Abstract
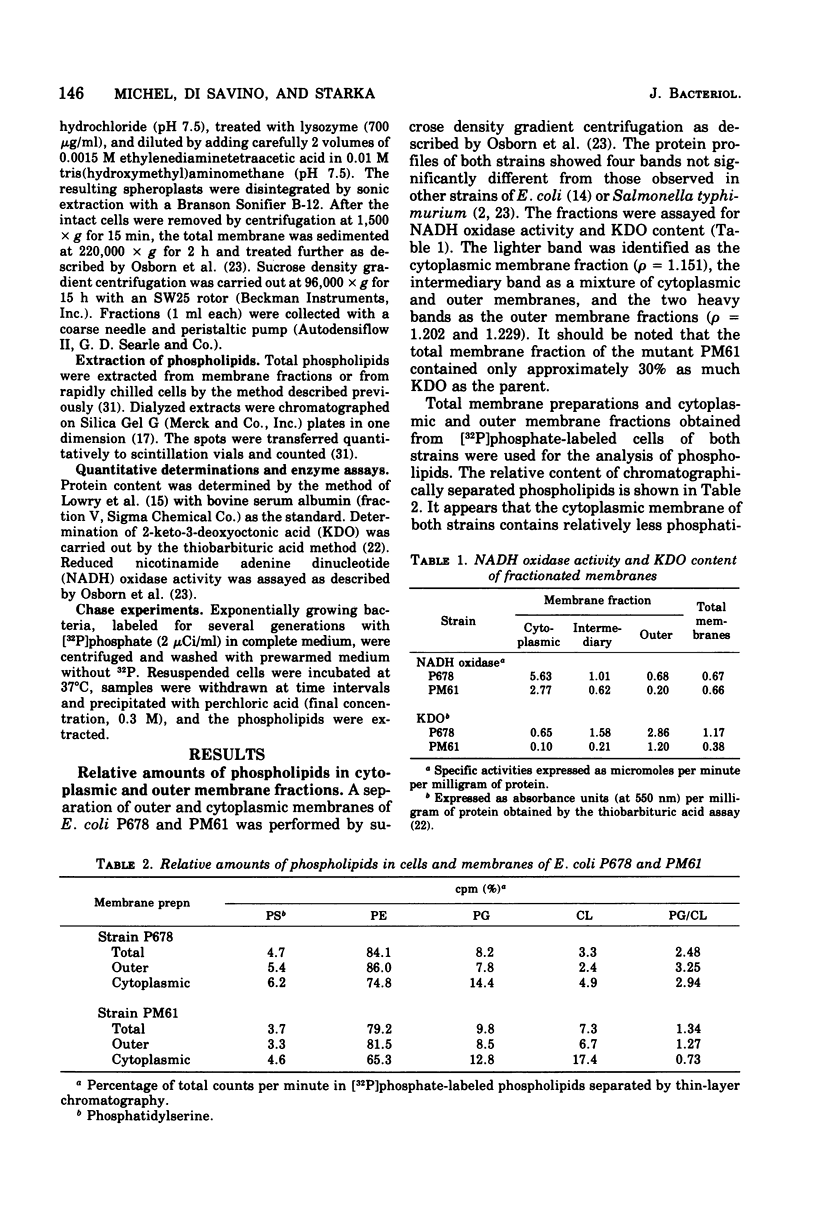
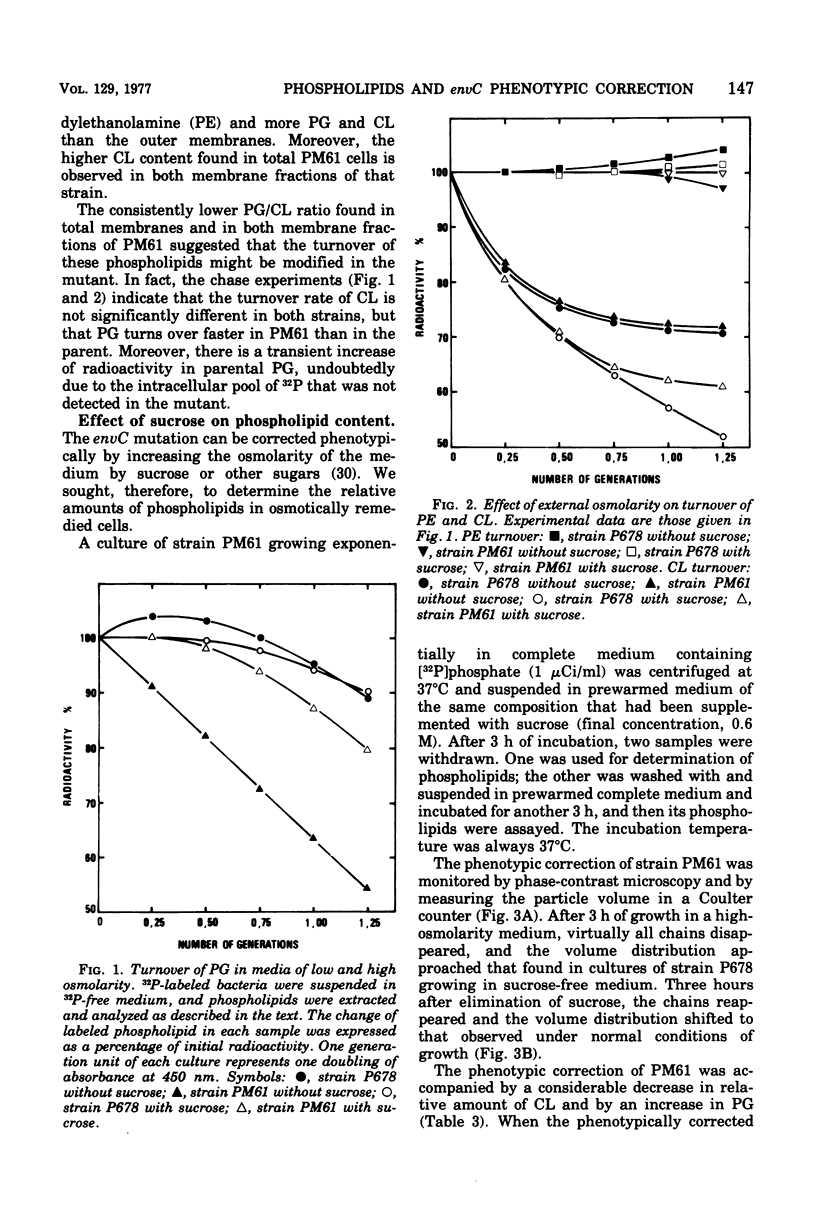
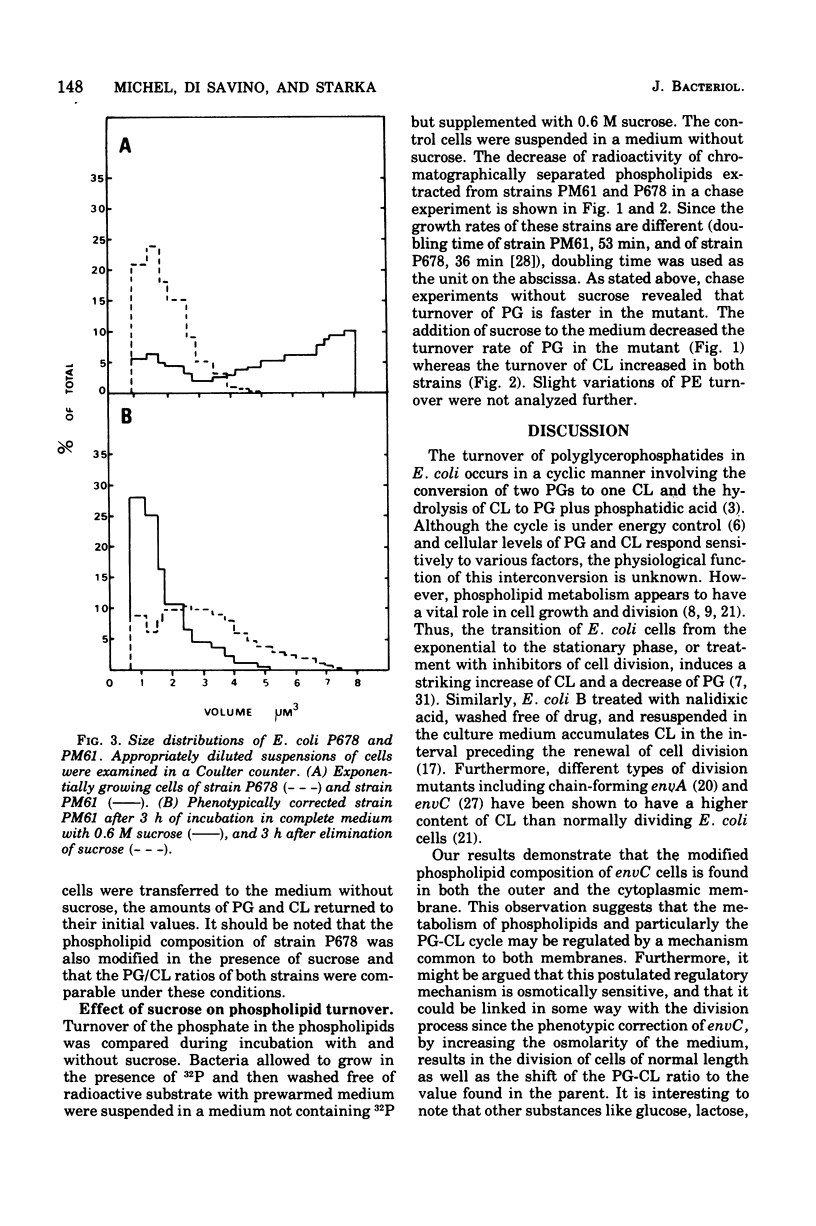
The cytoplasmic and outer membranes of a nonconditional chain-forming mutant, Escherichia coli PM61 envC, were separated by sucrose density gradient centrifugation. The phosphatidylglycerol/cardiolipin ratio in both membrane fractions was about one-third as high as in the parental strain P678. The increased level of cardiolipin in PM61 membranes is the result of an alteration of the polyglycerophosphatide cycle. It was found that the turnover rate of phosphatidylglycerol is more rapid in PM61 than in the parental strain but that its cardiolipin turnover is not significantly different. The envC mutation can be corrected phenotypically by increasing the osmolarity of the medium. In the presence of 0.6 M sucrose, the population of PM61 is composed of short rods, and the phosphatidylglycerol/cardiolipin ratio is shifted to that of the parent. The phosphatidylglycerol turns over more slowly, whereas the cardiolipin turns over more rapidly in both strains. Thus, the increase of external osmolarity acts on phospholipid metabolism as well as on an unknown step involved in the mechanism of cell division of the envC mutant.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler H. I., Fisher W. D., Cohen A., Hardigree A. A. MINIATURE escherichia coli CELLS DEFICIENT IN DNA. Proc Natl Acad Sci U S A. 1967 Feb;57(2):321–326. doi: 10.1073/pnas.57.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ames G. F., Spudich E. N., Nikaido H. Protein composition of the outer membrane of Salmonella typhimurium: effect of lipopolysaccharide mutations. J Bacteriol. 1974 Feb;117(2):406–416. doi: 10.1128/jb.117.2.406-416.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audet A., Cole R., Proulx P. Polyglycerophosphatide metabolism in Escherichia coli. Biochim Biophys Acta. 1975 Mar 24;380(3):414–420. doi: 10.1016/0005-2760(75)90109-5. [DOI] [PubMed] [Google Scholar]
- Bilsky A. Z., Armstrong J. B. Osmotic reversal of temperature sensitivity in Escherichia coli. J Bacteriol. 1973 Jan;113(1):76–81. doi: 10.1128/jb.113.1.76-81.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broekman J. H., Steenbakkers J. F. Effect of the osmotic pressure of the growth medium on fabB mutants of Escherichia coli. J Bacteriol. 1974 Mar;117(3):971–977. doi: 10.1128/jb.117.3.971-977.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole R., Benns G., Proulx P. Cardiolipin specific phospholipase D activity in Escherichia coli extracts. Biochim Biophys Acta. 1974 Mar 28;337(3):325–332. doi: 10.1016/0005-2760(74)90107-6. [DOI] [PubMed] [Google Scholar]
- Cronan J. E., Jr Phospholipid alterations during growth of Escherichia coli. J Bacteriol. 1968 Jun;95(6):2054–2061. doi: 10.1128/jb.95.6.2054-2061.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronan J. E., Vagelos P. R. Metabolism and function of the membrane phospholipids of Escherichia coli. Biochim Biophys Acta. 1972 Feb 14;265(1):25–60. doi: 10.1016/0304-4157(72)90018-4. [DOI] [PubMed] [Google Scholar]
- Daniels M. J. Lipid synthesis in relation to the cell cycle of Bacillus megaterium KM and Escherichia coli. Biochem J. 1969 Dec;115(4):697–701. doi: 10.1042/bj1150697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- Inoue K., Imagawa T., Amano T. Temperature-sensitive mutants of Escherichia coli B which can grow in high-osmotic medium at the nonpermissive temperature. Biken J. 1974 Dec;17(4):149–159. [PubMed] [Google Scholar]
- Kanemasa Y., Akamatsu Y., Nojima S. Composition and turnover of the phospholipids in Escherichia coli. Biochim Biophys Acta. 1967 Oct 2;144(2):382–390. [PubMed] [Google Scholar]
- Kopelovich L., Sweetman L., Nisselbaum J. S. Regulation of aspartate aminotransferase isozymes by D-erythrose 4-phosphate and glycolaldehyde phosphate, the naturally occurring homologues of D-glyceraldehyde 3-phosphate. J Biol Chem. 1972 May 25;247(10):3262–3268. [PubMed] [Google Scholar]
- Koplow J., Goldfine H. Alterations in the outer membrane of the cell envelope of heptose-deficient mutants of Escherichia coli. J Bacteriol. 1974 Feb;117(2):527–543. doi: 10.1128/jb.117.2.527-543.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matsuzawa H., Matsuhashi M., Oka A., Sugino Y. Genetic and biochemical studies on cell wall peptidoglycan synthesis in Escherichia coli K-12. Biochem Biophys Res Commun. 1969 Aug 15;36(4):682–689. doi: 10.1016/0006-291x(69)90360-x. [DOI] [PubMed] [Google Scholar]
- Michel G., Rodolakis A., Starka J. Sythesis and turnover of phospholipids in Escherichia coli cells with inhibited DNA synthesis. Ann Microbiol (Paris) 1975 Oct-Nov;126(3):273–285. [PubMed] [Google Scholar]
- Munro G. F., Bell C. A. Effects of external osmolarity on phospholipid metabolism in Escherichia coli B. J Bacteriol. 1973 Oct;116(1):257–262. doi: 10.1128/jb.116.1.257-262.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S., Boman H. G., Matsson E. Mutant of Escherichia coli with anomalous cell division and ability to decrease episomally and chromosomally mediated resistance to ampicillin and several other antibiotics. J Bacteriol. 1969 Mar;97(3):1334–1342. doi: 10.1128/jb.97.3.1334-1342.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S., Wolf-Watz H. Cell division and permeability of unbalanced envelope mutants of Escherichia coli K12. Ann Microbiol (Paris) 1974 Sep;125 B(2):211–226. [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard M., Hirota Y. Effet des sels et autres composés sur le phénotype de mutants thermosensibles de Escherichia coli. Ann Microbiol (Paris) 1973 Jan;124(1):29–43. [PubMed] [Google Scholar]
- Rodolakis A., Casse F., Starka J. Morphological mutants of Escherichia coli K12. Mapping of the env C mutation. Mol Gen Genet. 1974 May 21;130(2):177–181. doi: 10.1007/BF00269088. [DOI] [PubMed] [Google Scholar]
- Rodolakis A., Starka G. Sur un mutant d'Escherichia coli présentant des anomalies de la division et de la morphologie cellulaire. C R Acad Sci Hebd Seances Acad Sci D. 1973 Jan 22;276(4):663–665. [PubMed] [Google Scholar]
- Rodolakis A., Thomas P., Starka J. Morphological mutants of Escherichia coli. Isolation and ultrastructure of a chain-forming envC mutant. J Gen Microbiol. 1973 Apr;75(2):409–416. doi: 10.1099/00221287-75-2-409. [DOI] [PubMed] [Google Scholar]
- Russell R. R. Temperature-sensitive osmotic remedial mutants of Escherichia coli. J Bacteriol. 1972 Nov;112(2):661–665. doi: 10.1128/jb.112.2.661-665.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starka J., Di Savino D., Michel G., Rodolakis A., Thomas P. Phenotypic expression of an envC-division mutant of Escherichia coli K12. Ann Microbiol (Paris) 1974 Sep;125 B(2):227–232. [PubMed] [Google Scholar]
- Stárka J., Moravová J. Phospholipids and cellular division of Escherichia coli. J Gen Microbiol. 1970 Feb;60(2):251–257. doi: 10.1099/00221287-60-2-251. [DOI] [PubMed] [Google Scholar]



