Abstract
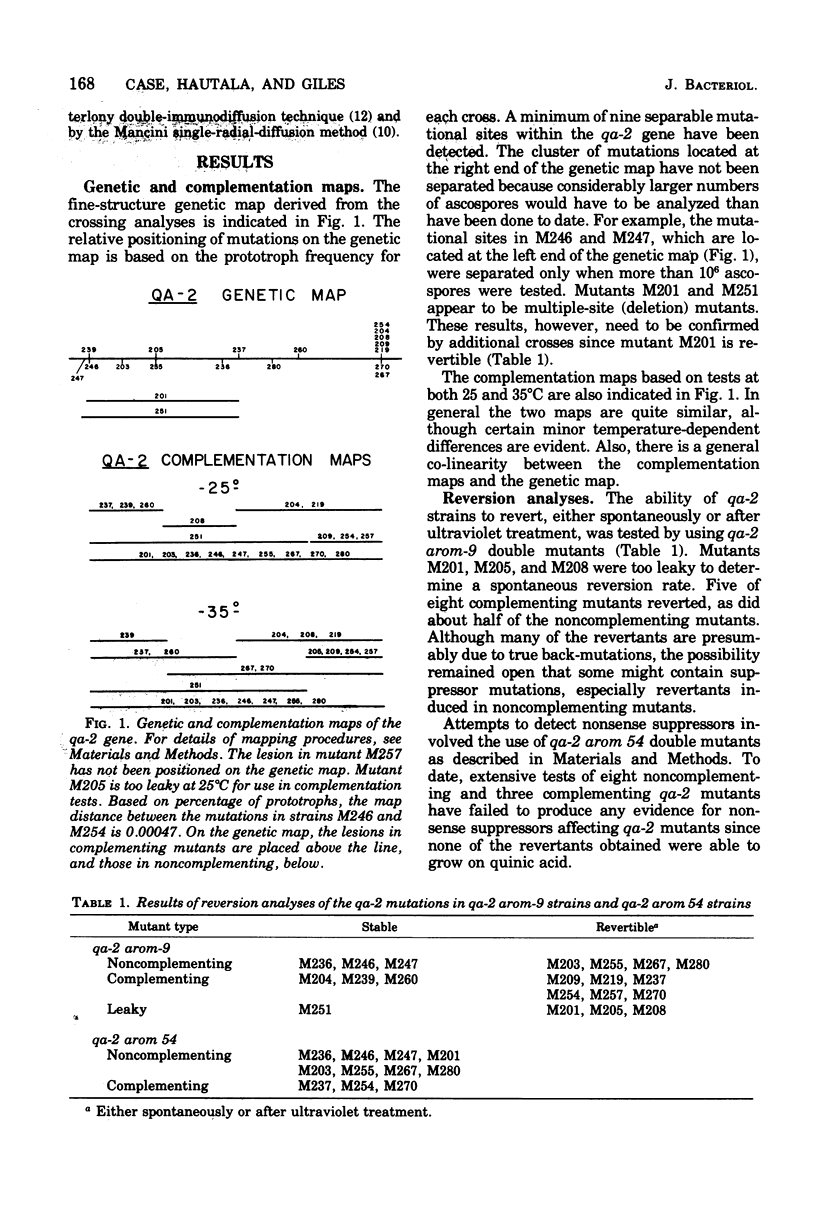
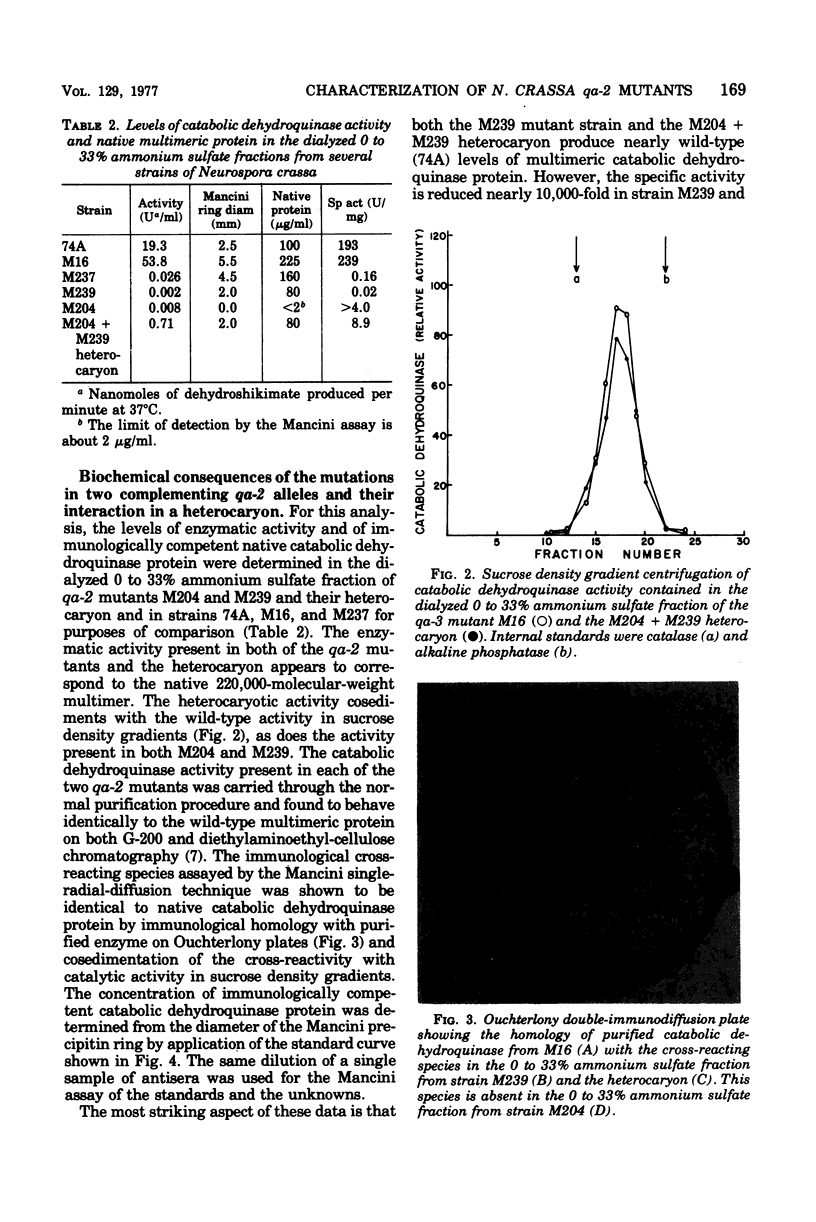
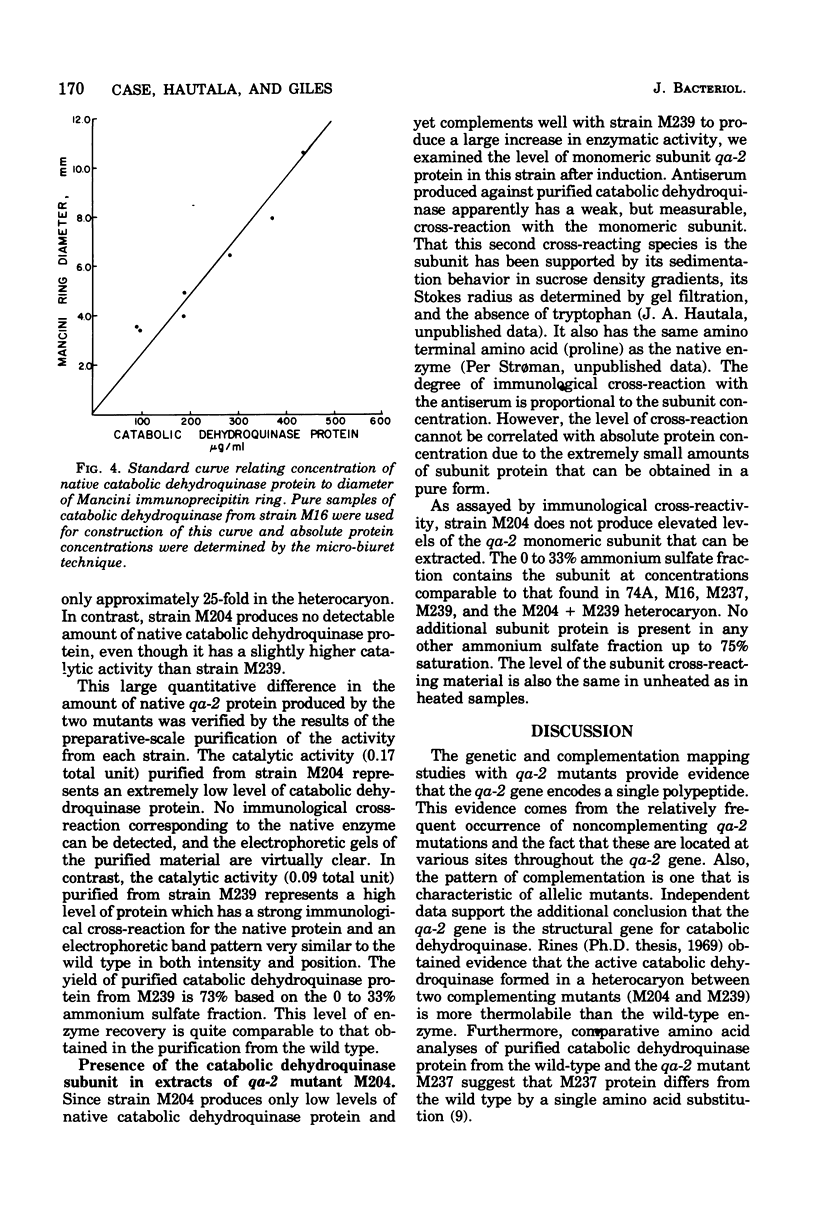
Genetic and complementation mapping studies using 20 qa-2 mutants defective for catabolic dehydroquinase indicate that the qa-2 gene encodes a single polypeptide chain and is the structural gene for catabolic dehydroquinase, a 220,000-molecular-weight protein composed of identical 10,000-molecular-weight subunits. Many qa-2 mutants are capable of reversion, but no evidence has yet been obtained for nonsense mutations in this gene. The biochemical consequences of the mutations in two complementing qa-2 strains (M239 and M204) have been determined. Both mutants have extremely low levels of catalytic activity and form a heterocaryon with about 4% of the wild-type activity. As assayed by immunological cross-reactivity, mutant M239 and the heterocaryon have nearly wild-type levels of native-molecular-weight catabolic dehydroquinase protein, whereas M204 has no detectable amount of this protein. Thus it is concluded that M239 has a mutation at or near the catalytic site which reduces the activity 10,000-fold but has little or no influence on the formation of the native multimeric structure. In contrast, M204 apparently has a mutation that severely inhibits aggregation and may have only a minor effect on the inherent potential for catalytic conversion at the reactive site. The heterocaryon would appear to form a mixed multimer with the monomeric subunits from M239 providing the aggregated structure and those from M204, the catalytically active moiety.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Case M. E., Giles N. H., Doy C. H. Genetical and biochemical evidence for further interrelationships between the polyaromatic synthetic and the quinate-shikimate catabolic pathways in Neurospora crassa. Genetics. 1972 Jul;71(3):337–348. doi: 10.1093/genetics/71.3.337. [DOI] [PubMed] [Google Scholar]
- Case M. E., Giles N. H. Evidence for nonsense mutations in the arom gene cluster of Neurospora crassa. Genetics. 1968 Sep;60(1):49–58. doi: 10.1093/genetics/60.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case M. E., Giles N. H. Genetic evidence on the organization and action of the qa-1 gene product: a protein regulating the induction of three enzymes in quinate catabolism in Neurospora crassa. Proc Natl Acad Sci U S A. 1975 Feb;72(2):553–557. doi: 10.1073/pnas.72.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaleff R. S. The inducible quinate-shikimate catabolic pathway in Neurospora crassa: genetic organization. J Gen Microbiol. 1974 Apr;81(2):337–355. doi: 10.1099/00221287-81-2-337. [DOI] [PubMed] [Google Scholar]
- Chaleff R. S. The inducible quinate-shikimate catabolic pathway in Neurospora crassa: induction and regulation of enzyme synthesis. J Gen Microbiol. 1974 Apr;81(2):357–372. doi: 10.1099/00221287-81-2-357. [DOI] [PubMed] [Google Scholar]
- Hautala J. A., Jacobson J. W., Case M. E., Giles N. H. Purification and characterization of catabolic dehydroquinase, an enzyme in the inducible quinic acid catabolic pathway of Neurospora crassa. J Biol Chem. 1975 Aug 10;250(15):6008–6014. [PubMed] [Google Scholar]
- ITZHAKI R. F., GILL D. M. A MICRO-BIURET METHOD FOR ESTIMATING PROTEINS. Anal Biochem. 1964 Dec;9:401–410. doi: 10.1016/0003-2697(64)90200-3. [DOI] [PubMed] [Google Scholar]
- Jacobson J. W., Hautala J. A., Case M. E., Giles N. H. Effect of mutations in the qa gene cluster of Neurospora crassa on the enzyme catabolic dehydroquinase. J Bacteriol. 1975 Oct;124(1):491–496. doi: 10.1128/jb.124.1.491-496.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]



