Abstract
A flashbulb memory (FM) is a vivid, enduring memory for how one learned about a surprising, shocking event. It thus involves memory for the source of event information, as opposed to memory for the event itself. Which brain regions are involved in FM, however, is uncertain. Although medial temporal lobe/diencephalic (MTL/D) damage impairs content or item memory, frontal lobe (FL) damage has been associated with impaired source memory. One would therefore expect that FM should depend on the FLs, although two recent reports do not support this idea. In the current study, we examined memory for the events of September 11th, and memory for the source of that information, in MTL/D patients, FL patients, and healthy subjects. Only the MTL/D patients were impaired in long-term memory for the event itself, measured after a 6 month retention interval. The FL patients, on the other hand, showed a selective deficit in source memory, although their memory for the target event was unimpaired. MTL/D and FL structures appear to play different roles in memory for flashbulb events.
People often report vivid, long-lasting recollections of the circumstances in which they learned shocking, emotionally-arousing news. Examples of such cases, termed flashbulb memories (FMs; Brown & Kulik, 1977; for reviews, see Conway, 1995; Winograd & Neisser, 1992), include the attack on Pearl Harbor in 1941, the assassination of President Kennedy in 1963, and the explosion of the Space Shuttle Challenger in 1986. FMs appear to be more accurate and more consistent than memories for less emotional events that occurred around the same time, although FMs are still subject to forgetting and distortion over the long term (e.g., Christianson, 1989; Davidson & Glisky, 2002; Neisser & Harsch, 1992; Schmolck, Buffalo, & Squire, 2000). Cases of FM are of interest to neuropsychologists for at least two reasons. First, whereas much research has focused on memory for the content of an event, FMs concern memory for the source of news about that event, and these two aspects of memory may be dissociable. Second, memories for emotional events may depend on somewhat different brain mechanisms than memories for non-emotional events. Neuropsychological studies of FM are rare, however — only a few exist, as far as we know (Budson et al., 2004; Candel, Jelicic, Merckelbach, & Wester, 2003; Davidson & Glisky, 2002; Kapur, Abbott, Graham, & Simons, 2000), and so the brain regions associated specifically with FMs remain uncertain. Here we describe how lesions to the medial temporal lobe/diencephalon (MTL/D) or to the frontal lobe (FL) — two brain regions thought to play major roles in memory — may influence memory for an emotionally arousing public event, and memory for how news about that event was acquired.
Item versus Source Memory
Most memory studies emphasize people’s ability to report the content of an experience, including items encountered in the past, such as pictures, words, or facts (often referred to as the target event). FM research, however, is less concerned with memory for the content than with memory for the context in which the items were encountered, or the source of the information (often referred to as the reception event; Brewer, 1992). That is, a flash-bulb memory is by definition a type of source memory — in both cases participants must recollect when, where, and/or from whom they learned information. Thus, flashbulb memories may be supported by brain regions similar to those involved in source memory.
Item and source memory appear to be differentially dependent on MTL/D and FL structures. Memory for content or items is usually significantly impaired by MTL/D lesions, but is often much less affected by FL damage. In contrast, memory for source appears to be less affected by MTL/D lesions, but more dependent on the FLs. For example, several investigators have taught facts (e.g., “Bob Hope’s father was a fireman”; Schacter, Harbluk, & McLachlan, 1984) to MTL/D and FL patients, and later asked patients to report both the new facts, and the circumstances in which they learned them. The MTL/D patients had difficulty remembering the new items, whereas the FL patients had no problem doing so. In contrast, usually the MTL/D patients were not disproportionately impaired in source memory, whereas the FL patients were (e.g., Janowsky, Shimamura, & Squire, 1989a; Johnson, O’Connor, & Cantor, 1997; Milner, Corsi, & Leonard, 1991; Schacter et al., 1984; Shimamura & Squire, 1987, 1991; Shimamura, Janowsky, & Squire, 1990; but see Schwerdt & Dopkins, 2001; and Thaiss & Petrides, 2003).1 Moreover, source memory was often uncorrelated with neuropsychological measures of MTL/D function, but was instead predicted by measures of FL function (e.g., Glisky, Polster, & Routhieaux, 1995; Glisky, Rubin, & Davidson, 2001; Schacter et al., 1984; Shimamura & Squire, 1987; Simons et al., 2002).
Although frontal lobe pathology usually impairs source memory, there are two reports suggesting that this may not be the case in emotional situations. First, Kapur et al. (2000) asked patients with FL damage how they learned of the death of Diana, Princess of Wales. They appeared to have normal flashbulb memories, which was inconsistent with the aforementioned literature on FL damage impairing memory for source. Second, Davidson and Glisky (2002) asked older adults how they learned of the deaths of Princess Diana and Mother Teresa of Calcutta. They found no relation between flashbulb memory and FL function in the older adults, which again was contrary to the usually strong positive correlation found between source memory and FL function (e.g., Glisky et al., 1995; 2001). Together, these two studies (Davidson & Glisky, 2002; Kapur et al., 2000) suggest that FMs, perhaps because of their emotionality, comprise a special case of source memory, and may be less affected by FL pathology.
The tragic events of September 11, 2001, provided us the opportunity to study further the neuropsychology of FM. That morning, four American passenger jets were hijacked. Two of the planes flew into and destroyed New York City’s World Trade Center, the third crashed into the Pentagon building in Virginia, and the fourth crashed in a field in rural Pennsylvania. In total, approximately 3,000 people (http://www.cnn.com/SPECIALS/2001/trade.center) were killed. Soon after September 11th, we tested patients with MTL/D damage, patients with FL damage, and healthy people. Participants completed a questionnaire containing specific questions about what happened that day (the target event), and how they learned about it (the reception event). We then retested patients after approximately six months, to examine their long term retention. We had two main hypotheses, based on the literature outlined above. First, we predicted that memory for facts about the target event (namely, the September 11th terrorist attacks) would be reliably impaired in the MTL/D patients but not in the FL patients. Second, we expected memory for the reception event (that is, the source of information about the terrorist attacks) to be retained in the MTL/D patients to the extent that they were able to recall the target event itself. We were uncertain whether the FL patients would be impaired in FM, as they are in most source memory studies in the laboratory, or would show normal FM as did the patients in the Kapur et al. (2000) study and the older adults with reduced FL function in our previous study (Davidson & Glisky, 2002).
Method
Participants
We recruited 45 adults, who were divided into three groups: 14 patients with MTL/D lesions, 13 patients with FL lesions, and a comparison group of 18 healthy subjects. Of these participants, scores from 2 MTL/D patients, 1 FL patient, and 3 healthy subjects were omitted because they were unavailable for retention testing. Tables 1 and 2 show lesion, demographic, and psychometric information for the 24 patients; in the majority, structural damage was indicated by computerized tomography (CT) and/or magnetic resonance imaging (MRI).
Table 1.
Lesion, demographic, and psychometric data for the MTL/D patients
| WMS
|
WCST
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Locus | Etiology | Age | Education | VIQ | GM | WM | Cat. | PE | FAS | Trails B |
| JM | Bilateral hippocampus and parahippocampal gyrus; right amygdala | Anoxia | 52 | 12 | 82 | 52* | 91 | 4 | 63* | 29 | 195* |
| PD | Cerebral and cerebellar atrophy | Anoxia | 65 | 20 | 111 | 52* | 83 | 6 | 5 | 43 | 110 |
| PS | Bilateral hippocampus, cerebral atrophy | Anoxia | 44 | 14 | 90 | 45* | 93 | 6 | 12 | 40 | 69 |
| RL | Bilateral hippocampus, possibly left posterior putamen | Anoxia | 73 | 18 | 122 | 75* | 102 | 6 | 14 | 25 | 102 |
| WS | Bilateral hippocampus | Anoxia | 55 | 14 | 111 | 59* | 96 | 6 | 7 | 36 | 60 |
| SS | Bilateral hippocampus, parahippocampal gyrus, amygdala, putamen, insula, basal forebrain, septum, and nearby white matter; left frontal lobe | Encephalitis | 74 | 18 | 133 | 45* | 141 | 6 | 6 | 50 | 65 |
| TR | Bilateral hippocampus | Encephalitis | 71 | 16 | 112 | 47* | 88 | 1* | 8 | 19* | 211 |
| CW | Bilateral anterior thalamus; cortical atrophy | Stroke | 61 | 12 | 84 | 73 | 99 | 1* | 37* | 50 | 72 |
| PB | Cerebral atrophy | Korsakoff | 75 | 14 | 99 | 59* | 115 | 5 | 79* | 8* | 194 |
| RG | Cerebral atrophy | Korsakoff | 84 | 10 | 100 | 72 | 91 | 0* | 87* | 26 | 50 |
| RM | Not scanned | Korsakoff | 82 | 14 | 107 | 66* | 121 | 6 | 24 | 36 | 76 |
| WK | No significant abnormalities | Korsakoff | 61 | 16 | 92 | 47* | 85 | 4 | 29 | 15* | 58 |
| Mean | 66 | 15 | 104 | 58 | 100 | 4 | 31 | 31 | 105 | ||
Age and Education in Years. VIQ = Verbal Intelligence Quotient from the Wechsler Adult Intelligence Scale - III (Wechsler, 1997) or the Wechsler Abbreviated Scale of Intelligence (1999); GM = General Memory index, WM = Working Memory index, from the Wechsler Memory Scale - III (1997); WCST = Wisconsin Card Sorting Test (Heaton, 1981), Cat = Categories, PE = Perseverative Errors; FAS = Controlled Oral Word Association Test (Spreen & Benton, 1977); Trails B = Trailmaking test, Part B, in seconds (Lezak, 1995).
= at least two standard deviations away from the normative mean.
Table 2.
Lesion, demographic, and psychometric data for the FL patients
| WMS
|
WCST
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Locus | Etiology | Age | Education | VIQ | GM | WM | Cat. | PE | FAS | Trails B |
| DH | Left VM | AcoA aneurysm | 71 | 13 | 98 | 66* | 63* | 1* | 48* | 22 | 141 |
| DJ | Right VM, right temporo-parietal | AcoA aneurysm | 65 | 11 | |||||||
| JS | Bilateral VM + right DL | AcoA aneurysm | 70 | 16 | 102 | 60* | 2* | 64* | 12* | 420* | |
| WD | Left VM, left parieto-occipital | AcoA aneurysm | 40 | 14 | 91 | 50* | 6 | 19 | 4* | 188* | |
| PD | Bilateral VM + left DL, left temporal | Head trauma | 53 | 14 | |||||||
| RB | Bilateral VM | Head trauma | 66 | 14 | 106 | 117 | 105 | 6 | 7 | 23 | 79 |
| TS | Right DL, bilateral frontopolar | Head trauma | 40 | 12 | 94 | 95 | 85 | 6 | 12 | 28 | 59 |
| AS | Right DL | Stroke | 77 | 12 | |||||||
| GM | Left DL | Stroke | 58 | 21 | 86 | 85 | 1* | 48* | 8* | 284* | |
| BW | Right VM + DL, right temporal | Tumor | 54 | 12 | 133 | 100 | 105 | 6 | 0 | 30 | 160* |
| HZ | Right VM + DL | Tumor | 67 | 14 | |||||||
| JW | Left VM + DL | Tumor | 50 | 14 | 107 | 104 | 102 | 6 | 6 | 49 | 87 |
| Mean | 59 | 14 | 104 | 85 | 91 | 4 | 26 | 22 | 177 | ||
VM = ventromedial, DL = dorsolateral, AcoA = Anterior Communicating Artery. Age and Education in Years. VIQ = Verbal Intelligence Quotient from the Wechsler Adult Intelligence Scale - III (Wechsler, 1997) or the Wechsler Abbreviated Scale of Intelligence (1999); GM = General Memory index from the Wechsler Memory Scale - III (WMS-III, 1997) or Delayed Recall index from the Wechsler Memory Scale - Revised (1987); WM = Working Memory index from the WMS-III; WCST = Wisconsin Card Sorting Test (Heaton, 1981), Cat. = Categories, PE = Perseverative Errors; FAS = Controlled Oral Word Association Test (Spreen & Benton, 1977); Trails B = Trailmaking test, Part B, in seconds (Lezak, 1995).
= at least two standard deviations away from the normative mean.
The 12 MTL/D patients had a mean age of 66 years (range = 44 to 84), and a mean of 15 years of education (see Table 1). Four of the five patients with memory impairments attributed to anoxia had bilateral damage to the hippocampus and related medial temporal lobe structures. Only cerebral and cerebellar atrophy was visible on the scan of the fifth anoxic patient, but it seems reasonable to assume, based on the previous literature, that he had at least some damage to the hippocampus (Bachevalier & Mevnier, 1996; Squire & Zola, 1996). The two patients with encephalitis had bilateral damage to the hippocampus and adjacent medial temporal lobe areas. In one of these patients, there was also evidence of left frontal lobe involvement. The only patient with memory impairment due to stroke had sustained bilateral damage to the anterior thalamus. We also included in this group four patients with Korsakoff’s disease, which is associated with damage to the diencephalon, including the mammillary bodies and thalamus (Colchester et al., 2001; Shimamura, Jernigan, & Squire, 1988; Squire, Amaral, & Press, 1990). Some degree of cerebral and cerebellar atrophy was also present in these cases. Because there were no significant differences between the Korsakoff’s patients and the other MTL/D patients on any of the dependent measures of memory, we included them in the same group. However, all analyses involving the MTL/D group were performed both with and without the Korsakoff patients. All but one of the MTL/D patients (TR) were members of the Memory Disorders Research Center’s participant pool and were tested in Boston, and the rest of the participants were tested in Tucson. On clinical tests, the MTL/D patients showed normal verbal intelligence (M = 104) on the Wechsler Adult Intelligence Scale - III (WAIS -III; Wechsler, 1997) or the Wechsler Abbreviated Scale of Intelligence (WASI, 1999), although two of the patients (CW and JM) showed slightly depressed IQ scores (approximately 1 standard deviation below the mean). The MTL/D patients had impaired memory scores (M = 58) on the General Memory index from the Wechsler Memory Scale - III (WMS-III; 1997).
The 12 FL patients had a mean age of 59 years (range = 40 to 77), and a mean of 14 years of education (see Table 2). Their lesions were due to anterior communicating artery aneurysm rupture (n = 4), brain tumor (n = 3), head trauma (n = 3), and stroke (n = 2). Four patients had left hemisphere lesions, four had right hemisphere lesions, and four had bilateral lesions. In four patients there was also neuroradiological evidence of extra-frontal damage (Table 2). Because patients with AcoA lesions may also have basal forebrain damage and, as a result, impairments on the general memory index, analyses involving the FL group were performed both with and without the AcoA patients. The four FL patients with AcoA damage did show memory impairments in clinical testing, but none of the other FL patients appeared to have substantial memory impairments (see Table 2).2 The FL patients showed normal verbal intelligence (M = 104 on the WAIS -III, 1997; or WASI, 1999).
When we compared the two patient groups on the neuropsychological measures listed in Tables 1 and 2, independent samples t tests revealed that they were similar in Verbal IQ, but the MTL/D patient group showed significantly worse memory than the FL group, t (18) = 3.44, p = .003. We also administered tests of frontal lobe/executive function —the Wisconsin Card Sorting Test (Heaton, 1981; Spreen & Strauss, 1998), verbal fluency (Spreen & Benton, 1977; Spreen & Strauss, 1998), and the Trailmaking test (Lezak, 1995; Spreen & Strauss, 1998) to as many patients as possible. On only one of these tasks did the two patients groups differ reliably: the MTL/D group (M = 105 s) took marginally less time than the FL group (M = 177 s) to complete Part B of the Trailmaking test, t (18) = 1.77, p = .09.
The 15 healthy subjects had a mean age of 58 years (range = 37 to 78), and a mean of 14 years of education. Between-subjects analyses of variance (ANOVAs) revealed that the three groups were equivalent in age and education (Fs < 1.34).
Materials
The FM questionnaire included specific questions about the target event (i.e., information about September 11th), and the reception event (i.e., the source of news about September 11th). In each case we asked participants to answer in as much detail as possible, and at the end of each section of the questionnaire we asked them to include anything that they had omitted in earlier answers or that we had not asked about.
Memory for the target event was assessed using five questions common to the FM literature (Brown & Kulik, 1977; Cohen, Conway, & Maylor, 1994; Conway et al., 1994; Davidson & Glisky, 2002; Neisser & Harsch, 1992), involving place (i.e., “where did the event occur?”), what occurred before the event, what occurred after the event, people involved, and time of day. Memory for the reception event was measured using five questions analogous to those above (e.g., “where were you when you heard the news?”, “what did you do after you heard the news?”), plus an additional question about the source of the news (i.e., “how did you hear the news, i.e., from what source?”).
Procedure
Between 3 and 30 days after September 11th, we interviewed the healthy subjects and all but one FL patient (patient JS, who was tested approximately 90 days later) by telephone, and tested the remaining patients in person. The procedure was similar for all participants: We read questions to them one at a time, encouraged them to answer in as much detail as they could, and recorded responses as close to verbatim as possible. After the initial test, we did not warn participants about the retention test, which was conducted approximately six months later. On the retention test, we again interviewed the healthy subjects and all but one FL patient by telephone, and tested the remaining patients in person. The same questionnaire was used at both the initial test and the retention test.
On both the initial test and the retention test, we asked participants at the beginning of the questionnaire if they could tell us the most important news event that had occurred during the second week of September, 2001. If they gave no answer, or recalled another event, we read them a series of cues (“World Trade Center”; “New York”; “airplane”; “terrorism”), one at a time in fixed order, until they provided the correct answer.
Scoring
For the initial test, we computed similar scores to evaluate memory for the target event and reception event. For each, participants were assigned a 0 if they did not provide an answer to a relevant question or gave a wrong answer, and a 1 if they gave a correct response. The maximum possible score was thus based on five questions for the target event and six for the reception event. Because of the different number of questions contributing to the two summed scores, we converted all scores to proportions. For the target event, we could know whether participants’ answers were accurate, because these were factual statements about the terrorist attacks that could be evaluated as true or false. For the reception event, we verified patient responses with a relative or caregiver whenever possible. If we found that a patient’s response to a relevant question was inaccurate, we assigned it a score of 0.
Retention of the target event was scored as a proportion of information recalled on the initial test. Retention of the reception event was scored (as in previous FM studies; e.g., Cohen et al., 1994; Conway et al., 1994; Davidson & Glisky, 2002; Neisser & Harsch, 1992) as a measure of congruence between a subject’s responses on the initial and those on the delayed tests. Two judges, who were blind to the group membership of each participant, rated the consistency of each persons’ answer on the initial and the delayed test using a scale of 0, 1, or 2. They assigned a score of 0 if on the delayed test the answer was forgotten or inconsistent with the initial test, 1 if at retest it was somewhat congruent with or less specific than the initial test (e.g., for place, “at home, in the living room chair” on the initial test, and “home” on the retention test), or 2 if it was highly similar between tests. The raters agreed on 88% of answers; in cases of disagreement we computed the median rating. If a person failed to answer a relevant question on the initial test it was omitted from his or her congruence measure. We derived a summed congruence score for the reception event (based on a maximum of six questions) and converted it to a proportion for each individual. This score thus reflected the proportion of information originally reported about the reception event that was retained across the six-month delay, and as such was a measure of individuals’ consistency (not accuracy per se).3 Therefore, because we cannot be certain that each participant’s report about the reception event was accurate, it was the consistency data that were of primary importance.
Results
We compared memory in the three groups of participants, and all comparisons were performed both with and without the Korsakoff and AcoA patients. Because the healthy subjects’ scores were on the ceiling on most measures, and variances across groups were unequal, we conducted non-parametric tests (Mann-Whitney U tests) for comparisons between groups. For descriptive purposes, however, we report means and standard errors.
Memory on the Immediate Tests
Results of the immediate tests are shown in Table 3. On the initial test of memory for the target event, 7 out of the 12 MTL/D patients were unable to tell us the most important news event of the second week of September, 2001 without at least one of the four extra cues. In contrast, all of the FL patients and the healthy subjects could tell us something about the terrorist attacks without these cues. Even with the extra cues, four of the MTL/D patients (RM, SS, TR, and WK) reported absolutely no information about September 11th during either session; none of the other participants had this difficulty. These four patients were therefore excluded from the analyses of memory for the reception event. Overall, the MTL/D group reported significantly less information (M = .55) about what happened on September 11th than the healthy subjects (M = .97; U = 28.50, p = .001). The FL group (M = .83) was also impaired relative to the comparison group (U = 47.50, p = .01). When analyses were conducted on the smaller groups of patients (i.e., without the KA patients in the MTL/D group and without the AcoA patients in the FL group), the only change in the results was that the difference between the FL and MTL/D patients (U = 14.50, p = .07) was now marginally significant.
Table 3.
Mean (and Standard Deviation) proportion of information recalled for the target and reception event in MTL/D patients, FL patients, and healthy participants, on the immediate test
| MTL/D Patients
|
FL Patients
|
Healthy Participants
|
||||
|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | |
| Target Event | .55 | .43 | .83 | .19 | .97 | .07 |
| Reception Event | .73 | .27 | .83 | .31 | 1.00 | .00 |
Scores of the four MTL/D patients who reported nothing about the target event were omitted from the reception event.
On the initial test of memory for the reception event, the MTL/D patients (M = .73) and the FL patients (M = .83) were not significantly different from one another (U = 35.00, n.s.) but both were impaired relative to the healthy participants (M = 1.00, U = 22.50, p = .001, and U = 67.50, p = .04, respectively). When the smaller patient groups were analyzed, these differences were no longer significant.
Memory on the Delayed Tests
Six months later, when we examined memory for the target event, 6 of the 12 MTL/D patients were unable to tell us anything about September 11th without at least one of the four supplemental cues. Even with the extra cueing, the eight MTL/D patients who recalled the event initially still retained significantly less (M = .76) about the events of September 11th than the healthy participants (M = 1.00; U = 15.00, p < .001). The FL group (M = .94) was not reliably impaired relative to the healthy group, and retained significantly more target information across the delay than the MTL/D group (U = 20.50, p = .02), as shown in Figure 1. Analyses of the smaller groups produced identical findings.
Figure 1.
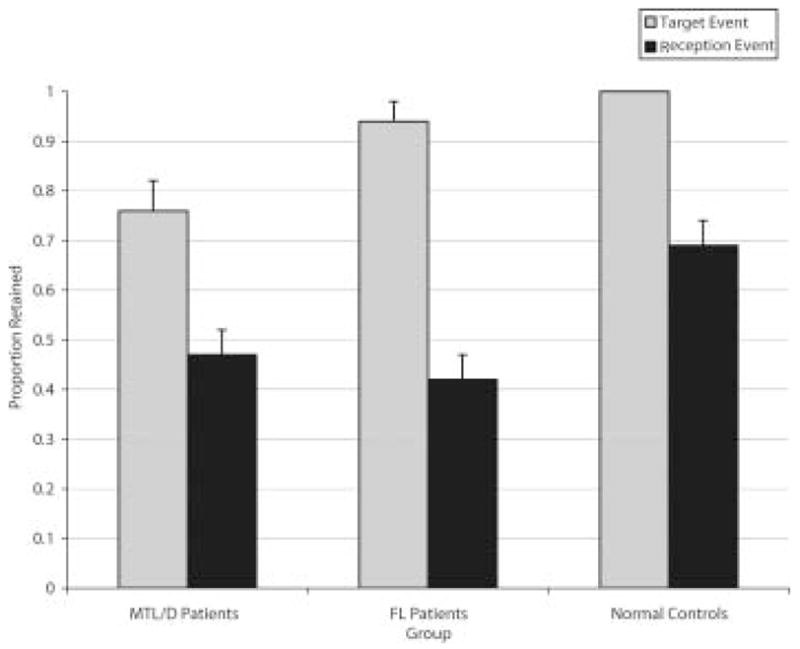
Mean (and standard error) retention of the target and reception event in MTL/D patients, FL patients, and healthy participants on the delayed test.
For the reception event, the MTL/D patients (M = .47) and the FL patients (M = .42) retained equivalent amounts of information (U = 44.00, n.s.), and both groups were impaired relative to the healthy group (M = .69, U = 26.00, p = .03, and U = 33.00, p = .005 respectively). Analyses of the smaller groups revealed the same findings.
Correlations between Psychometric Tests and Memory for the Target and Reception Events
We also examined whether performance on the psychometric tests listed in Tables 1 and 2 was related to memory for the target and reception events, using Pearson correlations. Because of ceiling levels of performance on the initial test, only results from the delayed test were examined. We collapsed all patients into one group for this analysis, in order to maximize statistical power (separate analyses on the two patient groups yielded similar patterns of results to those reported here). We included the four MTL/D patients who did not recall the event on the initial test in the target retention correlations, assigning them a score of 0. Retention of the target event was correlated with the General Memory index from the WMS- III (1997), r = .56, p = .01, but not with any of the frontal/executive function measures (WCST categories, WCST perseverative errors, FAS, and Trails B; all rs ≤ |.20|). Retention of the reception event was not reliably correlated with the General Memory index from the WMS - III (r = .005)4, nor was it significantly correlated with any of the measures of executive function (all rs ≤ |.24|).
Discussion
We evaluated the effects of medial temporal lobe/diencephalic (MTL/D) and frontal lobe (FL) lesions on flashbulb memory. We asked patients what they remembered about the September 11th terrorist attacks (the target event), and how they learned of the news (the reception event). Of primary interest was memory over the long term (i.e., after 6 months). We found, first, that long-term memory for what happened during the attacks was impaired only in the group with MTL/D damage. Second, despite the MTL/D group’s impaired memory for information about the terrorist attacks themselves, they actually retained slightly more source information over time than the FL group. Finally, we found that the FL patients were selectively impaired in recall of the reception event.
Long term memory for what happened during the September 11th terrorist attacks (i.e., the target event), was reliably poorer in the MTL/D group than in the other groups, consistent with previous findings of autobiographical or episodic memory impairment in such patients. On the other hand, long-term memory for the events of September 11th was relatively good in the FL group. The FL patients were indistinguishable from healthy participants, and had significantly better memory for the target event than the MTL/D group. All of the FL patients could tell us about what happened on September 11th without any of the additional cues, whereas many of the MTL/D patients needed these extra cues. Even with this extra help, four of the MTL/D patients could still report absolutely nothing about the target event, just a few days after it occurred. Long term memory for the target event was correlated with delayed memory indices from the WMS, suggesting that memory for the target event relied on processes required on standard tests of episodic memory.
In contrast, the FL patients were significantly impaired on memory for the reception event, that is, how they heard about September 11th. The MTL/D group was also impaired, but showed slightly better retention of the source of the news than the FL group, despite the FL group’s superior memory for the target event. Unlike memory for the target event, memory for how one learned the news about September 11th was not predicted by standard memory measures.
Taken together, these findings suggest that the MTL/D group had a global impairment that covered memory for both the central event and its source. The FL patients, on the other hand, appeared to have a more selective memory deficit specific to source. This pattern of results is consistent with laboratory studies contrasting item and source memory in neurological patients. When MTL/D pathology is severe, as in the present case, it may involve loss of all aspects of an experience in memory, including both item and source information (see Schacter et al., 1984; Shimamura & Squire, 1987, 1991). In contrast, FL injury does not usually produce amnesia for item information, but instead may impair processes necessary for attending to and integrating item and source information at encoding, and/or searching memory and evaluating item-source matches at retrieval (e.g., Glisky et al., 2001; Janowsky et al., 1989a; Senkfor & Van Petten, 1998; for a review, see Moscovitch, 1994). Despite the fact that FL pathology impaired source memory in the present study, we found no reliable correlations between the neuropsychological measures of FL function and source memory. This is consistent with some previous reports (Kopelman, 1989; Shimamura & Squire, 1991) but not others (e.g., Schacter et al., 1984; Shimamura & Squire, 1987; Simons et al., 2002). The failure to find reliable correlations may be attributable to the high degree of inter- and intra-subject variability among patients, and the relatively small sample sizes used in most patient studies. In addition, the various tests of FL function likely measure a variety of different processes, and may depend at least partly on nonfrontal regions as well. In general, however, source memory appears to be more consistently related to pathology and processes in the FL than in the MTL/D, although there may be exceptions to this rule.
Are flashbulb memories qualitatively different from other kinds of source memory? Based on previous findings, we thought that the FL patients might show intact source memory in the present study because of the high degree of emotional arousal associated with the event (Davidson & Glisky, 2002; Kapur et al., 2000). Although emotional arousal may have facilitated encoding of source information in the FL patients, leading to relatively normal memory for the reception event on the immediate test, there was no evidence that emotional arousal facilitated long term retention of source information in the FL group. It is therefore possible that the FL patients’ poor source memory after six months represents a frontally based retrieval deficit. This finding in FL patients stands in contrast with results from a previous study, in which older adults with below average FL function showed normal flashbulb memory (Davidson & Glisky, 2002). It could be that in older adults the FLs are functioning inefficiently, but their function can be enhanced or boosted by emotional arousal, thereby improving source memory. In neurological patients with substantial structural damage to the frontal lobes, however, such enhancements may have less effect. It could also be that some of the patients with FL damage suffered emotional dysfunction and were unable to benefit mnemonically from the emotional arousal that usually accompanies a flashbulb event. We were not able to obtain objective measures of emotional arousal in the present study. The self-report measures that we did collect showed little variability.
Regardless of whether FMs are qualitatively different from other kinds of source memory, overall flashbulb situations appear to be remembered better than other events. In several studies people have also been asked to report on a comparison event (i.e., the most interesting event that occurred in participants’ own lives around the same time as the flash-bulb event), in order to study potential differences between flashbulb and other autobiographical memories. People almost always remember more about the flashbulb reception event than about the comparison event (e.g., Christianson, 1989; Davidson & Glisky, 2002), perhaps because the former is usually more emotionally arousing than the latter. In the present study, we attempted to assess memory for a non-emotional event that occurred in the week before September 11th, but patients were largely unable to report such an event, and so we were unable to make a direct comparison. Even though we were not able to make this comparison, it is interesting to note that 8 out of the 12 MTL/D patients displayed relatively good memory for the target event, possibly because of its emotional aspects (see also Ogden & Corkin, 1991). It is also likely that the amygdala plays a key role in FM, given that it has been implicated in the beneficial effect of emotional arousal on memory in the laboratory (for reviews, see Buchanan & Adolphs, 2002; Hamann, 2001). Only a few patients in our study had evidence of amygdala damage, however, making it difficult to examine its hypothesized contribution to FM. To answer this question, it might be fruitful to investigate whether people with focal amygdala pathology have normal FMs, although, to our knowledge, this has not yet been reported.
Other variables may also have contributed to differences in memory among groups, or lack thereof (for example, if people in one group were especially surprised or aroused by the event, or had greater exposure to media coverage of it, than in the other groups). In this study, although we collected self ratings of surprise, arousal, and rehearsal from our participants, they appeared not to be reliable in the patient groups: The FL patients may have had poor metamemory (e.g., Janowsky, Shimamura & Squire, 1989b) and the MTL/D patients may have had difficulty making accurate retrospective ratings. We have no reason to think, however, that these factors differed across groups. For example, of the four MTL/D patients who remembered nothing, three spent at least a moderate amount of time watching television and/or reading newspapers, and so had many opportunities to re-experience and rehearse the event. The spouse of one of these patients, TR, reported having many conversations about the events of September 11th with him. Yet despite this, and the fact that on the initial test he was wearing a shirt with a logo referring to the September 11th attacks, he remembered nothing about them. On the delayed test, when cued with the terms World Trade Center, New York, airplane, and terrorism, he said that the obvious answer would be that terrorists had flown an airplane into the World Trade Center in New York, but he did not think this had ever occurred. Similarly, Kapur et al. (2000) reported no relation between such secondary variables and FM in MTL/D patients: How often they discussed and saw media coverage of a flashbulb event had no obvious bearing on their recall. Although many secondary factors are often associated with FM strength in normal individuals (for a review, see Conway 1995), their influence on FM in people with memory impairments may be less evident because of poor performance overall.
Future research could examine whether specific subregions of the MTL/D or FL are differentially important for FM. We saw no obvious relations between lesion locus and performance in either of the patient groups, but this may have been because they were relatively small in size and there was considerable heterogeneity with respect to lesion location. Future work might also explore whether there are certain conditions under which patients with MTL/D or FL pathology can show evidence of preserved FM. For example, patients might show intact implicit memory, or might be able to recognize information even if unable to recall it. Of course, all research on memory for real-world events must be interpreted with caution, because of the challenges inherent to such studies. Because these studies use retrospective reports, researchers can only examine test-retest consistency of participants’ responses, rather than their accuracy. As well, the relative rarity of public flashbulb events limits our opportunities to study the brain regions involved in memory for these situations. For these reasons, laboratory studies of emotion and source memory may be a more productive avenue for future research.
Acknowledgments
This research was supported by fellowships from the Natural Sciences and Engineering Research Council of Canada and the Canadian Institutes of Health Research to P. D., a National Institute on Aging grant (AG 14792) to E. G., and a National Institute on Neurological Disease and Stroke grant (NS 26985) to the Memory Disorders Research Center at Boston University and the Boston VA Healthcare System. We thank Lee Ryan for access to healthy participants; Lis Nielsen, Jasmeet Pannu, Pamela Perschler, and Sheryl Reminger for help contacting participants; Mick Alexander, Kelly Sullivan Giovanello, and David Schnyer for providing lesion data on the patients from Boston; Michael Robinson and Andrea Soulé for assistance with data collection, entry, and analysis; and members of the Memory Interest Group at the University of Arizona for helpful comments on a preliminary report. Preliminary findings were presented at the 2003 International Neuropsychological Society meeting in Honolulu, HI.
Footnotes
An exception may involve spatial context, which may be more dependent on the MTL/D than the FL region of the brain (e.g., Smith & Milner, 1981, 1984).
Four FL patients (AS, DJ, HZ, and PD) were not available for psychometric testing (see Table 2). Patient GM was not tested on VIQ because of a mild residual expressive language deficit.
This retention scoring method reflected consistency, which is necessary for, although not synonymous with, accuracy. That is, if a subject’s two reports were inconsistent, then we knew that at least one was inaccurate, and could score the data as such. However, even if reports were consistent over time, they were not necessarily accurate.
For two FL patients, we instead used a similar measure, the Delayed Recall index from the WMS-R (1987) — see Table 2.
References
- Bachevalier J, Meunier M. Cerebral ischemia: Are the memory deficits associated with hippocampal cell loss? Hippocampus. 1996;6:553–560. doi: 10.1002/(SICI)1098-1063(1996)6:5<553::AID-HIPO8>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Brewer WF. The theoretical and empirical status of the flashbulb memory hypothesis. In: Winograd E, Neisser U, editors. Affect and accuracy in recall. Cambridge, UK: Cambridge University Press; 1992. pp. 274–305. [Google Scholar]
- Brown R, Kulik J. Flashbulb memories. Cognition. 1977;5:73–99. [Google Scholar]
- Buchanan TW, Adolphs R. The role of the human amygdala in emotional modulation of long-term declarative memory. In: Moore S, Oaksford M, editors. Emotional Cognition: From brain to behavior. London, UK: John Benjamins; 2002. pp. 9–34. [Google Scholar]
- Budson AE, Simons JS, Sullivan AL, Beier JS, Solomon PR, Scinto LF, Daffner KR, Schacter DL. Memory and emotions for the September 11, 2001 terrorist attacks in patients with Alzheimer’s disease, patients with mild cognitive impairment, and healthy older adults. Neuropsychology. 2004;18:315–327. doi: 10.1037/0894-4105.18.2.315. [DOI] [PubMed] [Google Scholar]
- Candel I, Jelicic M, Merckelbach H, Wester A. Korsakoff patients’ memories of September 11, 2001. Journal of Nervous and Mental Disease. 2003;191:262–265. doi: 10.1097/01.NMD.0000061142.82435.BC. [DOI] [PubMed] [Google Scholar]
- Christianson SA. Flashbulb memories: Special, but not so special. Memory & Cognition. 1989;17:435–443. doi: 10.3758/bf03202615. [DOI] [PubMed] [Google Scholar]
- Cohen G, Conway MA, Maylor EA. Flashbulb memories in older adults. Psychology and Aging. 1994;9:454–463. doi: 10.1037//0882-7974.9.3.454. [DOI] [PubMed] [Google Scholar]
- Colchester A, Kingsley D, Lasserson D, Kendall B, Bello F, Rush C, Stevens TG, Goodman G, Heilpern G, Stanhope N, Kopelman MD. Structural MRI volumetric analysis in patients with organic amnesia, I: Methods and comparative findings across diagnostic groups. Journal of Neurology, Neurosurgery, and Psychiatry. 2001;71:13–22. doi: 10.1136/jnnp.71.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway MA. Flashbulb memories. Hove, UK: Lawrence Earlbaum Associates; 1995. [Google Scholar]
- Conway MA, Anderson SJ, Larsen SF, Donnelley CM, McDaniel MA, McClelland AGR, Rawles RE, Logie RH. The formation of flashbulb memories. Memory & Cognition. 1994;22:326–343. doi: 10.3758/bf03200860. [DOI] [PubMed] [Google Scholar]
- Davidson PSR, Glisky EL. Is flashbulb memory a special instance of source memory? Evidence from older adults. Memory. 2002;10:99–111. doi: 10.1080/09658210143000227. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kramer J, Kaplan E, Ober BA. The California Verbal Learning Test. San Antonio, TX: Psychological Corporation; 1987. [Google Scholar]
- Glisky EL, Polster MR, Routhieaux BC. Double dissociation between item and source memory. Neuropsychology. 1995;9:229–235. [Google Scholar]
- Glisky EL, Rubin SR, Davidson PSR. Source memory in older adults: An encoding or retrieval problem? Journal of Experimental Psychology: Learning, Memory, and Cognition. 2001;27:1131–1146. doi: 10.1037//0278-7393.27.5.1131. [DOI] [PubMed] [Google Scholar]
- Hamann SB. Cognitive and neural mechanisms of emotional memory. Trends in Cognitive Sciences. 2001;5:394–400. doi: 10.1016/s1364-6613(00)01707-1. [DOI] [PubMed] [Google Scholar]
- Heaton RK. Wisconsin Card Sorting Test manual. Los Angeles: Western Psychological Services; 1981. [Google Scholar]
- Janowsky JS, Shimamura AP, Squire LR. Source memory impairment in patients with frontal lobe lesions. Neuropsychologia. 1989a;27:1043–1056. doi: 10.1016/0028-3932(89)90184-x. [DOI] [PubMed] [Google Scholar]
- Janowsky JS, Shimamura AP, Squire LR. Memory and metamemory: Comparisons between patients with frontal lobe lesions and amnesic patients. Psychobiology. 1989b;17:3–11. [Google Scholar]
- Johnson MK, O’Connor M, Cantor J. Confabulation, memory deficits, and frontal dysfunction. Brain and Cognition. 1997;34:189–206. doi: 10.1006/brcg.1997.0873. [DOI] [PubMed] [Google Scholar]
- Kapur N. Paper presented at the meeting of the Memory Disorders Research Society; San Francisco. 1997. [Google Scholar]
- Kapur N, Abbott P, Graham KS, Simons JS. The neuropsychology of human flash-bulb memory. 2000. Manuscript submitted for publication. [Google Scholar]
- Kopelman MD. Remote and autobiographical memory, temporal context memory, and frontal atrophy in Korsakoff and Alzheimer patients. Neuropsychologia. 1989;27:437–460. doi: 10.1016/0028-3932(89)90050-x. [DOI] [PubMed] [Google Scholar]
- Lezak MD. Neuropsychological assessment. 2. New York: Oxford University Press; 1995. [Google Scholar]
- Milner B, Corsi P, Leonard G. Frontal-lobe contributions to recency judgements. Neuropsychologia. 1991;29:601–618. doi: 10.1016/0028-3932(91)90013-x. [DOI] [PubMed] [Google Scholar]
- Moscovitch M. Memory and working with memory: Evaluation of a component process model and comparisons with other models. In: Schacter DL, Tulving E, editors. Memory systems 1994. Cambridge, MA: MIT Press; 1994. pp. 269–310. [Google Scholar]
- Neisser U, Harsch N. Phantom flashbulbs: False recollections of hearing the news about Challenger. In: Winograd E, Neisser U, editors. Affect and accuracy in recall. Cambridge, UK: Cambridge University Press; 1992. pp. 9–31. [Google Scholar]
- Ogden JA, Corkin S. Memories of HM. In: Abraham WC, Corballis M, White GK, editors. Memory mechanisms: A tribute to G. V. Goddard. Hillsdale, NJ: Lawrence Erlbaum Associates; 1991. pp. 195–215. [Google Scholar]
- Schacter DL, Harbluk JL, McLachlan DR. Retrieval without recollection: An experimental analysis of source amnesia. Journal of Verbal Learning and Verbal Behavior. 1984;23:593–611. [Google Scholar]
- Schmolck H, Buffalo EA, Squire LR. Memory distortions develop over time: Recollections of the O.J. Simpson trial verdict after 15 and 32 months. Psychological Science. 2000;11:39–45. doi: 10.1111/1467-9280.00212. [DOI] [PubMed] [Google Scholar]
- Schwerdt PR, Dopkins S. Memory for content and source in temporal lobe patients. Neuropsychology. 2001;15:48–57. [PubMed] [Google Scholar]
- Senkfor AJ, Van Petten C. Who said what? An event-related potential investigation of source and item memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1998;24:1005–1025. doi: 10.1037//0278-7393.24.4.1005. [DOI] [PubMed] [Google Scholar]
- Shimamura AP, Janowsky JS, Squire LR. Memory for the temporal order of events in patients with frontal lobe lesions and amnesic patients. Neuropsychologia. 1990;28:803–813. doi: 10.1016/0028-3932(90)90004-8. [DOI] [PubMed] [Google Scholar]
- Shimamura AP, Jernigan TL, Squire LR. Korsakoff’s Syndrome: Radiological (CT) findings and neuropsychological correlates. Journal of Neuroscience. 1988;8:4400–4410. doi: 10.1523/JNEUROSCI.08-11-04400.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura AP, Squire LR. A neuropsychological study of fact memory and source amnesia. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1987;13:464–473. doi: 10.1037//0278-7393.13.3.464. [DOI] [PubMed] [Google Scholar]
- Shimamura AP, Squire LR. The relationship between fact and source memory: Findings from amnesic patients and normal subjects. Psychobiology. 1991;19:1–10. [Google Scholar]
- Simons JS, Verfaellie M, Galton CJ, Miller BL, Hodges JR, Graham KS. Recollection-based memory in frontotemporal dementia: Implications for theories of long-term memory. Brain. 2002;125:2523–2536. doi: 10.1093/brain/awf247. [DOI] [PubMed] [Google Scholar]
- Smith ML, Milner B. The role of the right hippocampus in the recall of spatial location. Neuropsychologia. 1981;19:781–793. doi: 10.1016/0028-3932(81)90090-7. [DOI] [PubMed] [Google Scholar]
- Smith ML, Milner B. Differential effects of frontal-lobe lesions on cognitive estimation and spatial memory. Neuropsychologia. 1984;22:697–705. doi: 10.1016/0028-3932(84)90096-4. [DOI] [PubMed] [Google Scholar]
- Spreen O, Benton AL. Neurosensory Center Comprehensive Examination for Aphasia (NCCEA) (Revised edition) Victoria, British Columbia, Canada: University of Victoria Neuropsychology Laboratory; 1977. [Google Scholar]
- Spreen O, Strauss E. A compendium of neuropsychological tests. New York: Oxford University Press; 1998. [Google Scholar]
- Squire LR, Amaral DG, Press GA. Magnetic resonance measurements of hippocampal formation and mammillary nuclei distinguish medial temporal lobe and diencephalic amnesia. Journal of Neuroscience. 1990;10:3106–3117. doi: 10.1523/JNEUROSCI.10-09-03106.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR, Zola SM. Ischemic brain damage and memory impairment: A commentary. Hippocampus. 1996;6:546–552. doi: 10.1002/(SICI)1098-1063(1996)6:5<546::AID-HIPO7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Thaiss L, Petrides M. Source versus content memory in patients with a unilateral frontal cortex or a temporal lobe excision. Brain. 2003;126:1112–26. doi: 10.1093/brain/awg112. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale -- III. San Antonio, TX: Psychological Corporation, Harcourt Brace; 1997. [Google Scholar]
- Wechsler D. Wechsler Memory Scale -- Revised manual. New York: Psychological Corporation; 1987. [Google Scholar]
- Wechsler D. Wechsler Memory Scale -- III. San Antonio, TX: Psychological Corporation, Harcourt Brace; 1997. [Google Scholar]
- Wechsler Abbreviated Scale of Intelligence. (1999) San Antonio, TX: Psychological Corporation, Harcourt Brace.; [Google Scholar]
- Winograd E, Neisser U, editors. Affect and accuracy in recall. Cambridge, UK: Cambridge University Press; 1992. [Google Scholar]


