Abstract
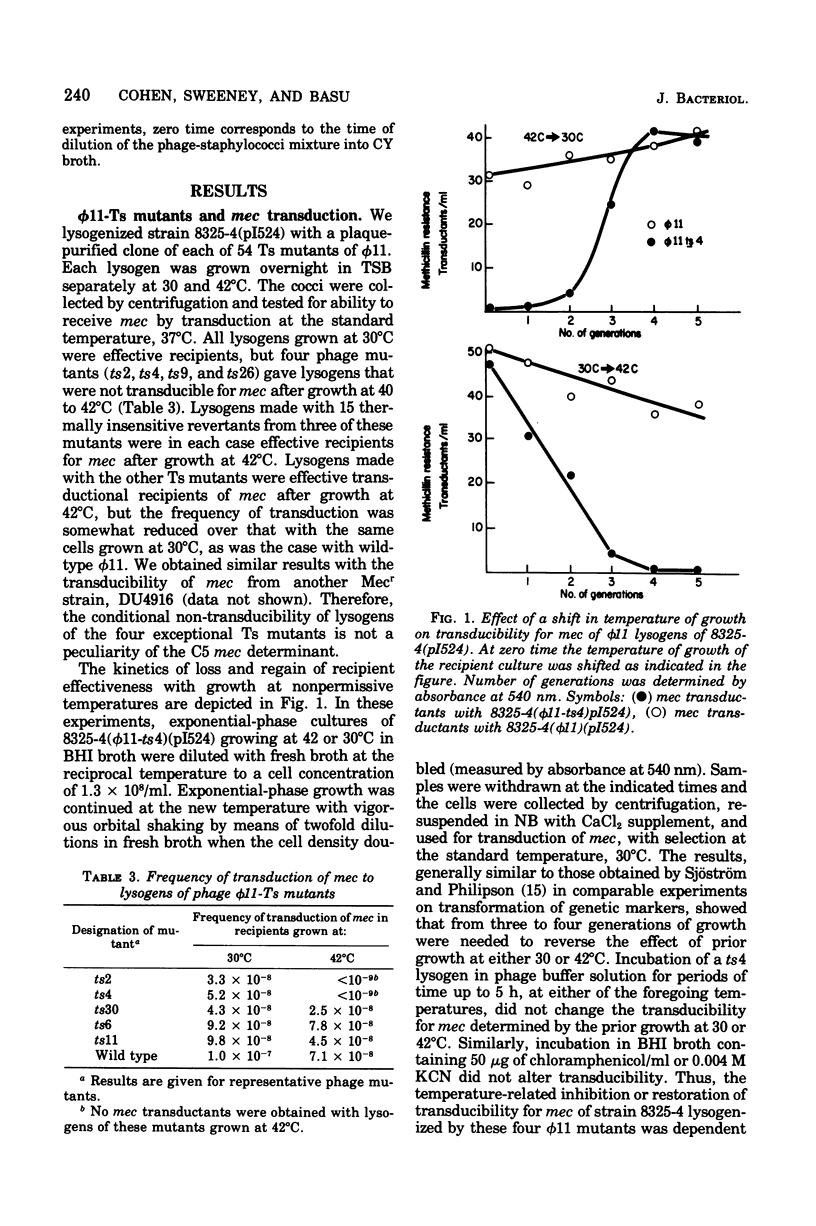
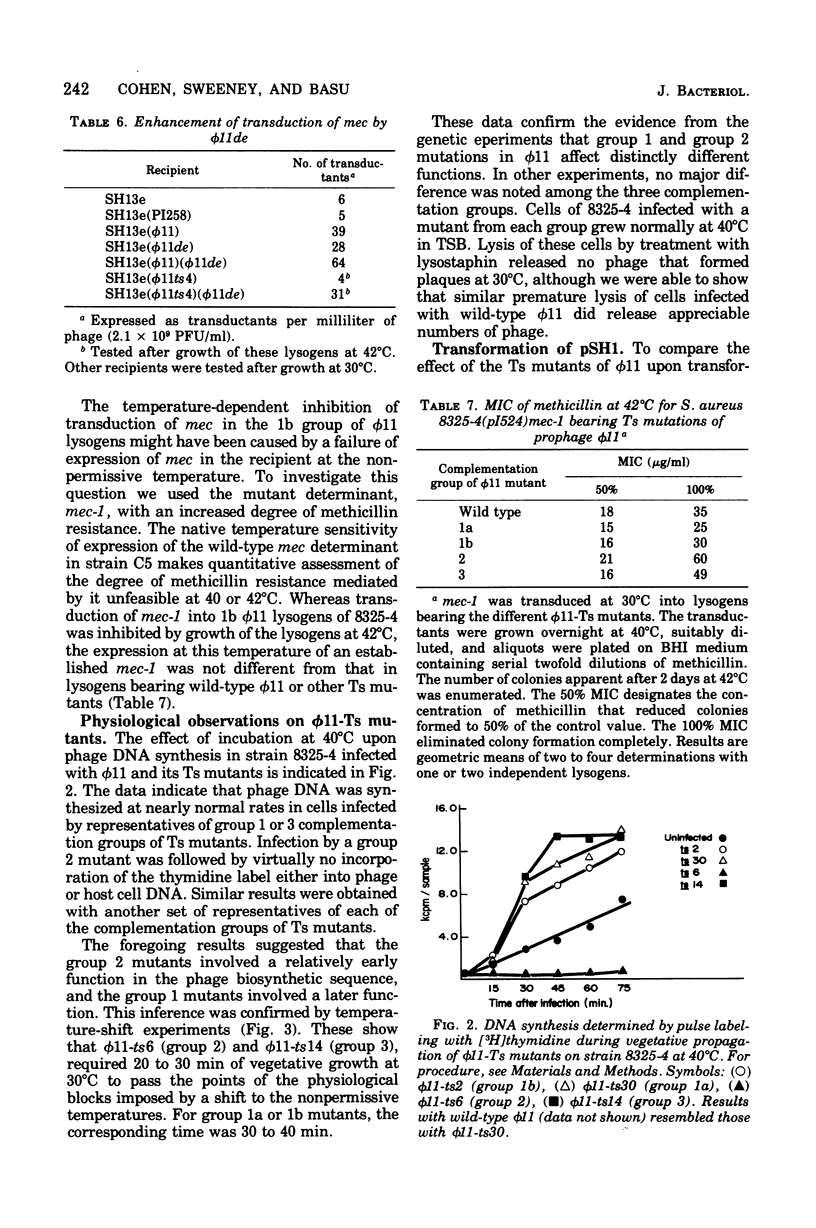
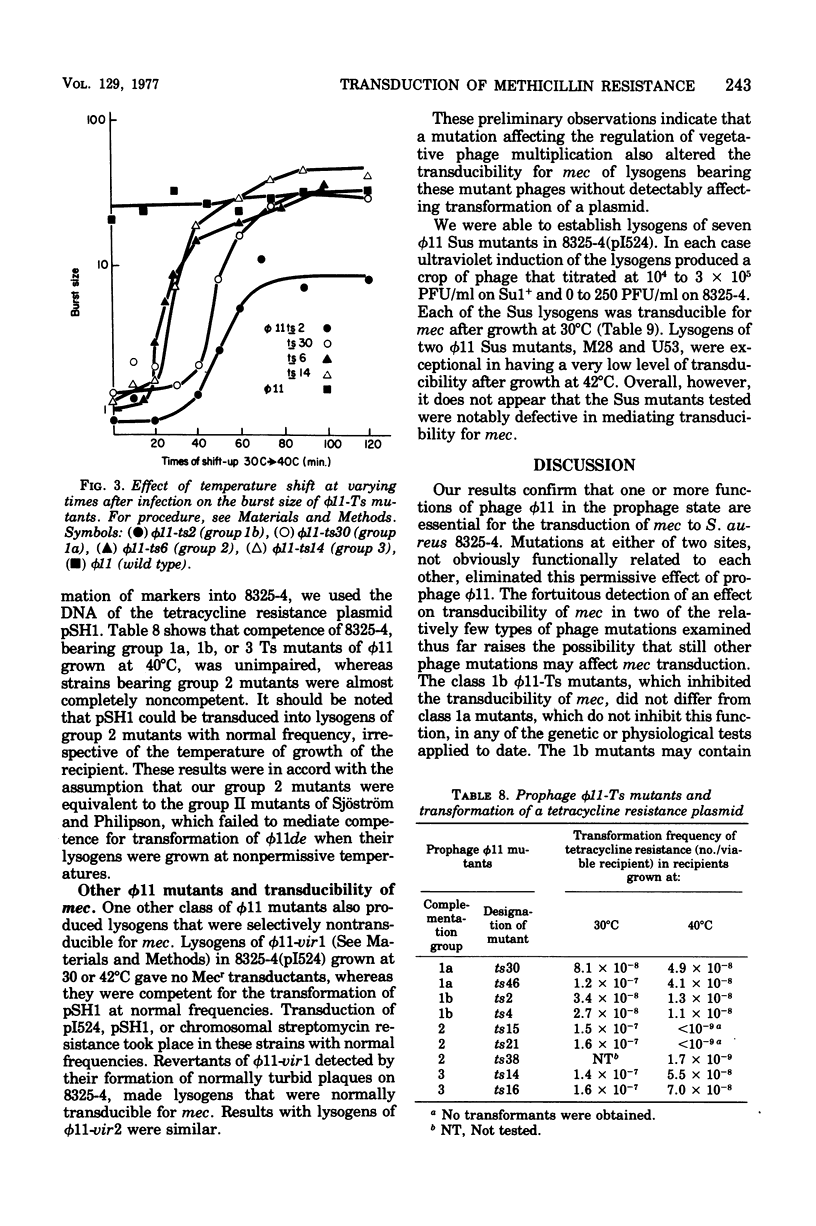
Methicillin resistance (mec) is not transduced into Staphylococcus aureus 8325-4, but is transduced into this host after it has been lysogenized with phage φ11 and has acquired the penicillinase plasmid pI524 by a separate transduction (Cohen and Sweeney, 1970, 1973). Strain 8325-4 is competent for transformation of typical plasmid or chromosomal markers and for mec only if it is lysogenic for φ11 or a related prophage (Sjöström et al., 1974, 1975). A mutant strain of φ11 that was temperature sensitive (Ts) for vegetative multiplication did not mediate competence for transformation of its 8325-4 lysogen if the lysogen had been grown at a nonpermissive temperature (Sjöström and Philipson, 1974). We isolated four Ts mutants of φ11 that did not mediate transducibility of their 8325-4(pI524) lysogens for mec after growth at nonpermissive temperatures (40 to 42°C). Transduction of typical plasmid or chromosomal markers was not affected. These φ11-Ts mutants mediated normal competence of their lysogens for transformation of a tetracycline resistance plasmid. Similarly, φ11-Ts mutants that rendered their lysogens temperature sensitive for transformation did not depress the frequency of transduction of mec. These two types of φ11-Ts mutants fell into two different genetic complementation groups that differed in the physiology of deoxyribonucleic acid synthesis and in the time of expression of the mutations during a single-burst growth cycle at a nonpermissive temperature. A virulent mutant of φ11, which plaqued with 100% efficiency on 8325(φ11), also failed to condition strain 8325-4 for transducibility of mec but retained the ability to confer competence for transformation of a tetracycline resistance plasmid. Different genetic loci and physiological functions are involved in φ11 mutations that affect transducibility of mec and those that affect competence for transformation of markers generally in S. aureus 8325-4.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Botstein D., Chan R. K., Waddell C. H. Genetics of bacteriophage P22. II. Gene order and gene function. Virology. 1972 Jul;49(1):268–282. doi: 10.1016/s0042-6822(72)80028-x. [DOI] [PubMed] [Google Scholar]
- Cohen S., Sweeney H. M. Effect of the prophage and penicillinase plasmid of the recipient strain upon the transduction and the stability of methicillin resistance in Staphylococcus aureus. J Bacteriol. 1973 Nov;116(2):803–811. doi: 10.1128/jb.116.2.803-811.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S., Sweeney H. M. Transduction of Methicillin Resistance in Staphylococcus aureus Dependent on an Unusual Specificity of the Recipient Strain. J Bacteriol. 1970 Dec;104(3):1158–1167. doi: 10.1128/jb.104.3.1158-1167.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornbusch K., Hallander H. O., Löfquist F. Extrachromosomal control of methicillin resistance and toxin production in Staphylococcus aureus. J Bacteriol. 1969 May;98(2):351–358. doi: 10.1128/jb.98.2.351-358.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebel-Tsipis J., Fox M. S., Botstein D. Generalized transduction by bacteriophage P22 in Salmonella typhimurium. II. Mechanism of integration of transducing DNA. J Mol Biol. 1972 Nov 14;71(2):449–469. doi: 10.1016/0022-2836(72)90362-2. [DOI] [PubMed] [Google Scholar]
- Kretschmer P. J., Egan J. B. Genetic map of the Staphylococcal bacteriophage phi11. J Virol. 1975 Sep;16(3):642–651. doi: 10.1128/jvi.16.3.642-651.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretschmer P. J., Egan J. B. Isolation of a suppressor host bacterium in Staphylococcus aureus. J Bacteriol. 1973 Oct;116(1):84–87. doi: 10.1128/jb.116.1.84-87.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacey R. W., Grinsted J. Genetic analysis of methicillin-resistant strains of Staphylococcus aureus; evidence for their evolution from a single clone. J Med Microbiol. 1973 Nov;6(4):511–526. doi: 10.1099/00222615-6-4-511. [DOI] [PubMed] [Google Scholar]
- NOVICK R. P. ANALYSIS BY TRANSDUCTION OF MUTATIONS AFFECTING PENICILLINASE FORMATION IN STAPHYLOCOCCUS AUREUS. J Gen Microbiol. 1963 Oct;33:121–136. doi: 10.1099/00221287-33-1-121. [DOI] [PubMed] [Google Scholar]
- Notani N. K., Setlow J. K. Mechanism of bacterial transformation and transfection. Prog Nucleic Acid Res Mol Biol. 1974;14(0):39–100. doi: 10.1016/s0079-6603(08)60205-6. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Bouanchaud D. The problems of drug-resistant pathogenic bacteria. Extrachromosomal nature of drug resistance in Staphylococcus aureus. Ann N Y Acad Sci. 1971 Jun 11;182:279–294. doi: 10.1111/j.1749-6632.1971.tb30664.x. [DOI] [PubMed] [Google Scholar]
- Novick R. Properties of a cryptic high-frequency transducing phage in Staphylococcus aureus. Virology. 1967 Sep;33(1):155–166. doi: 10.1016/0042-6822(67)90105-5. [DOI] [PubMed] [Google Scholar]
- Schwesinger M. D., Novick R. P. Prophage-dependent plasmid integration in Staphylococcus aureus. J Bacteriol. 1975 Aug;123(2):724–738. doi: 10.1128/jb.123.2.724-738.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström J. E., Löfdahl S., Philipson L. Transformation reveals a chromosomal locus of the gene(s) for methicillin resistance in Staphylococcus aureus. J Bacteriol. 1975 Sep;123(3):905–915. doi: 10.1128/jb.123.3.905-915.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström J. E., Philipson L. Role of the phi 11 phage genome in competence of Staphylococcus aureus. J Bacteriol. 1974 Jul;119(1):19–32. doi: 10.1128/jb.119.1.19-32.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiffler P. W., Sweeney H. M., Cohen S. Co-transduction of plasmids mediating resistance to tetracycline and chloramphenicol in Staphylococcus aureus. J Bacteriol. 1974 Nov;120(2):934–934. doi: 10.1128/jb.120.2.934-944.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]
- Wyman L., Goering R. V., Novick R. P. Genetic control of chromosomal and plasmid recombination in Staphylococcus aureus. Genetics. 1974 Apr;76(4):681–702. doi: 10.1093/genetics/76.4.681. [DOI] [PMC free article] [PubMed] [Google Scholar]


