Abstract
Wolbachia, a maternally transmitted microorganism of the Rickettsial family, is known to cause cytoplasmic incompatibility, parthenogenesis, or feminization in various insect species. The bacterium–host relationship is usually symbiotic: incompatibility between infected males and uninfected females can enhance reproductive isolation and evolution, whereas the other mechanisms enhance progeny production. We have discovered a variant Wolbachia carried by Drosophila melanogaster in which this cozy relationship is abrogated. Although quiescent during the fly’s development, it begins massive proliferation in the adult, causing widespread degeneration of tissues, including brain, retina, and muscle, culminating in early death. Tetracycline treatment of carrier flies eliminates both the bacteria and the degeneration, restoring normal life-span. The 16s rDNA sequence is over 98% identical to Wolbachia known from other insects. Examination of laboratory strains of D. melanogaster commonly used in genetic experiments reveals that a large proportion actually carry Wolbachia in a nonvirulent form, which might affect their longevity and behavior.
Keywords: symbiosis, microbial infection, parasite, Rickettsia, life-span
Deadly human diseases such as typhus, Rocky Mountain spotted fever, and Q fever are caused by Rickettsiae, obligate intracellular microorganisms associated with arthropod vectors (1). Wolbachia, a genus of the Rickettsial family, although not yet reported in mammals, is widespread among insects. More than a million insect species are estimated to contain the bacteria, which are maternally transmitted (2). Wolbachia are known, in various species, to cause cytoplasmic incompatibility (CI): crosses between bacteria-infected males and uninfected females produce few offspring, whereas infected females, due to an unknown mechanism, are resistant and produce ample progeny (3, 4). In the wasp, the bacteria can cause parthenogenesis: unfertilized eggs become diploid females instead of the usual haploid males (5). In the wood louse, Armadillidium vulgare, the bacteria feminize genetic males into functional females (6). These mechanisms provide advantages to the host in increasing progeny or accelerating evolution, while at the same time providing a venue for propagation of the host-dependent bacteria, which can grow in insect cells (7), but not in vitro. Therefore, the relationship between Wolbachia and host is generally regarded as symbiotic. Wolbachia can be transferred by injection, within or between species (8). Braig et al. (9) found that the bacteria from the mosquito Aedes albopictus, transferred by injection into uninfected embryos of Drosophila simulans, confer complete CI on the adults. The bacteria have been reported in a number of Drosophila species, associated with varied degrees of CI, including only weak or no effects (10–14). The various qualitative and quantitative effects seen in different Wolbachia–host combinations pose the question of what bacterium–host interactions are at play. Therefore it would be desirable to have a system for genetic analysis of these interactions.
MATERIALS AND METHODS
Electron Microscopy (EM) and Immunohistochemistry.
Flies were prepared by fixation in 1% paraformaldehyde, 1% glutaraldehyde, postfixation in 1% osmium tetroxide, dehydration in an ethanol series, and embedding in Epon 812. For EM, ultrathin sections (80 nm) were examined with a Philips 201 electron microscope at 60 kV. Cryostat sections (10 μm) of fly ovaries were stained with Wolbachia-specific monoclonal antibody (18) and visualized with Cy3-conjugated mouse secondary antibody.
PCR.
Total DNA was extracted from 20 adult fly ovaries and used as template for the PCR. Wolbachia-specific 16s rDNA primers were according to O’Neill et al. (15). Drosophila mitochondrial gene primers were used for a positive control of the reaction. PCR was performed with initial denaturation at 94°C for 5 min, followed by 30 cycles consisting of 94°C for 1 min, 50°C for 2 min, and 72°C for 3 min. The PCR product was cloned into the plasmid pCR-Script SK and sequenced.
With PCR using the same primers, we tested 10 D. melanogaster strains in our laboratory. A majority, including the commonly used wild-type Canton-S (13), FM7a, CyO/Sco, and TM3Sb/TM6Tb, tested positive for Wolbachia, carried in a nonvirulent form. Seven other infected strains have been reported by Bourtzis et al. (10).
RESULTS
In a screening project for gene mutations that cause brain degeneration, we serendipitously found that a D. melanogaster X-chromosome deficiency strain [originally isolated by Hannah (16)] has a notably reduced life-span compared with normal flies. To determine whether the chromosomal deficiency was responsible for the phenotype, we removed the deficiency by crosses with the white mutant w1118, which has normal life-span. To our surprise, even when the deficiency had been removed by genetic recombination, the short-life phenotype persisted, and was maternally transmitted to both male and female offspring. To find the cause of early death, we examined the adult fly eye and brain at various ages by light microscopy, using toluidine blue staining of semi-thin plastic sections. While newly eclosed adults showed essentially normal morphology, as age progressed there developed gross distortions of the brain and retina, associated with intensified staining. To investigate these abnormalities in greater detail, the brain and other tissues were examined by electron microscopy.
In the fly brain, neuronal and glial cell bodies are located in cortical regions that surround neuropils containing intermingled neuronal processes and their synaptic connections. The neuronal somata and axons are enveloped by glial processes that can be identified in the EM by their greater electron density (17). In normal flies, at least up to several weeks of age, few abnormalities are found in the various cortical and neuropil regions.
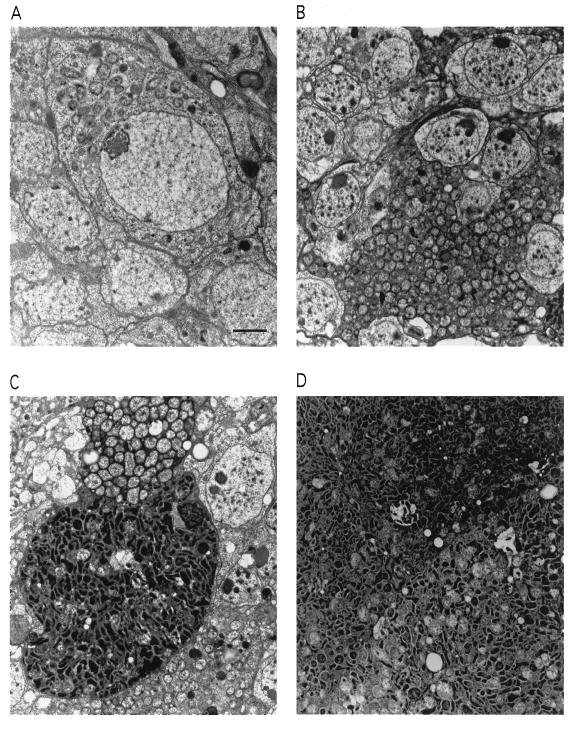
Fig. 1 illustrates the astonishing phenotype of the fly strain that shows early death. The adult flies, when newly hatched, have fairly well-formed brains and other tissues, but EM reveals the presence of intracellular bodies of a Rickettsia-like organism (RLO). Initially, these are seen in only small numbers within various cells, including brain neurons (Fig. 1A). With advancing age, the cell, including the nucleus, becomes packed with them, akin to a bag filled with popcorn (Fig. 1B). Cell membranes may rupture, joining neighboring cells in which the same process is occurring, to form a contiguous mass of the bodies (Fig. 1C). As seen in Fig. 1C, some groups of these RLOs develop smaller, electron-dense forms, resembling endospores, which eventually predominate (Fig. 1D).
Figure 1.
Proliferation of the Wolbachia popcorn strain in the adult Drosophila brain. (A) One-day-old fly carrying popcorn. Note bacteria in the cytoplasm of a neuronal cell body in the brain cortex. (B) In a fly at age 8 days, extreme proliferation of the bacteria is seen. (C) Fusion of two infected brain cells. The bacteria in one of the cells have assumed an electron-dense and elongated form, resembling endospores. (D) In a still older fly (11 days), the dense form predominates. (Bar = 2 μm.)
Similar pathology is observed in the retina, thoracic muscle, and ovary (Fig. 2). Remarkably, the bacteria are present in low numbers during development through the embryonic, larval, and pupal stages. However, as soon as the flies emerge as adults, the bacteria start to multiply rapidly, as if some repression mechanism has been removed, or a required growth factor has become available. The pervading destruction of tissue integrity is associated with early death (see Fig. 4). However, long before that occurs, the RLOs are transmissible to offspring via the ovary. Infected flies, whether raised at 25°C or 29°C, show similar pathology, although the higher temperature accelerates the process, with corresponding shortening of life-span.
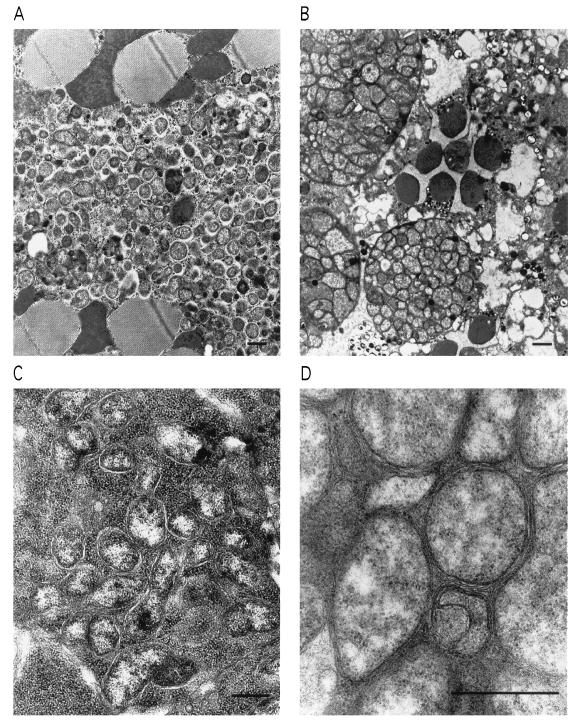
Figure 2.
Proliferation of popcorn in muscle, retina, and ovary. (A) Thoracic indirect flight muscle, seen in oblique section, showing invasion of the bacteria. The large, dark bodies are mitochondria, which deteriorate as the infection progresses. (B) Retina. Masses of the bacteria appear in both photoreceptor cells and surrounding pigment cells. The round, dark bodies are sections of the photoreceptor cell rhabdomeres. (C) Ovarian oocytes. (D) Higher magnification shows the bacterial cell wall and plasma membrane; the morphology is typical of Wolbachia (20). (Bars = 1 μm.)
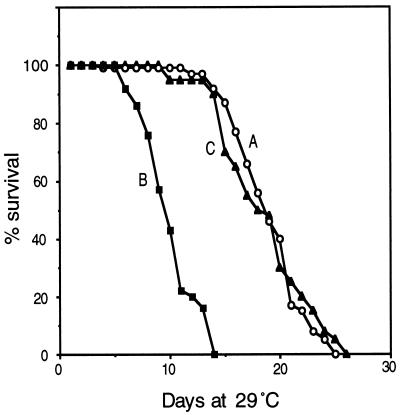
Figure 4.
Reduction of life-span by popcorn infection, and recovery by tetracycline treatment. (A) Survival curve of uninfected control flies (w1118). (B) w1118 flies after infection with popcorn. (C) Cured by tetracycline treatment. Each initial population was ≈100. These curves were obtained by culture at 29°C. The effects at 25°C are similar, with all lifetimes longer by a factor of 2.
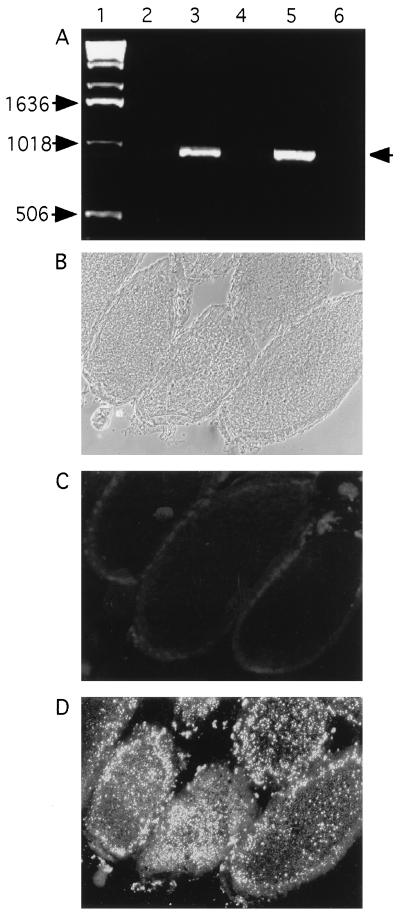
We were curious to know the relationship between these RLOs and the Wolbachia known to exist symbiotically in several Drosophila species. Therefore, we used PCR amplification with Wolbachia-specific 16s rDNA primers (15). Wolbachia-like sequences were indeed detected in ovaries of the infected flies (Fig. 3A). Using a monoclonal antibody against Wolbachia, MAbWolI (18), kindly donated by T. Karr, we could identify a profusion of Wolbachia in infected flies; these were spread widely within the brain and retina, as well as in the oocytes (Fig. 3D), in numbers commensurate with those seen by EM. No antibody staining was detected in other fly strains that were negative by PCR. These findings, as well as the ultrastructure of the RLO, lead us to conclude that the bacterium is a species of Wolbachia.
Figure 3.
Detection of popcorn in ovaries by PCR and immunostaining with monoclonal antibody. (A) Detection by PCR of Wolbachia 16s rDNA in ovaries of flies carrying popcorn, and elimination of the bacteria by tetracycline treatment. Lanes: 1, DNA size markers; 2, uninfected flies (w1118); 3, Wolbachia-infected flies (wild-type Canton-S); 4, Canton-S, after tetracycline treatment; 5, w1118 flies after infection with popcorn by maternal transmission; 6, same strain as lane 5, after tetracycline treatment. (B–D) Visualization of popcorn in cryostat section of ovary by monoclonal antibody. (B) As seen by Nomarski optics. (C) Uninfected strain (w1118) stained with Wolbachia-specific monoclonal antibody of Kose and Karr (18). (D) Strain carrying popcorn.
Based on the appearance in the EM of the heavily infected cells, we designate the bacterial strain by the name popcorn. Flies carrying popcorn were tested to determine whether the bacteria induce cytoplasmic incompatibility. Infected male flies were crossed with uninfected females, and vice versa. Both crosses resulted in similar numbers of progeny, showing that W. popcornia in these flies cause little, if any CI, as has also been observed in several Wolbachia-infected Drosophila species, including D. melanogaster (12–14). Table 1 shows the sequence comparison with the prototype Wolbachia, pipientis from mosquito, along with various reported Drosophila strains and two virulent Rickettsia species, R. prowazekii which causes typhus, and R. rickettsii which causes Rocky Mountain spotted fever. Data are also given for CI. Note that there are wide differences in the CI effect among the various Wolbachia-carrying Drosophila species. Despite its virulence, popcorn does not produce this effect. Of course, there is no reason to believe that the gene for rDNA, chosen for the comparison, is related to either virulence or CI. Identification of the relevant genes, in both bacterium and host, awaits further analysis.
Table 1.
Sequence comparison of popcorn with known Wolbachia strains
| Wolbachia strain (host) | Nucleotide changes compared to popcorn in 783-bp partial sequence of 16s rDNA | CI (ref.) |
|---|---|---|
| Wolbachia pipientis (Culex pipiens) (X61768)* | 15 | ++ (15) |
| Drosophila species | ||
| Wolbachia (D. simulans Hawaii strain) (X64265)* | 2 | ++ (23) |
| Wolbachia (D. simulans Riverside strain) (X64264)* | 2 | ++ (23) |
| Wolbachia (D. melanogaster) (Z28983)* | 4 | + (24) |
| Wolbachia (D. sechellia) (U17059)* | 9 | + (11) |
| Wolbachia (D. simulans Noumea strain) (X64267)* | 15 | ++ (23) |
| Wolbachia (D. simulans Mont D’Ambre strain) (X64266)* | 15 | ++ (23) |
| Wolbachia (D. mauritiana) (U17060)* | 17 | − (11) |
| Rickettsia species | ||
| Rickettsia prowazekii (M21789)* | 114 | (25) |
| Rickettsia rickettsii (M21293)* | 115 | (25) |
GenBank accession number.
To test whether popcorn is the cause of reduced life-span, we treated flies carrying the bacteria with an antibiotic, tetracycline, which eliminates Wolbachia (13). In flies treated by including 0.25 mg/ml of tetracycline in the corn meal–agar food for one or two generations, the PCR-amplified product was no longer detected. Their lifetime, as well, became normal (Fig. 4).
As with all Wolbachia strains, popcorn is maternally transmitted: when we crossed female flies containing popcorn to uninfected males, both male and female progeny acquired the popcorn syndrome. Transmission did not occur when infected males were crossed to uninfected females. In crosses that transfected popcorn into flies already carrying a nonvirulent form of Wolbachia, the full popcorn syndrome developed.
DISCUSSION
popcorn has characteristics different from other known strains. The original one described, W. pipientis, was detected in the ovary of the mosquito Culex pipiens. Since then, RLOs have been reported in the salivary glands or Malpighian tubules of mosquitoes (19) and flies (20). However, popcorn is seen to widely proliferate in the brain, muscle, and retina of Drosophila and to cause tissue degeneration and early death of its adult host. The dynamics of its growth, rapid in adults but not in earlier stages, presents an interesting system for the study of the host–symbiont relationship. It is conceivable that the availability of some essential substrate might be limited early on, due to the great developmental demands of the host, whereas in the adult, essentially postmitotic fly, nutrients might become more available, enabling massive proliferation of the bacteria to begin. Alternatively, a repressive mechanism of the host may be released. popcorn may have special features in its life cycle, as suggested by the presence of the peculiar, electron dense bodies that appear in the aged flies, which resemble endospores. Similar particles observed in C. pipiens were interpreted as degenerated forms of the bacteria (21). Different developmental stages have also been proposed in Coxiella burnetii, which causes the rickettsial disease, Q fever (22).
The virulence of popcorn apparently abrogates any symbiotic relationship; it does not appear to provide any positive evolutionary influence, and is deadly to its host. It does not induce CI, in spite of its high copy number in oocytes. Rather, the pathogenic aspects of popcorn are similar to those seen in typhus, where R. prowazekii accumulates within the cytoplasm until cell lysis occurs (1). In addition, R. prowazekii can be dormant for a long period until it meets favorable conditions for multiplication, as seen in recrudescent typhus or Brill–Zinsser disease (1). Indeed, R. prowazekii is also harmful to its louse vector, which is rare among pathogenic Rickettsiae; the Rickettsia-infected louse does not survive for more than 2 weeks (1).
Studies of rickettsial diseases have been hampered by a lack of in vitro culture methods and model systems. The similarity of the effects caused by popcorn in Drosophila to those caused by R. prowazekii in humans suggests that it might provide a useful model system for analyzing the interactions between invader and host, and the molecular genetic basis of transition from symbiosis to virulence.
As a final note, it should be pointed out that our sampling of D. melanogaster strains in common use for genetic experiments has revealed that many of them, indeed a majority of those we have so far tested, carry Wolbachia, albeit in a nonvirulent form, which can be maternally transmitted in crosses. Many fly strains are considered “weak” and difficult to maintain. The possible effects of Wolbachia on robustness and various phenotypes, including life-span and behavior, can no longer be ignored.
Acknowledgments
We thank Rosalind Young, Karen Chang, and Dawn Fuelberth for excellent technical support and members of our research group for helpful discussions. We are grateful to Timothy Karr for the Wolbachia-specific monoclonal antibody and an initial sample of 16s rDNA primers and to John Werren and Scott O’Neill for helpful comments on the manuscript. This research was supported by a fellowship to K-T.M from the Del Webb Foundation and grants to S.B. from the National Institutes of Health (EY 09278 and AG 12289), the National Science Foundation (MCB 9408718), the McKnight Foundation, the French Foundation, and the James G. Boswell Foundation.
ABBREVIATIONS
- CI
cytoplasmic incompatibility
- EM
electron microscopy
- RLO
Rickettsia-like organism
References
- 1.Hackstadt T. Infect Agents Dis. 1996;5:127–143. [PubMed] [Google Scholar]
- 2.Werren J H, Zhang W, Guo L R. Proc R Soc London B. 1995;261:55–71. [Google Scholar]
- 3.Karr T L. Curr Biol. 1994;4:537–540. doi: 10.1016/s0960-9822(00)00118-4. [DOI] [PubMed] [Google Scholar]
- 4.Werren J H. Annu Rev Entomol. 1997;42:587–609. doi: 10.1146/annurev.ento.42.1.587. [DOI] [PubMed] [Google Scholar]
- 5.Stouthamer R, Breeuwer J A J, Luck R F, Werren J H. Nature (London) 1993;361:66–68. doi: 10.1038/361066a0. [DOI] [PubMed] [Google Scholar]
- 6.Rigaud T, Juchault P. Genetics. 1993;133:247–252. doi: 10.1093/genetics/133.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Neill S L, Pettigrew M M, Sinkins S P, Braig H R, Andreadis T G, Tesh R B. Insect Mol Biol. 1997;6:33–39. doi: 10.1046/j.1365-2583.1997.00157.x. [DOI] [PubMed] [Google Scholar]
- 8.Boyle L, O’Neill S L, Robertson H M, Karr T L. Science. 1993;260:1796–1799. doi: 10.1126/science.8511587. [DOI] [PubMed] [Google Scholar]
- 9.Braig H R, Guzman H, Tesh R B, O’Neill S L. Nature (London) 1994;367:453–455. doi: 10.1038/367453a0. [DOI] [PubMed] [Google Scholar]
- 10.Bourtzis K, Nirgianaki A, Markakis G, Savakis C. Genetics. 1996;144:1063–1073. doi: 10.1093/genetics/144.3.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giordano R, O’Neill S L, Robertson H M. Genetics. 1995;140:1307–1327. doi: 10.1093/genetics/140.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoffmann A A, Clancy D, Duncan J. Heredity. 1996;76:1–8. doi: 10.1038/hdy.1996.1. [DOI] [PubMed] [Google Scholar]
- 13.Holden P R, Jones P, Brookfield J F Y. Genet Res. 1993;62:23–29. doi: 10.1017/s0016672300031529. [DOI] [PubMed] [Google Scholar]
- 14.Solignac M, Vautrin D, Rousset F. C R Acad Sci Paris. 1994;317:461–470. [Google Scholar]
- 15.O’Neill S L, Giordano R, Colbert A M E, Karr T L, Robertson H M. Proc Natl Acad Sci USA. 1992;89:2699–2702. doi: 10.1073/pnas.89.7.2699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hannah A M. Int Congr Genet. 1948;8:588–589. [Google Scholar]
- 17.Lane N J. In: Comparative Insect Physiology, Biochemistry, and Pharmacology. Kerkut G A, Gilbert L I, editors. Vol. 5. Oxford: Pergamon; 1985. pp. 1–47. [Google Scholar]
- 18.Kose H, Karr T L. Mech Dev. 1995;51:275–288. doi: 10.1016/0925-4773(95)00372-x. [DOI] [PubMed] [Google Scholar]
- 19.Yen J H, Barr A R. In: Incompatibility in Culex pipiens. Pal R, Whitten M J, editors. New York: Elsevier; 1974. pp. 97–118. [Google Scholar]
- 20.Thomopoulos G N, Neophytou E P, Limberi-Thomopoulos S. J Morphol. 1991;207:17–21. doi: 10.1002/jmor.1052070104. [DOI] [PubMed] [Google Scholar]
- 21.Wright J D, Barr A R. J Ultrastruct Res. 1980;72:52–64. doi: 10.1016/s0022-5320(80)90135-5. [DOI] [PubMed] [Google Scholar]
- 22.McCaul T F, Williams J C. J Bacteriol. 1981;147:1063–1076. doi: 10.1128/jb.147.3.1063-1076.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rousset F, Vautrin D, Solignac M. Proc R Soc London B. 1992;247:163–168. doi: 10.1098/rspb.1992.0023. [DOI] [PubMed] [Google Scholar]
- 24.Bourtzis K, Nirgianaki A, Onyango P, Savakis C. Insect Mol Biol. 1994;3:131–142. doi: 10.1111/j.1365-2583.1994.tb00160.x. [DOI] [PubMed] [Google Scholar]
- 25.Weisburg W G, Dobson M E, Samuel J E, Dasch G A, Mallavia L P, Baca O, Mandelco L, Sechrest J E, Weiss E, Woese C R. J Bacteriol. 1989;171:4202–4206. doi: 10.1128/jb.171.8.4202-4206.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]