Figure 2.
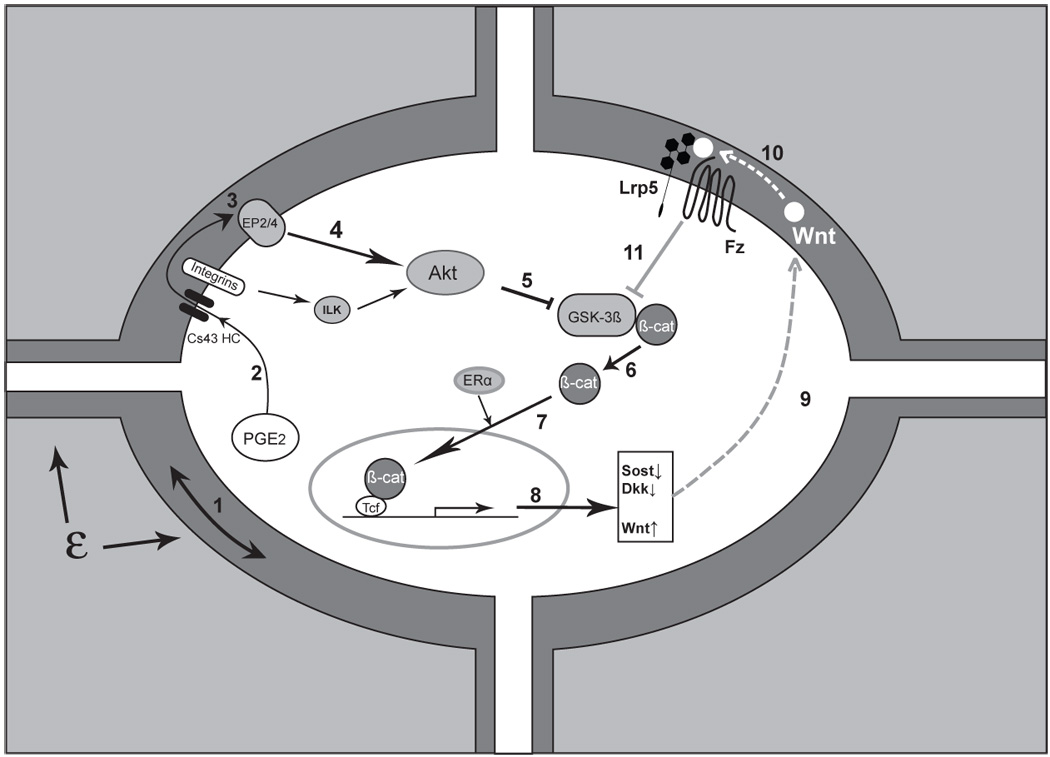
Triggering and amplification of the Wnt/β-catenin pathway in osteocytes in response to load. Mechanical load applied to bone (ε) is perceived by the osteocyte through an unknown mechanism, although fluid flow induced through the lacunarcanalicular system may be a critical component of this perception, ‘step 1’. Perception of load (strain) triggers a number of intracellular responses including the release of PGE2, ‘2’ through a poorly understood mechanism into the lacunar-canalicular fluid where it can act in an autocrine and/or paracrine fashion. Connexin-43 hemichannels (CX43 HC) in this PGE2 and integrin proteins appear to be involved. Binding of PGE2 to its EP2 and/or EP4 receptor, ‘3’, leads to a downstream inhibition of GSK-3β, ‘5’ (likely mediated by Akt, ‘4’) and the intracellular accumulation of free β-catenin, ‘6’. (Integrin activation can also lead to Akt activation and GSK-3β inhibition.) New evidence suggests that ER may participate in the nuclear translocation of β-catenin, ‘7’ which leads to changes in the expression of a number of key target genes ‘8’. One of the apparent consequences is the reduction in sclerostin and Dkk1,’9’ with increased expression of Wnt, ‘10’ (which one or ones is unknown). The net result of these changes is to create a permissive environment for the binding of Wnt to Lrp5-Fz and an amplification of the load signal, ‘11’. (See text for more details and references)
