Abstract
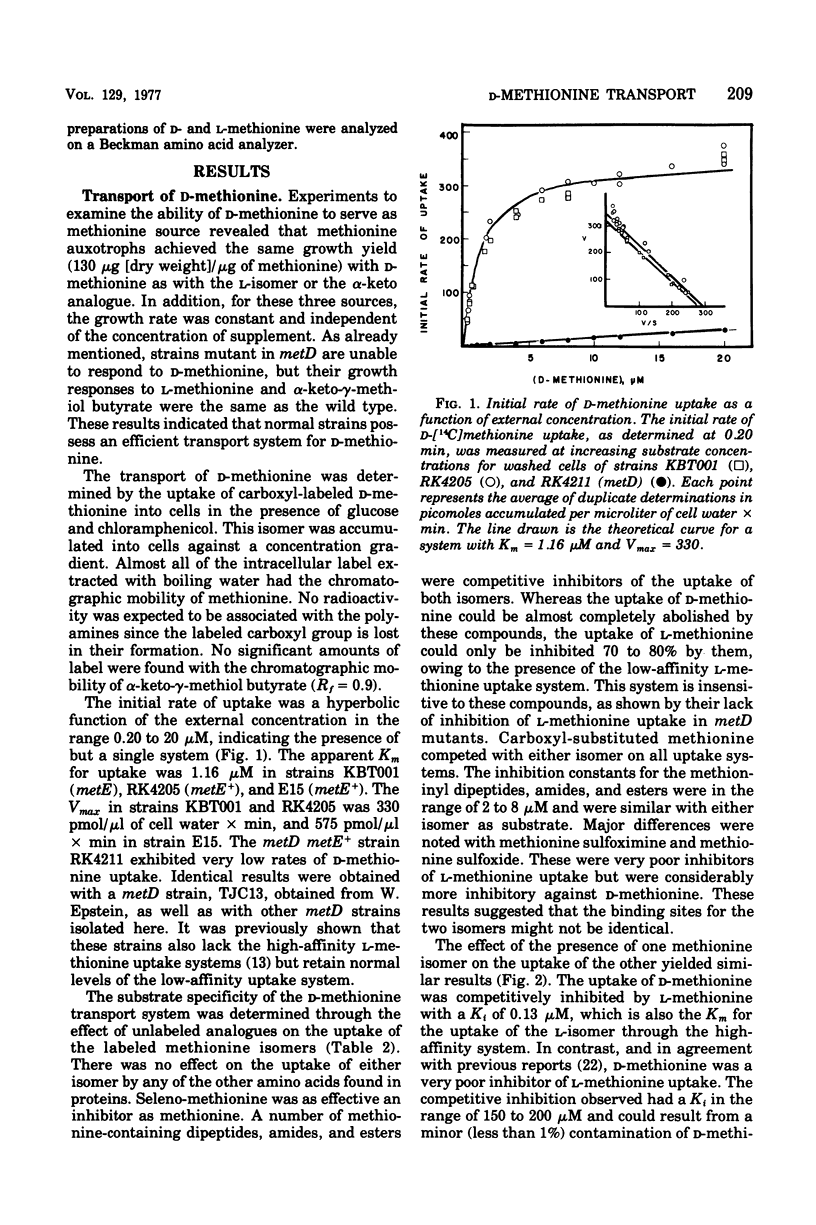
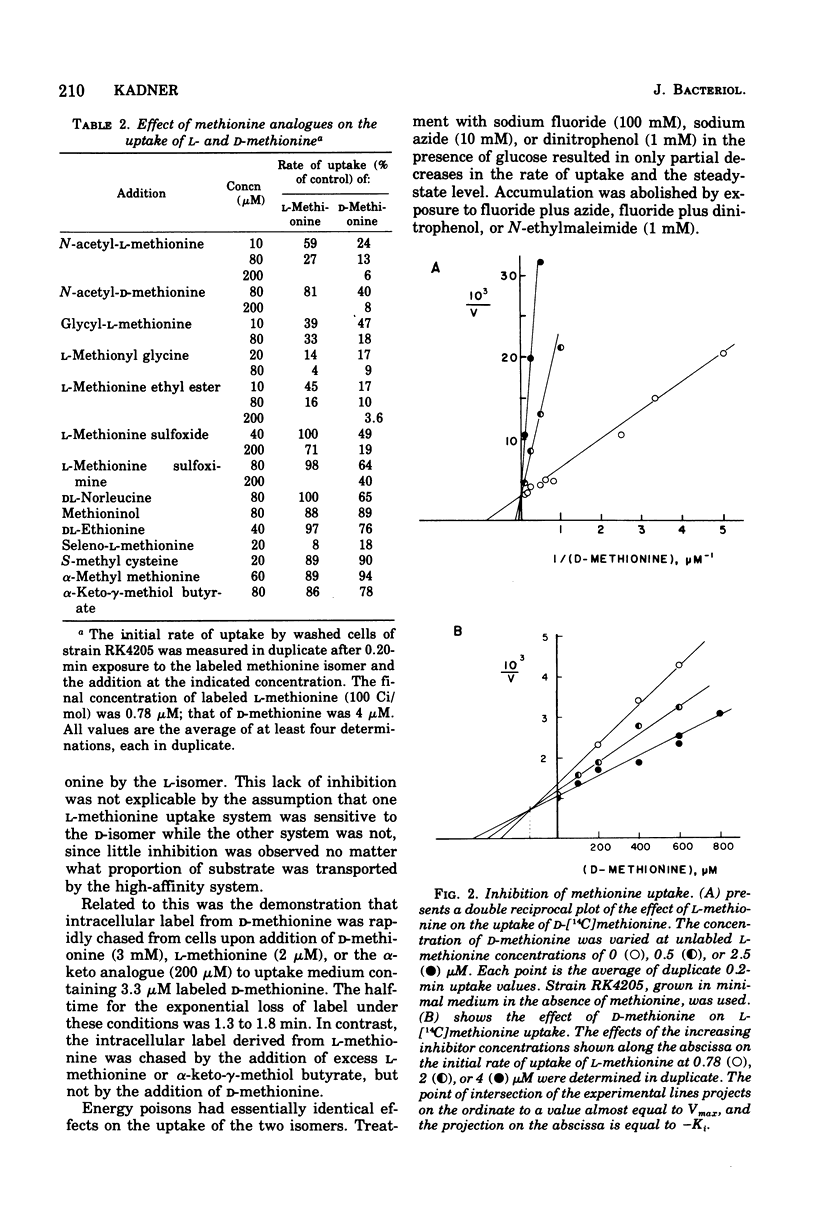
The transport and utilization of D-methionine was investigated in several strains of Escherichia coli K-12. Wild-type cells exhibit a single transport system with a Km of 1.16 muM. This activity exhibits a specificity similar to that of the uptake of L-methionine. The activity toward the D-isomer and the high-affinity uptake of L-methionine are lost in strains mutant in metD, along with the ability to utilize D-methionine as methionine source. Both activities respond identically to gene dosage of metD and are both restored in revertants or transductants. However, although L-methionine is a potent inhibitor of D-methionine uptake, D-methionine has little or no effect on the uptake of the L-isomer. No mutants altered in the uptake of only one of the two isomers were found in a screening. Regulation of both activities was similar in their response to the internal methionine pool, and some evidence was suggestive of partial repressive control of these activities. The evidence is most consistent with the role of the metD product as a common step for two methionine-specific uptake systems, but other gene products may represent the initial substrate binding sites. This system also appears to be involved in the uptake of N-acetyl methionine and methionine sulfoxide and methionine sulfoximine. The uptake of the keto analogue of methionine, alpha-keto-gamma-methiol butyrate, appears to be mediated by a separate system specific for alpha-keto straight-chain acids 5- to 6-carbon units in length.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Lever J. E. The histidine-binding protein J is a component of histidine transport. Identification of its structural gene, hisJ. J Biol Chem. 1972 Jul 10;247(13):4309–4316. [PubMed] [Google Scholar]
- Ayling P. D., Bridgeland E. S. Methionine transport in wild-type and transport-defective mutants of Salmonella typhimurium. J Gen Microbiol. 1972 Nov;73(1):127–141. doi: 10.1099/00221287-73-1-127. [DOI] [PubMed] [Google Scholar]
- Betteridge P. R., Ayling P. D. The role of methionine transport-defective mutations in resistance to methionine sulphoximine in Salmonella typhimurium. Mol Gen Genet. 1975;138(1):41–52. doi: 10.1007/BF00268826. [DOI] [PubMed] [Google Scholar]
- Brenchley J. E. Effect of methionine sulfoximine and methionine sulfone on glutamate synthesis in Klebsiella aerogenes. J Bacteriol. 1973 May;114(2):666–673. doi: 10.1128/jb.114.2.666-673.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clandinin M. T., Ahmed A. Mechanism of repression of methionine biosynthesis in Escherichia coli. II. The effect of metJ mutations on the free amino acid pool. Mol Gen Genet. 1973 Jul 16;123(4):325–331. doi: 10.1007/BF00433649. [DOI] [PubMed] [Google Scholar]
- Cooper S. Utilization of d-Methionine by Escherichia coli. J Bacteriol. 1966 Aug;92(2):328–332. doi: 10.1128/jb.92.2.328-332.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene R. C., Williams R. D., Kung H. F., Spears C., Weissbach H. Effect of methionine and vitamin B-12 on the activities of methionine biosynthetic enzymes in metJ mutants of Escherichia coli K12. Arch Biochem Biophys. 1973 Sep;158(1):249–256. doi: 10.1016/0003-9861(73)90619-x. [DOI] [PubMed] [Google Scholar]
- Halpern Y. S. Genetics of amino acid transport in bacteria. Annu Rev Genet. 1974;8:103–133. doi: 10.1146/annurev.ge.08.120174.000535. [DOI] [PubMed] [Google Scholar]
- Holloway C. T., Greene R. C., Su C. H. Regulation of S-adenosylmethionine synthetase in Escherichia coli. J Bacteriol. 1970 Nov;104(2):734–747. doi: 10.1128/jb.104.2.734-747.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaczorowski G., Shaw L., F-entes M., Walsh C. Coupling of alanine racemase and D-alanine dehydrogenase to active transport of amino acids in Escherichia coli B membrane vesicles. J Biol Chem. 1975 Apr 25;250(8):2855–2865. [PubMed] [Google Scholar]
- Kadner R. J. Regulation of methionine transport activity in Escherichia coli. J Bacteriol. 1975 Apr;122(1):110–119. doi: 10.1128/jb.122.1.110-119.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadner R. J. Transport systems for L-methionine in Escherichia coli. J Bacteriol. 1974 Jan;117(1):232–241. doi: 10.1128/jb.117.1.232-241.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadner R. J., Watson W. J. Methionine transport in Escherichia coli: physiological and genetic evidence for two uptake systems. J Bacteriol. 1974 Aug;119(2):401–409. doi: 10.1128/jb.119.2.401-409.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadner R. J., Winkler H. H. Energy coupling for methionine transport in Escherichia coli. J Bacteriol. 1975 Sep;123(3):985–991. doi: 10.1128/jb.123.3.985-991.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajewska-Grynkiewicz K., Walczak W., Klopotowski T. Mutants of Salmonella typhimurium able to utilize D-histidine as a source of L-histidine. J Bacteriol. 1971 Jan;105(1):28–37. doi: 10.1128/jb.105.1.28-37.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn J., Somerville R. L. Mutant strains of Escherichia coli K12 that use D-amino acids. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2484–2487. doi: 10.1073/pnas.68.10.2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montie D. B., Montie T. C. Methionine transport in Yersinia pestis. J Bacteriol. 1975 Oct;124(1):296–306. doi: 10.1128/jb.124.1.296-306.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oxender D. L. Membrane transport. Annu Rev Biochem. 1972;41(10):777–814. doi: 10.1146/annurev.bi.41.070172.004021. [DOI] [PubMed] [Google Scholar]
- Piperno J. R., Oxender D. L. Amino acid transport systems in Escherichia coli K-12. J Biol Chem. 1968 Nov 25;243(22):5914–5920. [PubMed] [Google Scholar]
- Rahmanian M., Oxender D. L. Derepressed leucine transport activity in Escherichia coli. J Supramol Struct. 1972;1(1):55–59. doi: 10.1002/jss.400010108. [DOI] [PubMed] [Google Scholar]
- Su C. H., Greene R. C. Regulation of methionine biosynthesis in Escherichia coli: mapping of the metJ locus and properties of a metJ plus-metJ minus diploid. Proc Natl Acad Sci U S A. 1971 Feb;68(2):367–371. doi: 10.1073/pnas.68.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild J., Walczak W., Krajewska-Grynkiewicz K., Klopotowski T. D-amino acid dehydrogenase: the enzyme of the first step of D-histidine and D-methionine racemization in Salmonella typhimurium. Mol Gen Genet. 1974;128(2):131–146. doi: 10.1007/BF02654486. [DOI] [PubMed] [Google Scholar]


