of central capsules in the Arthracanthida, an order that is considered more derived than other orders of acantharia that lack central capsules (37–39), suggests that “central capsules” may have evolved more than once.
The occurrence of SrSO4 in both acantharia and spumellaria is another feature cited as evidence of their common ancestry. SrSO4 constitutes the main skeletal material in acantharia. Vegetative adults of colonial spumellaria, however, also are known to contain crystals of SrSO4 in their central capsules, and the biflagellated swarmers of all spumellaria examined thus far contain crystals of SrSO4 in membrane-bound vesicles. However, metabolism of SrSO4 is not unique to acantharia and spumellaria. Crystals of SrSO4 have been observed in the desmid alga Closterium littorale (40), in Chara, the “stonewort” freshwater plant (40), and in loxodid ciliates (41). The role of SrSO4 in Chara and the loxodid ciliates appears to be graviperception (40, 41). A similar function has been proposed in the desmid algae (40). This function apparently has never been proposed for the membrane-bound crystals found in spumellarian swarmers. Instead, Anderson (18) proposed that SrSO4 crystals may serve a function in buoyancy control but noted that silica or calcium compounds, which occur at higher concentrations in sea water, would be better candidates for this purpose. Anderson also suggested that strontium may be of some physiological importance to the spumellaria but did not elaborate on what this requirement might be. One possibility is that strontium serves a similar function in spumellaria as in some gastropods where it is required for proper shell development (42). One inconsistency of the potential importance of strontium in spumellarian skeletal development is that spumellarian species that lack skeletal material (e.g., T. nucleata and Collozoum spp.) have swarmers with crystalline SrSO4 inclusions. Also, the lack of SrSO4 crystals in acantharian swarmer cells is inconsistent with the hypothesis that SrSO4 serves a similar function in both the Acantharea and the spumellarian polycystines.
The absence of SrSO4 crystals in swarmer cells of the Acantharea implies another difference between SrSO4 utilization in acantharia and spumellaria. Acantharian swarmer cells lack SrSO4 crystals, yet their reproductive life cycle stage occurs deeper in the euphotic zone (37). This suggests a need for buoyancy control. Perhaps acantharian cells use myonemes and other cellular structures as hydrostatic devices to regulate depth in the water column. In addition, many species of acantharia form cysts also comprised of SrSO4 that aid in the sinking of swarmer cells to depth. The greater density of SrSO4 relative to silica may also explain why polycystine radiolaria, which can possess siliceous skeletons, use the heavier SrSO4 in their swarmers. Of interest, phaeodarian radiolaria, which live deeper in the water column, have lost the capacity to deposit SrSO4 and, like acantharia, lack SrSO4-containing swarmer cells. Whatever function served, the presence of SrSO4 in these marine protists and its singular utilization as the structural compound in the skeletons of acantharia warrant further scrutiny in the evolution of this group as do their unique non-actin-containing myonemes.
Which protistan lineage shares the most recent ancestry with the Acantharea? The branching pattern of the acantharia was shallow relative to that of the spumellaria, so it is likely that acantharia diversified more recently than the spumellaria and that an extant relative shares some of the unique features possessed by the Acantharea. We conclude that the ability to deposit SrSO4 is a less reliable phylogenetic marker than the ability to construct elaborate skeletal architecture using SrSO4. For example, the closest relative to the acantharia may also possess strontium-containing skeletons. Among the possibilities is Podactinelius sessilis (43), first described on the Deutschen Südpolar Expedition of 1901–1903. This genus possesses spines of SrSO4 (400–500); however, these spines are not arranged in the characteristic geometric pattern observed in all members of the Acantharea. The genus was once included as a separate order Actineliida in the class Acantharea; however, it has been relegated recently to an uncertain taxonomic affinity (11).
The determination of the nearest relative of the Spumellarida remains uncertain. It may be difficult to determine the phenotype of the most recent common ancestor of the Spumellarida if the long branches in the spumellarian lineage are interpreted as evidence of a very ancient divergence. Although the fossil record of spicule-bearing forms (Sphaerozoidae) extends to the Lower Oligocene (44) and that of the Collosphaeridae to the base of the Miocene (45), even more ancient origins are possible in view of the existence of extant skeletonless forms that would not be preserved in the sediments. As an alternative hypotheses, the long branch lengths of the spumellaria may be explained as the result of a rapidly evolving lineage.
It is assumed that the Nassellarida that represent the second order included in the Polycystinea are closely related to the Spumellarida (46). The molecular phylogenetic position of the Phaeodarea is also unknown and deserves consideration. As for Haeckel’s Radiolaria and the definition of the Radiolaria sensu lato (Polycystinea, Phaeodarea, and Acantharea), continued use of this definition in anything but a historical perspective is unjustified in view of the results described herein.
Acknowledgments
We thank Drs. Annika Sanfilippo and William Riedel for reprints and translated Russian references, Dr. Rebecca Gast for critical reading of this manuscript, Dr. Alexi Shalapyonok for assistance with translating Russian manuscripts, and Gaspar Taroncher–Oldenburg for assistance in German translation. We also thank Drs. Anthony Michaels, O. Roger Anderson, and Neil Swanberg for help with identifications of specimens and helpful discussions. Drs. Anthony Michaels and Hank Trapido-Rosenthal opened their labs at the Bermuda Biological Station for Research. Frances Howes and numerous other volunteers assisted with blue water diving collection of specimens. This report is based upon work supported under a National Science Foundation Graduate Fellowship. Further financial support is acknowledged in part from the National Science Foundation (OCE-9314533) (to D.A.C.) and from the Scurlock Fund for research at the Bermuda Biological Station for Research, the Grant-In-Aid Fund of the Bermuda Biological Station for Research, funds from the Woods Hole Coastal Research Center, the Education Office of the Woods Hole Oceanographic Institution, and the Lerner Gray Fund for Marine Research of the American Museum of Natural History (to L.A.A.Z.), National Institutes of Health (GM32964), and the Unger G. Vettlesen Foundation (to M.L.S.). This is Woods Hole Oceanographic Institution Contribution No. 9547.
ABBREVIATIONS
- rRNA
ribosomal RNA
- SrSO4
strontium sulfate
- FITC
fluorescein isothiocyanate
Footnotes
References
- 1.Calkins G N. Protozoölogy. New York: Lea & Febiger; 1909. pp. 1–349. [Google Scholar]
- 2.Cavalier-Smith T. Microbiol Rev. 1993;57:953–994. doi: 10.1128/mr.57.4.953-994.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corliss J O. Acta Protozool. 1994;33:1–51. [Google Scholar]
- 4.Müller J. In: Abhandlungen der Königlische Akademie der Wissenschaften zu Berlin. Dummler F, editor; Dummler F, editor. Berlin: Deutsche Akademie der Wissenschaften zu Berlin; 1858. pp. 1–62. [Google Scholar]
- 5.Haeckel E. In: The Voyage of the H. M. S. Challenger. Thompson C W, Murray J, editors; Thompson C W, Murray J, editors. London: Her Majesty’s Stationary Office; 1887. pp. 1–1760. [Google Scholar]
- 6.Anderson O R. Radiolaria. New York: Springer; 1983. pp. 1–355. [Google Scholar]
- 7.Deflandre G. In: Traité de Paléontologie. Piveteau J, editor; Piveteau J, editor. Paris: Masson; 1952. pp. 303–313. [Google Scholar]
- 8.Deflandre G. In: Traité de Zoologie. Grassé P P, editor; Grassé P P, editor. Paris: Masson; 1953. pp. 389–436. [Google Scholar]
- 9.Tregouboff G. In: Traité de Zoologie. Grassé P P, editor; Grassé P P, editor. Paris: Masson; 1953. pp. 271–320. [Google Scholar]
- 10.Goll R M, Merinfeld E G. In: The Encyclopedia of Paleontology. Fairbridge R W, Jablonski D, editors; Fairbridge R W, Jablonski D, editors. Hutchinson, & Ross, Stroudsburg: Dowden; 1979. pp. 673–684. [Google Scholar]
- 11.Levine N D, Corliss J O, Cox F E G, Deroux G, Grain J, Honigberg B M, Leedale G F, Loeblich A R I, Lom J, Lynn D, Merinfeld E G, Page F C, Poljansky G, Sprague V, Vavra J, Wallace F G. J Protozool. 1980;27:37–58. doi: 10.1111/j.1550-7408.1980.tb04228.x. [DOI] [PubMed] [Google Scholar]
- 12.Lee J J, Hutner S H, Bovee E C. An Illustrated Guide to the Protozoa. Lawrence, KS: Society of Protozoologists; 1985. pp. 1–629. [Google Scholar]
- 13.Febvre J. In: Handbook of Protoctista. Margulis L, Corliss J O, Melkonian M, Chapman D J, editors; Margulis L, Corliss J O, Melkonian M, Chapman D J, editors. Boston: Jones and Bartlett; 1990. pp. 363–379. [Google Scholar]
- 14.Hollande A, Enjumet M. Arch Museum Paris. 1960;VII:1–134. [Google Scholar]
- 15.Massera Bottazzi E. Boll Zool. 1978;45:133–144. [Google Scholar]
- 16.Dogel V A. Zoologicheski Zhurnal (Zoological Journal) 1950;29:562–565. [Google Scholar]
- 17.Hollande A, Martoja R. Protistologica. 1974;X:603–609. [Google Scholar]
- 18.Anderson O R. In: Silicon and Siliceous Structures in Biological Systems. Simpson T L, Volcani B E, editors; Simpson T L, Volcani B E, editors. New York: Springer; 1981. pp. 347–379. [Google Scholar]
- 19.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. Plainview, NY: Cold Spring Harbor Lab. Press; 1984. [Google Scholar]
- 20.Medlin L, Elwood H J, Stickel S, Sogin M L. Gene. 1988;71:491–499. doi: 10.1016/0378-1119(88)90066-2. [DOI] [PubMed] [Google Scholar]
- 21.Elwood H J, Olsen G J, Sogin M L. Mol Biol Evol. 1985;2:399–410. doi: 10.1093/oxfordjournals.molbev.a040362. [DOI] [PubMed] [Google Scholar]
- 22.Middendorf L R, Bruce J C, Bruce R C, Eckles R D, Grone D L, Roemer S C, Slonicker G D, Steffens D L, Sutter S L, Brumbaugh J A, Patonay G. Electrophoresis. 1992;13:487–494. doi: 10.1002/elps.11501301103. [DOI] [PubMed] [Google Scholar]
- 23.Olsen, G. J., Overbeek, R., Larsen, N., Marsh, T. L., McCaughey, M. J., Maciukenas, M. A., Kuan, W. -M., Macke, T. J., Xing, Y. & Woese, C. R. (1992) Nucleic Acids Res. 20, Suppl., 2199–2200. [DOI] [PMC free article] [PubMed]
- 24.Amann R I, Binder B J, Olson S W, Devereux R, Stahl D A. Appl Environ Microbiol. 1990;56:1919–1925. doi: 10.1128/aem.56.6.1919-1925.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Giovannoni S J, DeLong E F, Olsen G J, Pace N R. J Bacteriol. 1988;170:720–726. doi: 10.1128/jb.170.2.720-726.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim E L, Amaral L A, Caron D A, DeLong E F. Appl Environ Microbiol. 1993;59:1647–1655. doi: 10.1128/aem.59.5.1647-1655.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jukes T H, Cantor C R. In: Mammalian Protein Metabolism. Munro H N, editor; Munro H N, editor. New York: Academic; 1969. pp. 21–32. [Google Scholar]
- 28.Olsen G J. Methods Enzymol. 1988;164:793–812. doi: 10.1016/s0076-6879(88)64084-5. [DOI] [PubMed] [Google Scholar]
- 29.Felsenstein J. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 30.Olsen G J, Matsuda H, Hagstrom R, Overbeek R. Comput Appl Biosci. 1994;10:41–48. doi: 10.1093/bioinformatics/10.1.41. [DOI] [PubMed] [Google Scholar]
- 31.Kishino H, Hasegawa M. J Mol Evol. 1989;29:170–179. doi: 10.1007/BF02100115. [DOI] [PubMed] [Google Scholar]
- 32.Swofford D L. paup: Phylogenetic Analysis Using Parsimony. Champaign, IL: Illinois Natural History Survey; 1991. [Google Scholar]
- 33.Knoll A H. Science. 1992;256:622–627. doi: 10.1126/science.1585174. [DOI] [PubMed] [Google Scholar]
- 34.Merinfeld E G. Biosystems. 1978;10:29–30. [Google Scholar]
- 35.Shulman S S, Reshetnyak V V. In: Realno li cushchestvovanie v cisteme prosteishikh nadklassa Actinopoda? (About the Possibility of the Superclass Actinopoda Existence in the System of Protozoa?) Skarlato O A, editor; Skarlato O A, editor. Proceedings of the Zoological Institute, Leningrad, USSR: Academy of Science of the USSR; 1980. pp. 23–41. [Google Scholar]
- 36.Merinfeld E G. Phylogenetic Relationships Among the Actinopoda. Poland: Warszawa; 1981. p. 244. [Google Scholar]
- 37.Reshetnyak V V. Acantharia (Acanthariea, Protozoa) from the World Ocean. SSSR, Leningrad: Nauka; 1981a. p. 224. [Google Scholar]
- 38.Hollande A, Cachon J, Cachon-Enjumet M. Protistologica. 1965;1:91–112. [Google Scholar]
- 39.Strelkov A A, Reshetnyak V V. In: Chemoradiology of the Pelagical and Benthal (Metals and Their Radionuclei in Hydrobionts and in Their Environment Medium) Polikarpov G G, editor; Polikarpov G G, editor. Kiev: Naukova Dumka; 1974. pp. 95–191. [Google Scholar]
- 40.Raven J A, Smith F A, Walker N A. In: Biomineralization in Lower Plants and Animals. Leadbeater B S C, Riding R, editors; Leadbeater B S C, Riding R, editors. New York: Clarendon; 1986. pp. 125–139. [Google Scholar]
- 41.Fenchel T, Finlay B J. J Protozool. 1986;33:69–79. [Google Scholar]
- 42.Bidwell J P, Paige J A, Kuzirian A M. Biol Bull. 1986;170:75–90. [Google Scholar]
- 43.Schröder O. Deutsche Südpolar-Expedition 1901–1903. 1907;9:227–236. [Google Scholar]
- 44.Bjørklund K R, Goll R M. J Paleontol. 1979;53:1293–1326. [Google Scholar]
- 45.Riedel W R. In: The Fossil Record. Harland W B, editor; Harland W B, editor. London: Geol. Soc. London; 1967. pp. 291–298. [Google Scholar]
- 46.Cachon J, Cachon M, Estep K W. In: Handbook of Protoctista. Margulis L, Corliss J O, Melkonian M, Chapman D J, editors; Margulis L, Corliss J O, Melkonian M, Chapman D J, editors. Boston: Jones and Bartlett; 1990. pp. 334–346. [Google Scholar]