Abstract
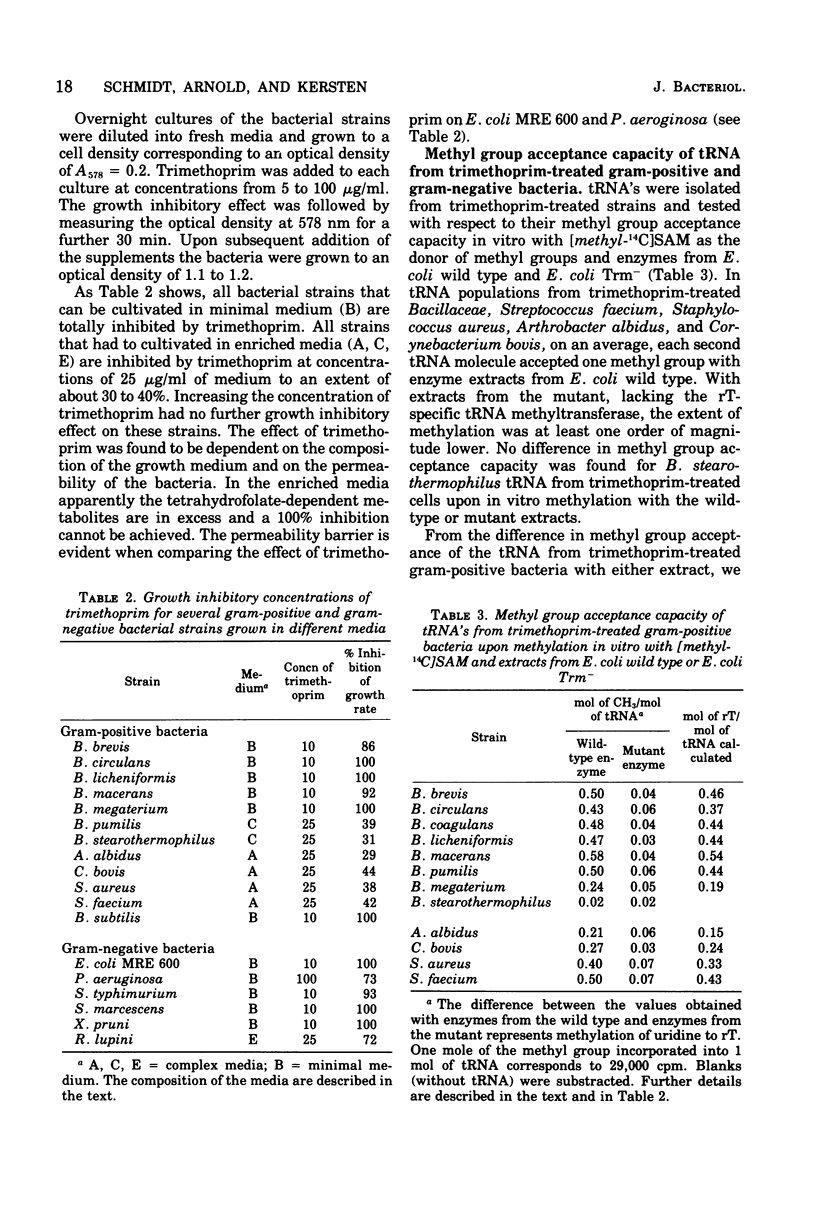
Trimethoprim, an inhibitor that prevents tetrahydrofolate-dependent transmethylation reactions inbacteria, was used in a comparative study to discriminate between two possible biosynthetic pathways, either the S-adenosylmethionine or the tetrahydrofolate-dependent formation of ribothymidine (rT) in transfer ribonucleic acids (tRNA's) of several strains of gram-positive and gram-negative microorganisms. rT-deficient tRNA's accumulate in trimethoprim-treated gram-positive Streptococcus faecium, Staphylococcus aureus, Corynebacterium bovis, Arthrobacter albidus, and all examined Bacillaceae, except Bacillus stearothermophilus. The rT-deficient rT-deficient tRNA's accept the methyl moiety from S-adenosylmethionine in vitro, with extracts from Escherichia coli (wild type) as a source of methylating enzymes; 90% of the incorporated methyl groups are present in rT. Trimethoprim does not inhibit the biosynthesis of rT in tRNA of gram-negative Enterobacteriaceae, Rhizobium lupini, and Pseudomonadaceae, suggesting that the rT-specific tRNA methyltransferases of these gram-negative strains use S-adenosylmethionine as coenzyme.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agris P. F., Koh H., Söll D. The effect of growth temperatures on the in vivo ribose methylation of Bacillus stearothermophilus transfer RNA. Arch Biochem Biophys. 1973 Jan;154(1):277–282. doi: 10.1016/0003-9861(73)90058-1. [DOI] [PubMed] [Google Scholar]
- Arnold H. H., Kersten H. Inhibition of the tetrahydrofolate-dependent biosynthesis of ribothymidine in tRNAs of B. subtilis and M. lysodeikticus by trimethoprim. FEBS Lett. 1975 May 1;53(2):258–261. doi: 10.1016/0014-5793(75)80032-9. [DOI] [PubMed] [Google Scholar]
- Arnold H. H., Schmidt W., Kersten H. Occurrence and biosynthesis of ribothymidine in tRNAs of B. subtilis. FEBS Lett. 1975 Mar 15;52(1):62–65. doi: 10.1016/0014-5793(75)80638-7. [DOI] [PubMed] [Google Scholar]
- Arnold H. H., Schmidt W., Raettig R., Sandig L., Domdey H., Kersten H. S-Adenosylmethionine and tetrahydrofolate-dependent methylation of tRNA in Bacillus subtilis. Incomplete methylations caused by trimethoprim, pactamycin, or chloramphenicol. Arch Biochem Biophys. 1976 Sep;176(1):12–20. doi: 10.1016/0003-9861(76)90135-1. [DOI] [PubMed] [Google Scholar]
- Arnold H., Kersten H. The occurrence of ribothymidine, 1-methyladenosine, methylated guanosines and the corresponding methyltransferases in E. coli and Bacillus subtilis. FEBS Lett. 1973 Oct 1;36(1):34–38. doi: 10.1016/0014-5793(73)80331-x. [DOI] [PubMed] [Google Scholar]
- Björk G. R., Isaksson L. A. Isolation of mutants of Escherichia coli lac king 5-methyluracil in transfer ribonucleic acid or 1-methylguanine in ribosomal RNA. J Mol Biol. 1970 Jul 14;51(1):83–100. doi: 10.1016/0022-2836(70)90272-x. [DOI] [PubMed] [Google Scholar]
- Björk G. R., Neidhardt F. C. Physiological and biochemical studies on the function of 5-methyluridine in the transfer ribonucleic acid of Escherichia coli. J Bacteriol. 1975 Oct;124(1):99–111. doi: 10.1128/jb.124.1.99-111.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delk A. S., Rabinowitz J. C. Biosynthesis of ribosylthymine in the transfer RNA of Streptococcus faecalis: a folate-dependent methylation not involving S-adenosylmethionine. Proc Natl Acad Sci U S A. 1975 Feb;72(2):528–530. doi: 10.1073/pnas.72.2.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann V. A., Sprinzl M., Pongs O. The involvement of 5S RNA in the binding of tRNA to ribosomes. Biochem Biophys Res Commun. 1973 Oct 1;54(3):942–948. doi: 10.1016/0006-291x(73)90785-7. [DOI] [PubMed] [Google Scholar]
- Kersten H., Sandig L., Arnold H. H. Tetrahydrofolate-dependent 5-methyluracil-tRNA transferase activity in B. subtilis. FEBS Lett. 1975 Jul 15;55(1):57–60. doi: 10.1016/0014-5793(75)80956-2. [DOI] [PubMed] [Google Scholar]
- Kuchino Y., Nishimura S. Nucleotide sequence specificities of guanylate residue-specific tRNA methylases from rat liver. Biochem Biophys Res Commun. 1970 Jul 27;40(2):306–313. doi: 10.1016/0006-291x(70)91010-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Ofengand J., Henes C. The function of pseudouridylic acid in transfer ribonucleic acid. II. Inhibition of amino acyl transfer ribonucleic acid-ribosome complex formation by ribothymidylyl-pseudouridylyl-cytidylyl-guanosine 3'-phosphate. J Biol Chem. 1969 Nov 25;244(22):6241–6253. [PubMed] [Google Scholar]
- Schmidt W., Arnold H. H., Kersten H. Biosynthetic pathway of ribothymidine in B. subtilis and M. lysodeikticus involving different coenzymes for transfer RNA and ribosomal RNA. Nucleic Acids Res. 1975 Jul;2(7):1043–1051. doi: 10.1093/nar/2.7.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S., Reinitz E. R., Gefter M. L. Role of modifications in tyrosine transfer RNA. II. Ribothymidylate-deficient tRNA. Arch Biochem Biophys. 1973 Jul;157(1):55–62. doi: 10.1016/0003-9861(73)90389-5. [DOI] [PubMed] [Google Scholar]
- Yang W. K., Novelli G. D. Isoaccepting +RNA's in mouse plasma cell tumors that synthesize different myeloma protein. Biochem Biophys Res Commun. 1968 May 23;31(4):534–539. doi: 10.1016/0006-291x(68)90510-x. [DOI] [PubMed] [Google Scholar]



