Abstract
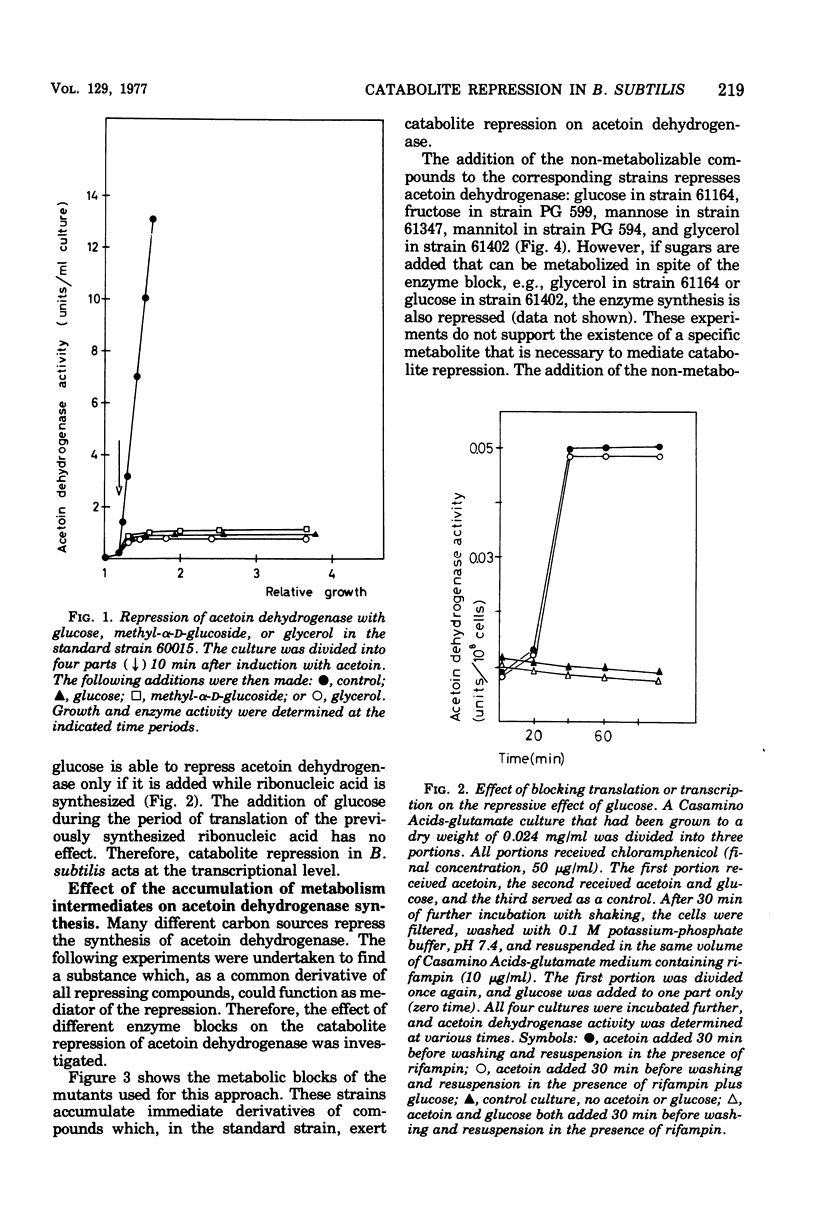
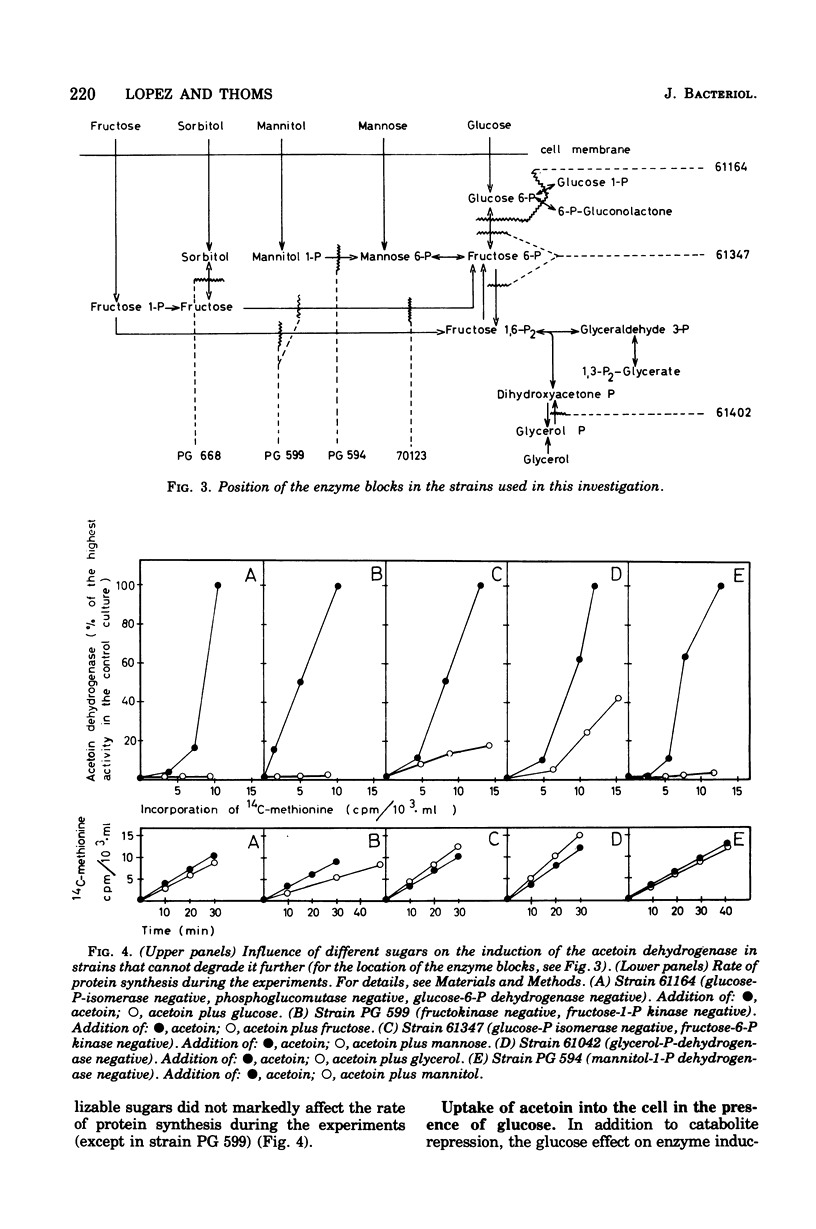
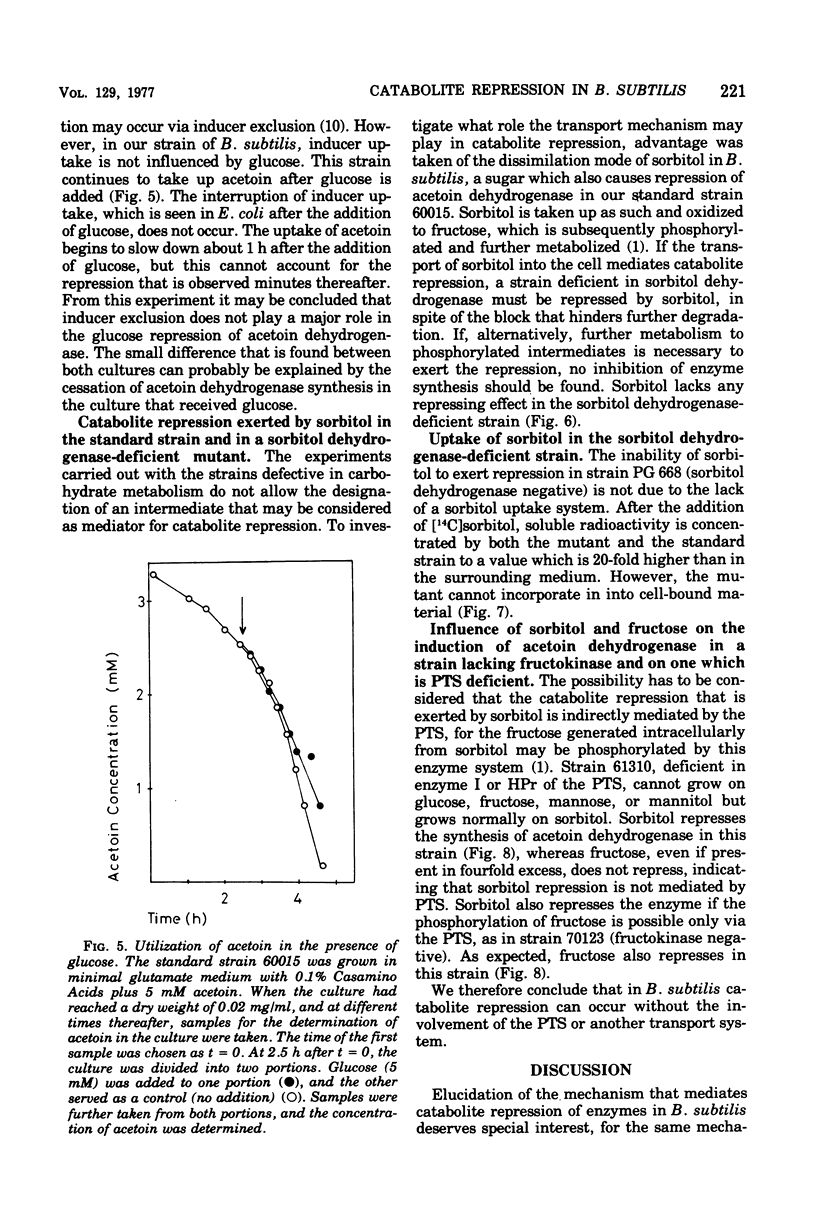
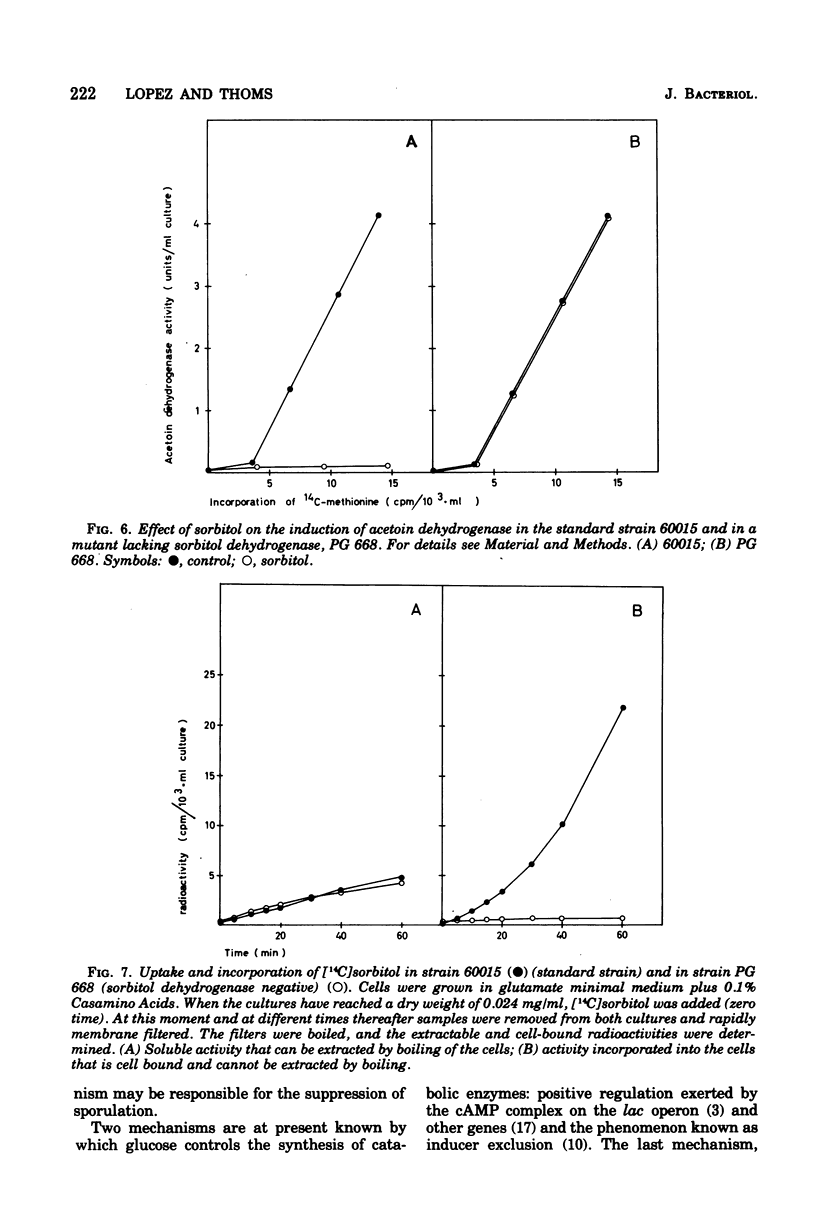
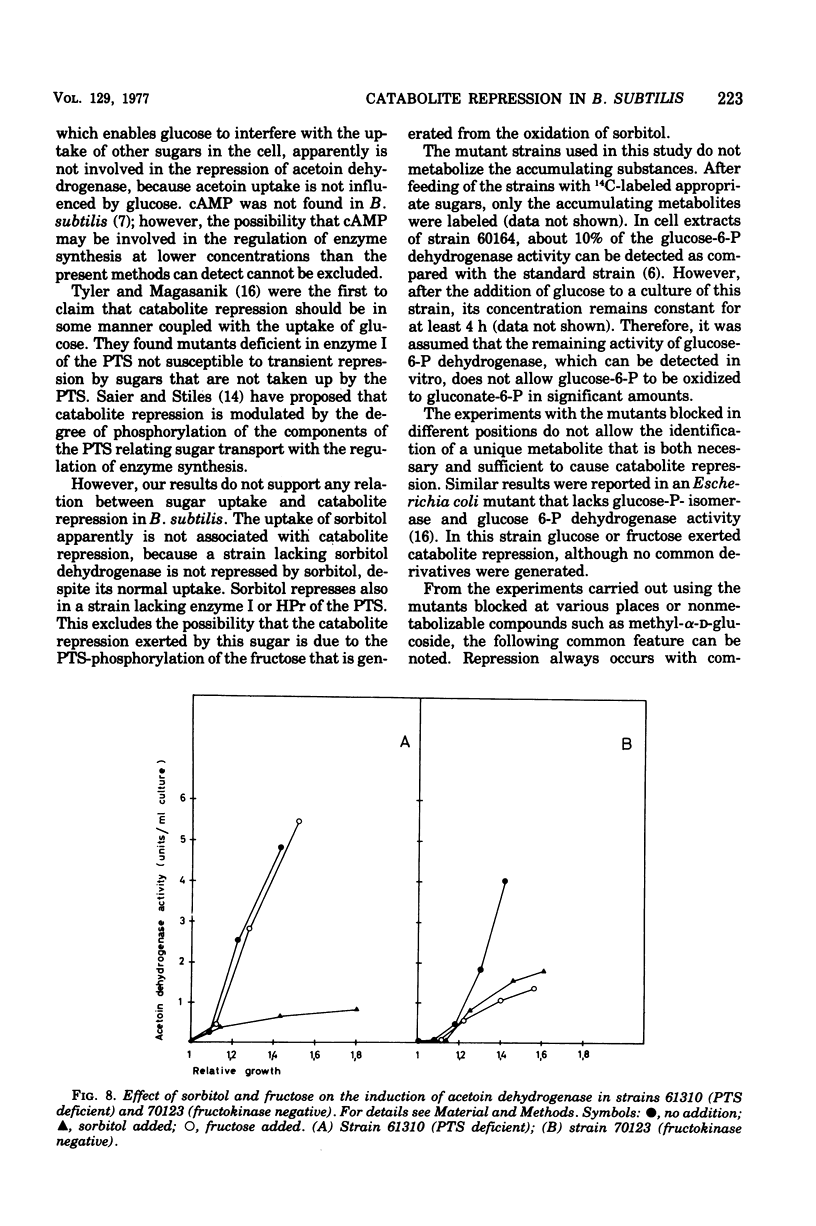
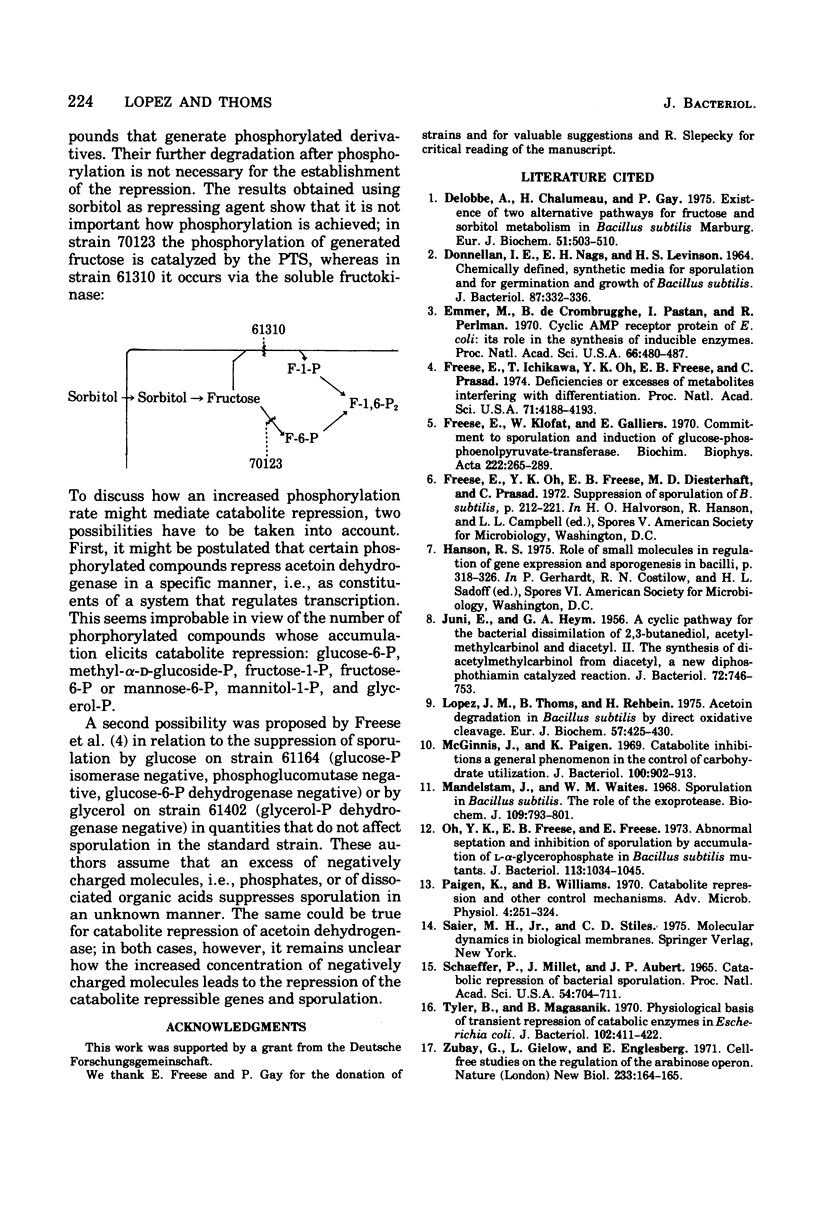
Many phosphorylated intermediates exert catabolite repression on the enzyme acetoin dehydrogenase in Bacillus subtilis. This was shown with strains that are blocked at different positions in central metabolism when they receive sugars that cannot be metabolized past enzymatic block(s). In the case of sorbitol, transport events were not involved in catabolite repression, for this sugar cannot repress acetoin dehydrogenase in a strain lacking sorbitol dehydrogenase but otherwise able to take up sorbitol. The presence of glucose did not markedly influence the uptake of acetoin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DONNELLAN J. E., Jr, NAGS E. H., LEVINSON H. S. CHEMICALLY DEFINED, SYNTHETIC MEDIA FOR SPORULATION AND FOR GERMINATION AND GROWTH OF BACILLUS SUBTILIS. J Bacteriol. 1964 Feb;87:332–336. doi: 10.1128/jb.87.2.332-336.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delobbe A., Chalumeau H., Gay P. Existence of two alternative pathways for fructose and sorbitol metabolism in Bacillus subtilis Marburg. Eur J Biochem. 1975 Feb 21;51(2):503–510. doi: 10.1111/j.1432-1033.1975.tb03950.x. [DOI] [PubMed] [Google Scholar]
- Emmer M., deCrombrugghe B., Pastan I., Perlman R. Cyclic AMP receptor protein of E. coli: its role in the synthesis of inducible enzymes. Proc Natl Acad Sci U S A. 1970 Jun;66(2):480–487. doi: 10.1073/pnas.66.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E., Ichikawa T., O Y. K., Freese E. B., Prasad C. Deficiencies or excesses of metabolites interfering with differentiation. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4188–4193. doi: 10.1073/pnas.71.10.4188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freese E., Klofat W., Galliers E. Commitment to sporulation and induction of glucose-phosphoenolpyruvate-transferase. Biochim Biophys Acta. 1970 Nov 24;222(2):265–289. doi: 10.1016/0304-4165(70)90115-7. [DOI] [PubMed] [Google Scholar]
- HEYM G. A., JUNI E. A cyclic pathway for the bacterial dissimilation of 2, 3-butanediol, acetylmethylcarbinol and diacetyl. II. The synthesis of diacetylmethylcarbinol from diacetyl, a new diphosphothiamin catalyzed reaction. J Bacteriol. 1956 Dec;72(6):746–753. doi: 10.1128/jb.72.6.746-753.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López J. M., Thoms B., Rehbein H. Acetoin degradation in Bacillus subtilis by direct oxidative cleavage. Eur J Biochem. 1975 Sep 15;57(2):425–430. doi: 10.1111/j.1432-1033.1975.tb02317.x. [DOI] [PubMed] [Google Scholar]
- Mandelstam J., Waites W. M. Sporulation in Bacillus subtilis. The role of exoprotease. Biochem J. 1968 Oct;109(5):793–801. doi: 10.1042/bj1090793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinnis J. F., Paigen K. Catabolite inhibition: a general phenomenon in the control of carbohydrate utilization. J Bacteriol. 1969 Nov;100(2):902–913. doi: 10.1128/jb.100.2.902-913.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O Y. K., Freese E. B., Freese E. Abnormal septation and inhibition of sporulation by accumulation of L- -glycerophosphate in Bacillus subtilis mutants. J Bacteriol. 1973 Feb;113(2):1034–1045. doi: 10.1128/jb.113.2.1034-1045.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P., Millet J., Aubert J. P. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci U S A. 1965 Sep;54(3):704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler B., Magasanik B. Physiological basis of transient repression of catabolic enzymes in Escherichia coli. J Bacteriol. 1970 May;102(2):411–422. doi: 10.1128/jb.102.2.411-422.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubay G., Gielow L., Englesberg E. Cell-free studies on the regulation of the arabinose operon. Nat New Biol. 1971 Oct 6;233(40):164–165. doi: 10.1038/newbio233164a0. [DOI] [PubMed] [Google Scholar]



