Abstract
Retrovirus packaging cell lines expressing the Moloney murine leukemia virus gag and pol genes but lacking virus envelope genes produce virus-like particles constitutively, whether or not they express a transcript from an integrated retroviral provirus. In the absence of a proviral transcript, the assembled particles contain processed gag and reverse transcriptase, and particles made by cells expressing an integrated lacZ provirus also contain viral RNA. The virus-like particles from both cell types are enveloped and are secreted/budded into the extracellular space but are noninfectious. Their physicochemical properties are similar to those of mature retroviral particles. The noninfectious gag pol RNA particles can readily be made infectious by the addition of lipofection reagents to produce preparations with titers of up to 105 colony-forming units per ml.
The role in assembly of two retrovirus-encoded proteins, gag and env, have been extensively studied and are reasonably well understood. The retrovirus gag protein is central to the mechanisms of virus assembly because of its ability to interact with both the viral RNA and with the virus envelope proteins. In the case of the C-type retroviruses, as represented by Moloney murine leukemia virus (MoMLV), virion assembly and maturation include intracellular oligomerization of gag, its association with viral RNA via a recognition of the packaging signal Ψ on the RNA, and the subsequent specific interaction of the resulting complex with plasma membrane-embedded env protein. In addition to viral RNA, env, and gag, a number of other viral components, including the virus-encoded pol gene products protease, reverse transcriptase (RT), and integrase, participate in the full assembly of mature fully packaged and infectious virus particles.
The assembly of virus-like particles, however, does not require the presence of all these components. Retroviral gag protein alone can oligomerize and assemble into virus-like particles, both in vitro and in vivo (1–9), even in the absence of genomic RNA, RT, viral protease, or envelope (10–20). Similarly, gag particle assembly can occur not only intracellularly but even in an in vitro translation system (2). In addition, expression of HIV gag and pol genes results in the production of virus-like particles that contain gag protein and RT activity and that associate with virus RNA molecules, even in the absence of virus env (4). The role, if any, that these particles play in the final assembly of mature virus particles is unknown.
In addition to virus assembly, the env protein performs at least two other important viral functions: a receptor recognition function that allows the specific binding of the virus to a cell surface receptor required for uptake into an endosome and a fusion function that permits the release of the uncoated virus core and the viral RNA from the endosome into the cytoplasm. Through its interaction with specific cellular receptors, the env glycoprotein of each retrovirus defines the tropism of the virus. Recently, a number of groups have shown that the envelope component of one retrovirus can be substituted by the envelope of another virus to produce “pseudotyped” virus particles that exhibit a cell tropism specified by the new env component (21–24). These kinds of envelope modifications have been further extended to the development of targetable retrovirus vectors through the insertion of tissue-specific ligands into the env molecule (25–27).
Recently, a number of packaging cells capable of producing high-titer retrovirus vectors have been developed that very efficiently express the gag and pol genes but express no virus envelope. Because they are such efficient factories for the overexpression of retroviral proteins, these cells provide practical reagents not only for the production of virus vectors but also potentially for the study of mechanisms of virus assembly and of the virus–cell membrane fusion events required for virus infection. In the present studies, we have characterized noninfectious virus-like particles produced constitutively by packaging cell lines derived from the human embryonal kidney cell line 293 that expresses the MoMLV gag and pol genes in the absence of MoMLV envelope. We demonstrate that such particles are produced constitutively in large amounts, that they can associate with viral RNA, and strikingly, that they can be made infectious by the addition of lipofection reagents.
MATERIALS AND METHODS
Producer Cell Lines and Plasmid Constructs.
The 208F cells and HT1080 cells were obtained from the American Type Culture Collection. The previously described retrovirus packaging cell line 293GP expressing the Moloney gag and pol genes (28), its derivative containing an integrated LZRNL provirus (LTR-lacZ-RSV-neoR-LTR) (29), and the amphotropic producer cell line 293GP-LZRNL/amphotropic expressing the Moloney amphotropic envelope from a stably transfected amphotropic envelope expression plasmid have been reported from our laboratory (30). For production of vesicular stomatitis virus (VSV)-G pseudotyped virus, 293GP-LZRNL cells were transfected with the expression plasmid pCMV-VSV-G, as described (31).
Preparation of gag pol, gag pol RNA Particles and Fully Assembled Virus Particles.
For all preparations of particles, 200 ml of conditioned medium from 60–80% confluent 293GP and 293GP/LZRNL cells, respectively, was collected, filtered through a 0.45-μm (pore size) filter, and centrifuged at 25,000 rpm, 4°C, in an SW28 rotor for 2 h. The pellets were washed once with DMEM, recentrifuged as above, resuspended in 500 μl of DMEM, and stored at −70°C. Particles from 293GP cells are designated gag pol particles, and those from 293GP-LZRNL cells are designated gag pol RNA particles. Amphotropic virus and VSV-G pseudotyped virus were also concentrated by centrifugation as described above. The pellets were resuspended in 500 μl of DMEM and were assayed, along with unconcentrated conditioned medium, for RT activity as described below.
RT Activity.
The RT activity of virus particles and sucrose gradient fractions was assayed by established methods (32). A reaction volume of 100 μl of RT reaction mixture contained 50 mM Tris⋅HCl (pH 8.3), 10 mM dithiothreitol, 1 mM MnCl2, 60 mM NaCl, 20 μM dTTP, 2.5 μCi of [3H]dTTP (1 Ci = 37 GBq), 0.25% Nonidet P-40, and 0.04 unit of poly(rA)⋅(dT)10. The reaction mixture was incubated at 37°C for 30 min, spotted onto filter paper disks, and air-dried. The filter discs were washed with 5% Na2HPO4 and then with water, rinsed with ethanol, and dried, and radioactivity was measured in a scintillation counter.
Metabolic Labeling of Virus Particles and SDS/PAGE Analysis.
The 293GP, 293GP-LZRNL, and 293GP-LZRNL/amphotropic envelope producer cells were grown to subconfluency in 10-cm dishes. Cells were washed once with methionine-free medium and incubated with methionine-free medium supplemented with 5% fetal bovine serum for 1 h. [35S]Methionine was added to a final concentration of 100 μCi per ml of medium in a total volume of 5 ml. After labeling for 18 h, the conditioned medium was collected, filtered through a 0.45-μm filter, centrifuged twice at 25,000 rpm in a SW28 rotor at 4°C, washed, and repelleted as described above. The pellet was suspended in 100 μl of DMEM, and equal numbers of counts were loaded onto a 12.5% SDS/PAGE gel. Electrophoresis was carried out at 150 V constant voltage, after which the gel was fixed in acetic acid/methanol/water, 10:20:70 (vol/vol) for 30 min, dried on a slab-gel dryer, and exposed to x-ray film.
Sucrose Density Gradient Centrifugation.
Equilibrium centrifugation was carried out on a 15–40% (wt/wt) continuous sucrose gradient in PBS. Particles pelleted as above were centrifuged in a SW41 rotor at 30,000 rpm at 4°C for 18 h. Aliquots of 500 μl each were collected from the top and analyzed for sucrose density and for RT activity. Velocity centrifugation was carried out in a SW41 rotor with a 5–30% (wt/wt) continuous sucrose gradient. The gradient was centrifuged at 30,000 rpm in a SW41 rotor for 30 min at 4°C and analyzed as described above.
Particle Morphology by Electron Microscopy.
Concentrated gag pol and gag pol RNA particles were diluted in PBS and 20 μl was placed on a pure carbon film substrate on a 300-mesh copper grid that had previously been glow-discharged. After 1 min, several drops of 1% aqueous uranyl acetate were added, excess stain was removed, and the sample was allowed to air dry. The samples were examined in a JEOL 100cx or 2000fx electron microscope operating at 80 kev. For thin-section electron microscopy, producer cells were grown on a Mat Tek culture plate and fixed with 2% glutaraldehyde in 0.1 M sodium cacodylate buffer for 20 min. Cells were washed in buffer for 10 min and postfixed in 1% osmium tetroxide for 30 min. The cells were washed with distilled water, dehydrated in ethanol, and infiltrated with Durcupan ACM resin (Electron Microscopy Sciences, Fort Washington, PA). After polymerization at 60°C for 24 h, the coverslip was removed, and 70-nm-thick sections were cut with a diamond knife (Diatome, Fort Washington, PA) and viewed at 80 kev with a JEOL 100cx electron microscope.
Infectivity of Particles.
Pelleted gag pol RNA particles prepared as described above were suspended in 300 μl of serum-free optiMEM medium (GIBCO/BRL) containing 8 μl of a stock solution (1 mg/ml) of Cellfectin, Lipofectin, Lipofectamine, or Transfectamine (all from GIBCO/BRL). These mixtures were incubated at room temperature for 15 min and then added to subconfluent monolayers of 293GP, HT1080, or 208F cells in six-well dishes containing 2 ml of medium per well. Alternatively, the concentrated gag pol or gag pol RNA particles were added to cells grown as described above immediately after the lipofection agents (8 μl) had been added directly to the cell monolayers. In all cases, G418 was added 24 h after infection to a final concentration of 400 μg/ml, and resulting G418-resistant colonies were counted 2 weeks later. Infection of cells with amphotropic and VSV-G-pseudotyped virus preparations was carried out in the presence of Polybrene (8 μg/ml) as described (19).
LacZ expression in transduced cells was determined by flow cytometric analysis of infected cells. The fluorescent lacZ substrate from Molecular Probes (fluorescein d-galactopyranoside) was used to identify LacZ-expressing cells by a protocol described by the manufacturer, using a FACScan (Becton Dickinson) instrument.
RESULTS
RT Activity and gag Subunits in Particles from Conditioned Medium.
Table 1 summarizes the levels of RT activity in untransfected and transfected preparations of 293GP and 293GP-LZRNL cells. Indistinguishable levels of total RT activity were present in undiluted medium from cells deficient in envelope protein expression as well as from cells expressing either the amphotropic envelope or the VSV-G protein, indicating that the release of RT into the medium is constitutive and does not require full assembly of intact infectious virus particles. The yield of RT activity in the pellet was variable, ranging from 25% to 53% of starting material.
Table 1.
RT activity in conditioned medium from packaging cell lines
| Cell | Total RT, dpm | RT in pellet, % of total RT | RT in supernatant after centrifugation, % of total RT |
|---|---|---|---|
| 293GP | 3.8 × 107 | 50 | 1.8 |
| 293GP (mock transfection) | 3.9 × 107 | 25 | 2.2 |
| 293GP LZRNL (mock transfection) | 4.4 × 107 | 36 | 0.3 |
| 293GP LZRNL (amphotropic env) | 4.7 × 107 | 53 | 2.3 |
| 293GP LZRNL (VSV-G env) | 5.8 × 107 | 33 | 2.9 |
Total RT in sample before pelleting is expressed in dpm units. RT in pellet is the percent of total RT recovered in the pellet after centrifugation.
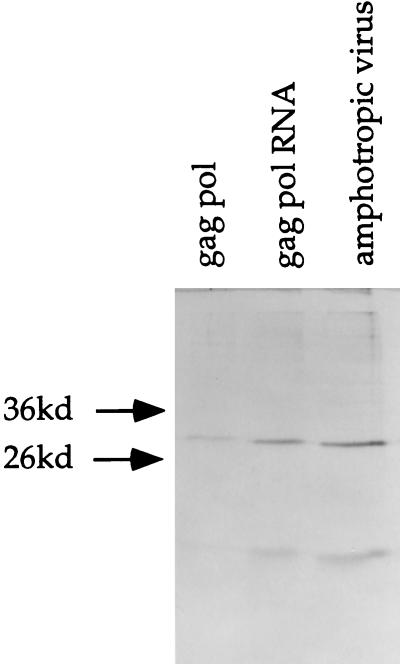
Fig. 1 presents results of an SDS/PAGE analysis of pellets from conditioned medium from 293GP, 293GP-LZRNL, and 293GP-LZRNL/amphotropic cells labeled with [35S]methionine. Pellets from all three cell types contained the gag-derived p30 and p15 gag proteins in approximately equal ratios, indicating that the appropriately processed gag proteins were present in the form of sedimentable particles.
Figure 1.
Autoradiograph of 35S-methionine-labeled virus particles from producer cells. The 293GP, 293GP-LZRNL, and 293GP-LZRNL/amphotropic cells were labeled and the conditioned medium from each was concentrated. Samples were electrophoresed in a SDS/PAGE gel and exposed. Two bands seen in each lane represent fully processed p30 and p15 gag proteins. The closed arrows indicate the position of the 36-kDa and 26-kDa molecular mass markers (prestained markers, Sigma).
Infectivity of gag pol RNA Particles.
Table 2 summarizes the infectivity assays of the gag pol RNA particles on HT1080 cells. Because the 239GP cells contain no viral RNA, the assembled gag pol particles did not generate any G418-resistant colonies in the exposed HT1080 cells. Similarly, infection with gag pol RNA particles failed to produce G418-resistant colonies in the presence or absence of Polybrene. However, in the presence of Lipofectin, the gag pol RNA particles became infectious, as indicated by the appearance of large numbers of G418-resistant colonies. Particles mixed with Cellfectin gave rise to more G418-resistant colonies than Lipofectin, but Lipofectamine gave rise to a low colony count (data not shown). In the presence of Lipofectin, the unconcentrated conditioned medium from 293GP-LZRNL cells produced 1–4 × 102 G418-resistant colonies per ml. After concentrating the particles approximately 400-fold by centrifugation and after exposure to Lipofectin, the gag pol RNA particles demonstrated titers of colony-forming units exceeding 105 G418-resistant colonies per ml, representing complete recovery of the infectious particles present in the unconcentrated medium. Exposure of the cells to gag pol RNA particles and Lipofectin without preincubation reduced the efficiency of infection by approximately 10-fold. Pretreatment of the particles with DNase or RNase had no significant effect on the efficiency of gene transfer, suggesting that the viral RNA present in the assembled particles is largely inaccessible to the enzymes and, therefore, apparently not present merely in loose nonspecific complexes with the assembled gag proteins. Infectivity with Lipofectin-treated particles was approximately 10-fold less efficient than with authentic amphotropic vector and 100-fold less efficient than pseudotyped LZRNL virus, all produced in the same 293GP packaging cell line and normalized to identical RT levels (data not shown).
Table 2.
Infectivity on HT1080 cells of pelleted gag pol RNA particles*
| Experiment | G418-resistant colonies, no./ml |
|---|---|
| gag pol RNA only† | 0 |
| gag pol RNA + Polybrene‡ | 0 |
| gag pol RNA + Lipofectin§ | 3.6 × 105 |
| gag pol RNA + Lipofectin + Polybrene | 1.5 × 105 |
| gag pol RNA + RNase + Lipofectin¶ | 3.2 × 105 |
| gag pol RNA + DNase + Lipofectin¶ | 3.5 × 105 |
| Lipofectin on cells + gag pol RNA‖ | 2.0 × 104 |
In a number of experiments, the unpelleted conditioned medium from 293GP-LZRNL cells in the presence of Lipofectin contained 100–400 G418-resistant colony-forming units/ml.
gag pol RNA particles used in this experiment are from 293GP-LZRNL cells and were concentrated as described in the text.
Polybrene was used at 8 μg/ml (final concentration).
Lipofectin (GIBCO/BRL) was used (8 μg; 8 μl of 1 mg/ml) for infecting cells in 2 ml of medium.
gag pol RNA particles were treated with RNase and DNase at a final concentration of 10 μg/ml and were incubated at 37°C for 30 min.
Lipofectin and gag pol RNA particles were added separately to the cells without preincubation.
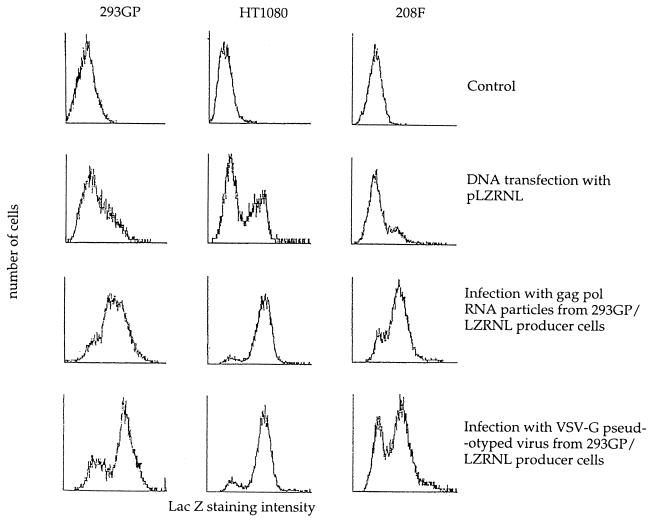
To characterize further the mechanism of gene transfer by the Lipofectin-treated gag pol RNA particles, we examined the expression pattern of lacZ in HT1080, 208F, and 293GP cells transfected with the pLZRNL retrovirus plasmid, infected as described above, or infected with mature VSV-G pseudotyped LZRNL virus obtained from the same producer cell line (28). Pooled G418-resistant cells were analyzed for lacZ expression by flow cytometry by using the flourescent lacZ substrate assay. In Fig. 2, the pattern of lacZ expression as indicated by the level of gene expression and the number of lacZ-positive cells is very similar to that of cells infected with authentic LZRNL virus and markedly different from that of cells transfected with the pLZRNL plasmid. The majority of cells exposed to infectious gag pol RNA particles express the lacZ transgene at the same intensity as retrovirally infected cells, whereas cells transfected with pLZRNL DNA demonstrate a much smaller percentage of G418-resistant cells expressing lacZ.
Figure 2.
Flow cytometry analysis of lacZ expression in 293GP, HT1080, or 208F cells transfected by (i) Lipofectin-mediated gene transfer with pLZRNL DNA, (ii) infected with gag pol RNA particles from cell line 293GP-LZRNL in the presence of Lipofectin, or (iii) infected with VSV-G pseudotyped retrovirus prepared from the packaging cell line 293GP-LZRNL. Infected and transfected cells selected by growth in G418 were pooled and assayed for lacZ activity by flow cytometry analysis. In each case, 10,000 live cells were analyzed. The ordinate represents cell numbers and the abscissa represents arbitrary fluorescence emission intensity units.
Physicochemical Properties of Virus-Like Particles.
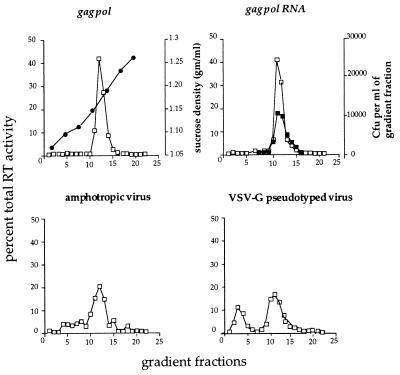
We further examined the structure of the assembled gag pol particles, gag pol RNA particles, amphotropic virus, and VSV-G pseudotyped particles by equilibrium and velocity sedimentation centrifugation. In parallel equilibrium sucrose gradients, fractions were analyzed for sucrose density and for RT activity. Fig. 3 demonstrates that the peak RT activity of both gag pol and gag pol RNA preparations corresponds to a density of 1.15–1.16 g/ml, similar to that previously reported for MoMLV (33). No significant amounts of RT activity were detected at other positions in the gradient, either in nonsedimentable form at the top of the gradients or as aggregates at or near the bottom of the gradients. Recovery of RT activity was nearly complete (data not shown). Interestingly, the peak RT activities of both gag pol and gag pol RNA particles were indistinguishable from each other and from that of mature fully packaged MoMLV, indicating either that the viral genomic RNA present in the gag pol RNA particles or in intact virus makes very little contribution to the particle density or that the particles produced by 293GP cells may encapsidate cellular RNAs (see Discussion). Fig. 3 also indicates that the Lipofectin-induced particle infectivity in HT1080 cells cosediments with the RT activity. As expected, the majority of infectious pseudotyped viral particles (>90%) also cosediment with the major RT peak.
Figure 3.
RT activity (□) in fractions from equilibrium sucrose density gradients of pelleted gag pol particles, gag pol RNA particles, amphotropic virus, and VSV-G pseudotyped virus. The gag pol RNA figure also shows the infectivity of the fractions, as measured by the generation of G418-resistant colonies [colony-forming units (cfu)/ml] after infection of HT1080 cells with aliquots of the gradient fraction and Lipofectin (▪). •, Density of sucrose from gradient fractions in the gag pol figure.
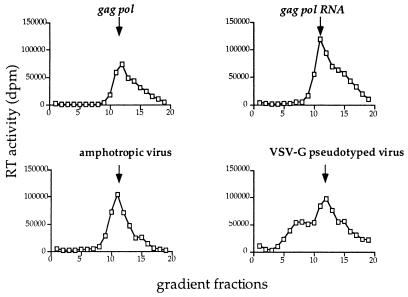
An overall structural similarity between assembled gag pol and gag pol RNA particles is further indicated by their similar sedimentation by velocity gradient centrifugation (Fig. 4). Both kinds of particles exhibit a major RT peak at approximately 580S with a large leading edge, presumably containing larger or aggregated material. Authentic VSV-G pseudotyped virus also demonstrated a somewhat heterogeneous particle distribution as assayed by RT activity, with a major peak at approximately 580S and an inconsistent minor peak sedimenting at approximately 370S.
Figure 4.
RT activity (□) of fractions from velocity gradient sedimentation analysis of pelleted gag pol particles, gag pol RNA particles, amphotropic virus, and VSV-G pseudotyped virus. For each sample, RT values represent the percentage fraction of total RT loaded onto the gradient. The arrows indicate the approximate position of the 580S major peak. For the pseudotyped particles, the slower sedimenting material with a sedimentation coefficient of approximately 370S was not present in all preparations of VSV-G pseudotyped virus.
Morphology by Electron Microscopy.
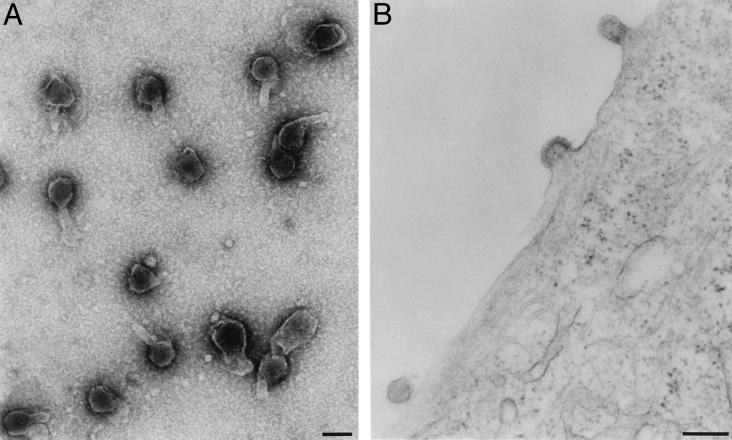
Electron microscope examination of gag pol particles from 293GP producer cells and of gag pol RNA particles from 293GP-LZRNL cells (Fig. 5A) revealed large numbers of similar, apparently enveloped particles in centrifuged samples of conditioned medium. Most particles were roughly spherical with a diameter of 80–100 nm and with an electron-dense core. Thin section electron micrograph examination of 293GP-LZRNL cells also revealed typical type C retrovirus particles in a variety of stages of budding at the plasma membrane (Fig. 5B) and with no morphological evidence of subvirus assembled complexes in the cytoplasm.
Figure 5.
Electron micrographs of 293GP-LZRNL particles (A) and 293GP-LZRNL packaging cells (B). (A) Preparation of gag pol RNA particles from 293GP/LZRNL cells negatively stained with uranyl acetate. (B) Virus particles at different stages of budding from the plasma membrane. (Bar = 100 nm.)
DISCUSSION
The striking finding in the present study is that the noninfectious virus-like particles produced by provirus-expressing packaging cell lines readily become infectious by addition of lipofection reagents. The structural role of such reagents, if any, in the particles is still unclear, and they may function simply by replacing the fusogenic function of authentic envelope constituents such as MoMLV env or surrogate envelopes such as VSV-G protein, thereby facilitating fusion of the virus envelope with the plasma or endosomal membranes of the infected cell. In contrast, we have recently found that the VSV-G glycoprotein does form a complex with the noninfectious virus-like gag pol particles and, therefore, may play a structural role during the induction of infectivity during cell-free modification of RNA particles (unpublished results).
The physical and hydrodynamic properties of gag pol RNA particles closely resemble mature virus particles (Figs. 3 and 4). By the criteria of RNase inaccessibility, the content of RT and processed gag, and their similar buoyant densities, the particles from 293GP-LZRNL cells closely resemble fully assembled enveloped virus. Since the production and secretion of the particles is equally efficient in 293GP cells and 293GP-LZRNL cells, these studies suggest that the presence of a packagable virus genome is not required for particle production. However, it is possible that some of the particles, particularly those derived from cells lacking an integrated LZRNL provirus, may contain transcripts derived from such endogenous retrovirus-like elements or may even contain other cellular RNAs. It has recently been shown that MoMLV gag protein alone can assemble intracellularly into capsid-like particles (34, 35). Furthermore, because the 293GP-LZRNL cells contain both gag pol and packagable viral RNA, it is not surprising that the assembled particles should associate with viral RNA to produce particles that require only a reconstituted mechanism for cell entry to become infectious.
Even with this demonstration of their potential infectivity, the noninfectious virus-like particles made by the 293GP packaging cell line and by 293GP-LZRNL producer cells would be of much less potential interest if they were present in small numbers or seemed to be structural dead ends. However, evidence presented herein indicates not only that they are potentially infectious but also that they are produced in large amounts. Evidence for their abundance and constitutive production in both cell types is provided by the identical levels of RT activity in the conditioned medium from 293GP packaging cells and from 293GP-LZRNL/ampho or 293GP-LZRNL cells producing high titers of either amphotropic or VSV-G pseudotyped virus (Table 1). The present studies suggest that the large numbers of such noninfectious particles may constitute a potentially useful store of “previrus” particles that can become infectious by addition in vitro of an env surrogate able to provide the needed fusiogenic function ordinarily provided in a mature virus particle by a virus env protein. We anticipate that the development of more efficient techniques for the conversion in vitro of noninfectious particles into infectious virus particles could be useful for studies of the mechanisms of retrovirus assembly and cell entry and possibly for the high-titer preparation of improved retrovirus vectors with modified or surrogate envelopes.
Acknowledgments
We thank Dr. Thomas J. Deerinck and Dr. Mark H. Ellisman for their assistance with the electron microscope studies. We are also grateful to Drs. Shin-Tai Chen and Akihiro Abe for many helpful discussions. The work was supported by the National Institutes of Health Grants DK-49023-01 and HL-53680-01 and grants from the Charles and Anna Stern Foundation and the Del Webb Foundation.
ABBREVIATIONS
- MoMLV
Moloney murine leukemia virus
- RT
reverse transcriptase
- VSV
vesicular stomatitis virus
References
- 1.Dickson C, Eisenman R, Fan H, Hunter E, Teich N. In: Protein Biosynthesis and Assembly in RNA Tumor Viruses. Weiss R, Teich N, Varmus H, Coffin J, editors. Cold Spring Harbor, NY: Cold Spring Harbor Lab. Press; 1984. pp. 513–648. [Google Scholar]
- 2.Sakalian M, Parker S D, Weldon R A, Jr, Hunter E. J Virol. 1996;70:3706–3715. doi: 10.1128/jvi.70.6.3706-3715.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sommerfelt M A, Roberts C R, Hunter E. Virology. 1993;192:298–306. doi: 10.1006/viro.1993.1033. [DOI] [PubMed] [Google Scholar]
- 4.Smith A J, Cho M I, Hammarskjold M L, Rekosh D. J Virol. 1990;64:2743–2750. doi: 10.1128/jvi.64.6.2743-2750.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gheysen D, Jacobs E, de Foresta F, Thiriart C, Francotte M, Thines D, De Wilde M. Cell. 1989;59:103–112. doi: 10.1016/0092-8674(89)90873-8. [DOI] [PubMed] [Google Scholar]
- 6.Gottlinger H G, Dorfman T, Sodroski J G, Haseltine W A. Proc Natl Acad Sci USA. 1991;88:3195–3199. doi: 10.1073/pnas.88.8.3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Srinivasakumar N, Hammarskjold M-L, Rekosh D. J Virol. 1995;69:6106–6114. doi: 10.1128/jvi.69.10.6106-6114.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spearman P, Ratner L. J Virol. 1996;70:8187–8194. doi: 10.1128/jvi.70.11.8187-8194.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garnler L, Wills J W, Verderame M E, Sudol M. Nature (London) 1996;381:744–745. doi: 10.1038/381744a0. [DOI] [PubMed] [Google Scholar]
- 10.Shields A, Witte W N, Rothenberg E, Baltimore D. Cell. 1978;14:601–609. doi: 10.1016/0092-8674(78)90245-3. [DOI] [PubMed] [Google Scholar]
- 11.Hanafusa H, Baltimore D, Smoler D, Watson K F, Yaniv A, Spiegelman S. Science. 1972;177:1188–1191. doi: 10.1126/science.177.4055.1188. [DOI] [PubMed] [Google Scholar]
- 12.Levin J G, Grimley P M, Ramseur J M, Berezesky I K. J Virol. 1974;14:152–161. doi: 10.1128/jvi.14.1.152-161.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wills J W, Cameron C E, Wilson C B, Xiang Y, Bennett R P, Leis J. J Virol. 1994;68:6605–6618. doi: 10.1128/jvi.68.10.6605-6618.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parent L J, Wilson C B, Resh M D, Wills J W. J Virol. 1996;70:1016–1026. doi: 10.1128/jvi.70.2.1016-1026.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDermott J, Farrell L, Ross R, Barklis E. J Virol. 1996;70:5106–5114. doi: 10.1128/jvi.70.8.5106-5114.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiang Y, Ridky T W, Krishna N K, Leis J. J Virol. 1997;71:2083–2091. doi: 10.1128/jvi.71.3.2083-2091.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parent L J, Bennett R P, Craven R C, Nelle T D, Krishna N K, Bowzard J B, Wilson C B, Puffer B A, Montelaro R C, Wills J W. J Virol. 1995;69:5455–5460. doi: 10.1128/jvi.69.9.5455-5460.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheele C M, Hanafusa H. Virology. 1971;45:401–410. doi: 10.1016/0042-6822(71)90341-2. [DOI] [PubMed] [Google Scholar]
- 19.Bassin R H, Phillips L A, Kramer M J, Haapala D K, Peebles P T, Nomura S, Fischinger P J. Proc Natl Acad Sci USA. 1971;68:1520–1524. doi: 10.1073/pnas.68.7.1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dougherty R M, Di Stefano H S. Virology. 1965;27:351–359. doi: 10.1016/0042-6822(65)90115-7. [DOI] [PubMed] [Google Scholar]
- 21.Emi N, Friedmann T, Yee J K. J Virol. 1991;65:1202–1207. doi: 10.1128/jvi.65.3.1202-1207.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yee, J. K., Friedmann, T. & Burns, J. C. (1994) Methods in Cell Biology, Vol. 43, Pt. A (Academic, San Diego), pp. 99–112. [DOI] [PubMed]
- 23.Miller D G, Edwards R H, Miller A D. Proc Natl Acad Sci USA. 1994;91:78–82. doi: 10.1073/pnas.91.1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong J, Roth M G, Hunter E. J Virol. 1992;66:7374–7382. doi: 10.1128/jvi.66.12.7374-7382.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Young J A T, Bates P, Willert K, Varmus H E. Science. 1990;250:1421–1423. doi: 10.1126/science.2175047. [DOI] [PubMed] [Google Scholar]
- 26.Kasahara N, Dozy A M, Kan Y W. Science. 1994;266:1373–1376. doi: 10.1126/science.7973726. [DOI] [PubMed] [Google Scholar]
- 27.Somia N V, Zoppe M, Verma I M. Proc Natl Acad Sci USA. 1995;92:7570–7574. doi: 10.1073/pnas.92.16.7570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burns J C, Friedmann T, Driever W, Burrascano M, Yee J K. Proc Natl Acad Sci USA. 1993;90:8033–8037. doi: 10.1073/pnas.90.17.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu L, Yee J K, Wolff J A, Friedmann T. Virology. 1989;171:331–341. doi: 10.1016/0042-6822(89)90600-4. [DOI] [PubMed] [Google Scholar]
- 30.Sharma S, Cantwell M, Kipps T J, Friedmann T. Proc Natl Acad Sci USA. 1996;93:11842–11847. doi: 10.1073/pnas.93.21.11842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yee J K, Miyanohara A, LaPorte P, Bouic K, Burns J C, Friedmann T. Proc Natl Acad Sci USA. 1994;91:9564–9568. doi: 10.1073/pnas.91.20.9564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goff S, Traktman P, Baltimore D. J Virol. 1981;38:239–248. doi: 10.1128/jvi.38.1.239-248.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gregoriades A, Old L J. Virology. 1969;37:189–202. doi: 10.1016/0042-6822(69)90198-6. [DOI] [PubMed] [Google Scholar]
- 34.Hansen M, Jelinek L, Whiting S, Barklis E. J Virol. 1990;64:5306–5316. doi: 10.1128/jvi.64.11.5306-5316.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones T A, Blaug G, Hansen M, Barklis E. J Virol. 1990;64:2265–2279. doi: 10.1128/jvi.64.5.2265-2279.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]