Abstract
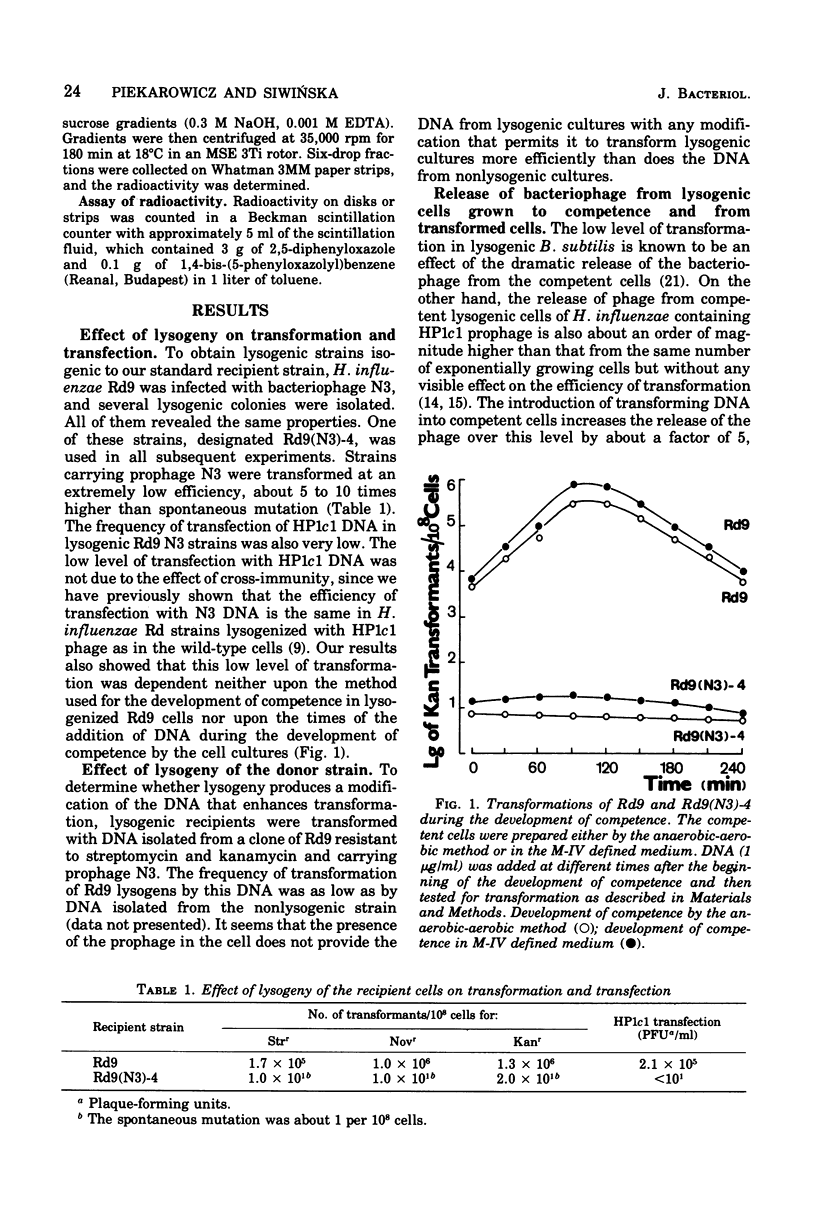
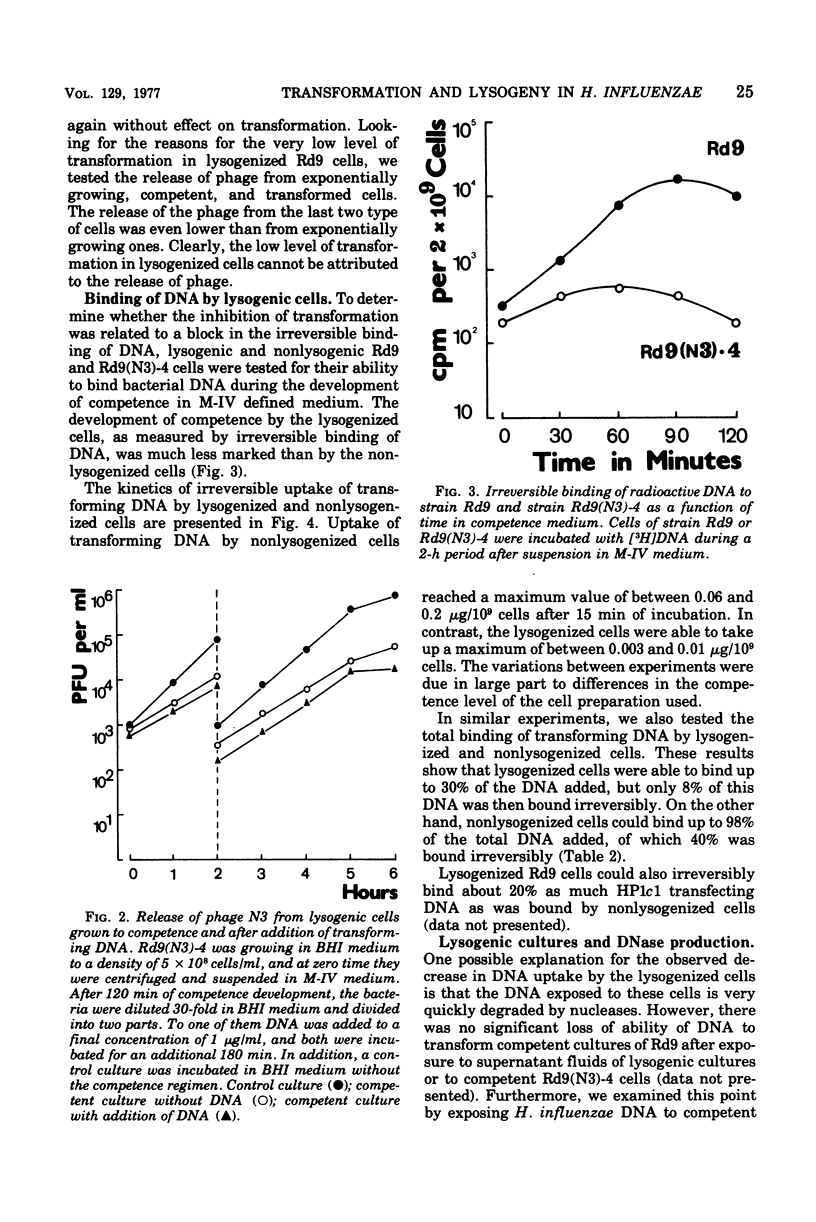
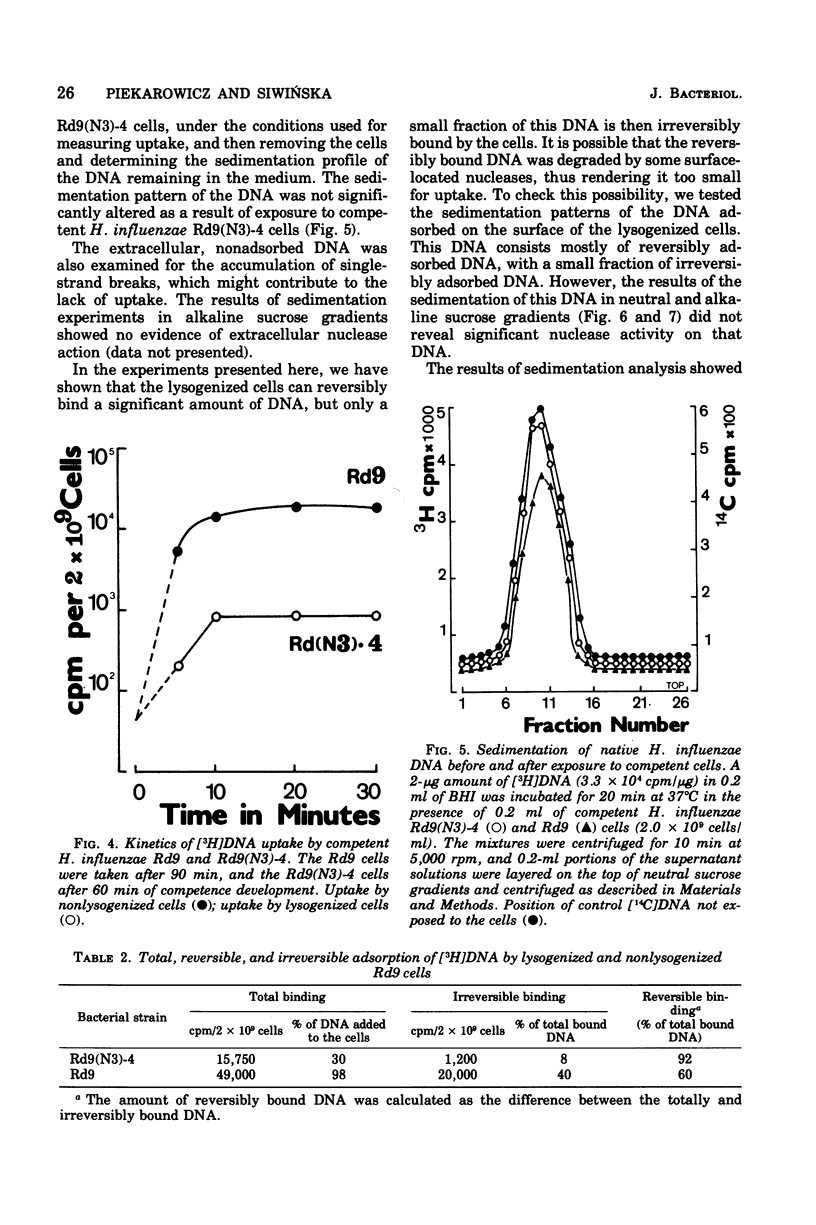
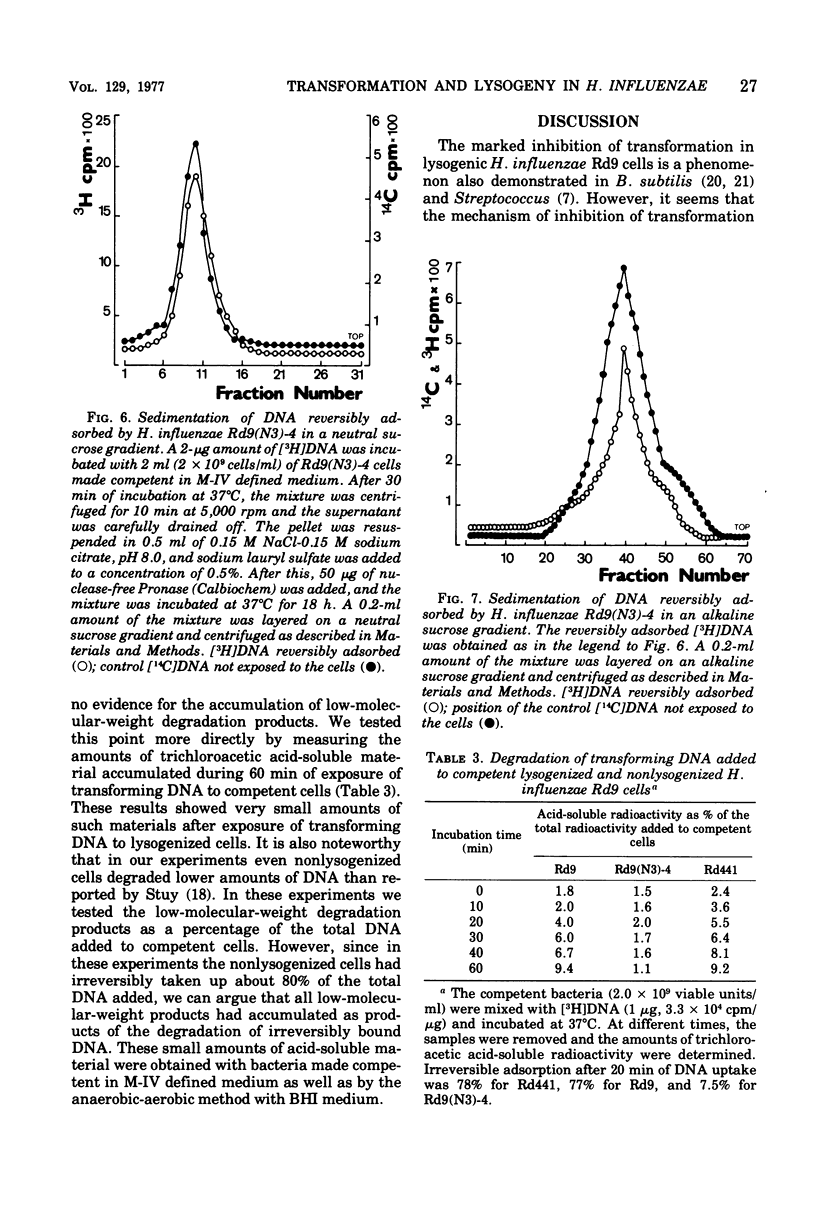
Haemophilus influenzae Rd9 lysogenic for temperate bacteriophage N3 was found to be virtually nontransformable and nontransfectable. This inhibition of transformation and transfection was due partly to the decreased capacity of competent lysogenic cells for irreversible binding of deoxyribonucleic acid (DNA) and partly to some events taking place after adsorption of the DNA. The unadsorbed DNA was not degraded by the competent lysogenic cells.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boling M. E., Setlow J. K. Dependence of Vegetative Recombination Among Haemophilus influenzae Bacteriophage on the Host Cell. J Virol. 1969 Sep;4(3):240–243. doi: 10.1128/jvi.4.3.240-243.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOODGAL S. H., HERRIOTT R. M. Studies on transformations of Hemophilus influenzae. I. Competence. J Gen Physiol. 1961 Jul;44:1201–1227. doi: 10.1085/jgp.44.6.1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover S. W., Piekarowicz A. Host specificity of DNA in Haemophilus influenzae: restriction and modification in strain Rd. Biochem Biophys Res Commun. 1972 Feb 25;46(4):1610–1617. doi: 10.1016/0006-291x(72)90793-0. [DOI] [PubMed] [Google Scholar]
- Herriott R. M., Meyer E. Y., Vogt M., Modan M. Defined medium for growth of Haemophilus influenzae. J Bacteriol. 1970 Feb;101(2):513–516. doi: 10.1128/jb.101.2.513-516.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablońska E., Piekarowicz A. Bacteriophage N3 of Haemophilus influenzae. II. Infection of transformable cells by bacteriophage DNA. Acta Microbiol Pol. 1976;25(3):175–186. [PubMed] [Google Scholar]
- Joenje H., Venema G. Different nuclease activities in competent and noncompetent Bacillus subtilis. J Bacteriol. 1975 Apr;122(1):25–33. doi: 10.1128/jb.122.1.25-33.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons L. C., Ranhand J. M., Leonard C. G., Colon A. E., Cole R. M. Inhibition of transformation in group H streptococci by lysogeny. J Bacteriol. 1973 Mar;113(3):1217–1222. doi: 10.1128/jb.113.3.1217-1222.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piekarowicz A., Baj J. Host specificity of DNA in haemophilus influenzae: The physiological and genetical bases of instability of restriction and modification of DNA in strain Rd. Acta Microbiol Pol A. 1975;8(3):119–130. [PubMed] [Google Scholar]
- Piekarowicz A., Brzeziński R., Kauc L. Host specificity of DNA in Haemophilus influenzae: the in vivo action of the restriction endonucleases on phage and bacterial DNA. Acta Microbiol Pol A. 1975;7(2):51–65. [PubMed] [Google Scholar]
- Piekarowicz A., Glover S. W. Host specificity of DNA in Haemophilus influenzae: the two restriction and modification systems in strain Ra. Mol Gen Genet. 1972;116(1):11–25. doi: 10.1007/BF00334255. [DOI] [PubMed] [Google Scholar]
- Pizer L. I., Smith H. S., Miovic M., Pylkas L. Effect of prophage W on the propagation of bacteriophages T2 and T4. J Virol. 1968 Nov;2(11):1339–1345. doi: 10.1128/jvi.2.11.1339-1345.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rettenmier C. W., Hemphill H. E. Prophage-mediated interference affecting the development of Bacillus subtilis bacteriophage phi e. J Virol. 1973 Mar;11(3):372–377. doi: 10.1128/jvi.11.3.372-377.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels J., Clarke J. K. New bacteriophage of Haemophilus influenzae. J Virol. 1969 Nov;4(5):797–798. doi: 10.1128/jvi.4.5.797-798.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick B., Setlow J. K., Boling M. E., Allison D. P. Minicell production and bacteriophage superinducibility of thymidine-requiring strains of Haemophilus influenzae. J Bacteriol. 1975 Sep;123(3):1208–1217. doi: 10.1128/jb.123.3.1208-1217.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Boling M. E., Allison D. P., Beattie K. L. Relationship between prophage induction and transformation in Haemophilus influenzae. J Bacteriol. 1973 Jul;115(1):153–161. doi: 10.1128/jb.115.1.153-161.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto H., Lopez R., Garrigan O., Tomasz A. Nucleolytic degradation of homologous and heterologous deoxyribonucleic acid molecules at the surface of competent pneumococci. J Bacteriol. 1975 May;122(2):676–685. doi: 10.1128/jb.122.2.676-685.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinhart W. L., Herriott R. M. Fate of recipient deoxyribonucleic acid during transformation in Haemophilus influenzae. J Bacteriol. 1968 Nov;96(5):1718–1724. doi: 10.1128/jb.96.5.1718-1724.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuy J. H. Acid-soluble breakdown of homologous deoxyribbonucleic acid adsorbed by Haemophilus influenzae: its biological significance. J Bacteriol. 1974 Nov;120(2):917–922. doi: 10.1128/jb.120.2.917-922.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T., Takano T., Arai T., Nishida H., Sato S. Episome-mediated Transfer of Drug Resistance in Enterobacteriaceae X. Restriction and Modification of Phages by fi R Factors. J Bacteriol. 1966 Aug;92(2):477–486. doi: 10.1128/jb.92.2.477-486.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasbin R. E., Wilson G. A., Young F. E. Transformation and transfection in lysogenic strains of Bacillus subtilis 168. J Bacteriol. 1973 Feb;113(2):540–548. doi: 10.1128/jb.113.2.540-548.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasbin R. E., Wilson G. A., Young F. E. Transformation and transfection in lysogenic strains of Bacillus subtilis: evidence for selective induction of prophage in competent cells. J Bacteriol. 1975 Jan;121(1):296–304. doi: 10.1128/jb.121.1.296-304.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoon K. C., Scocca J. J. Constitution of the cell envelope of Haemophilus influenzae in relation to competence for genetic transformation. J Bacteriol. 1975 Aug;123(2):666–677. doi: 10.1128/jb.123.2.666-677.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



