Abstract
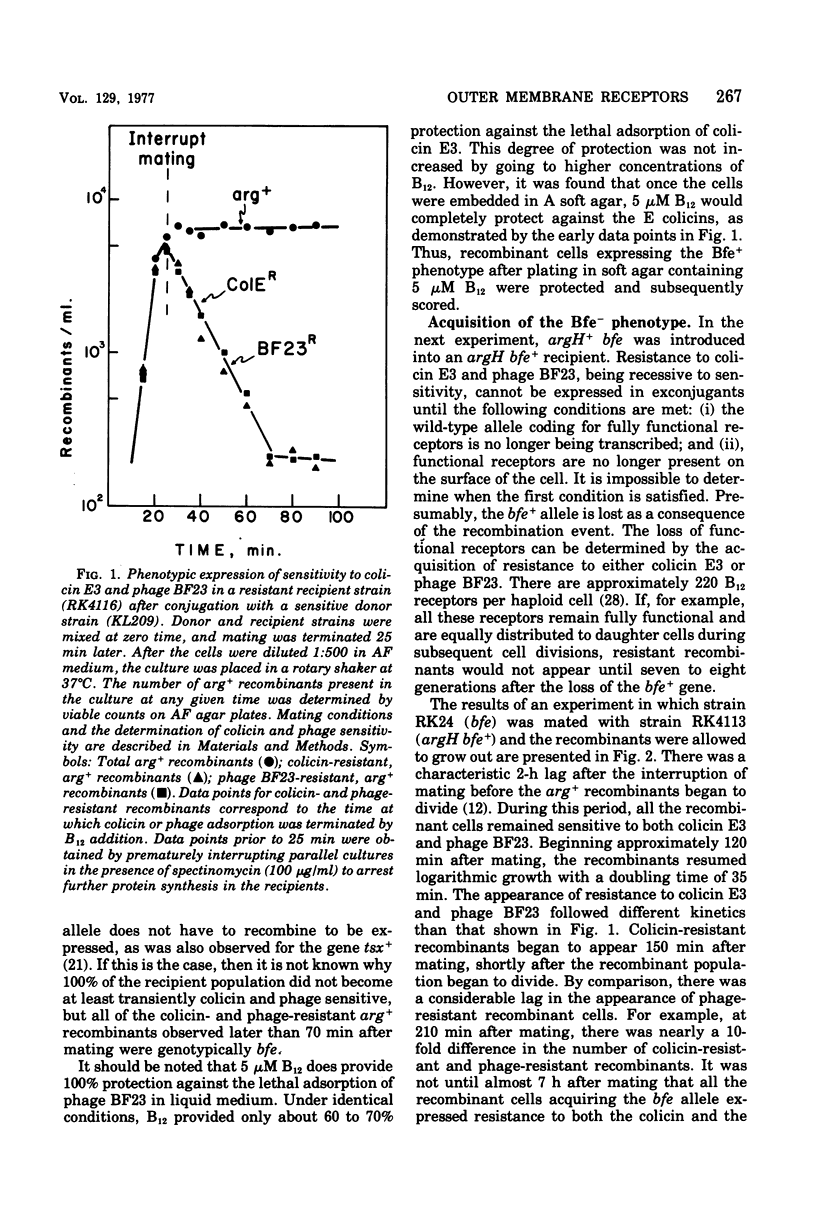
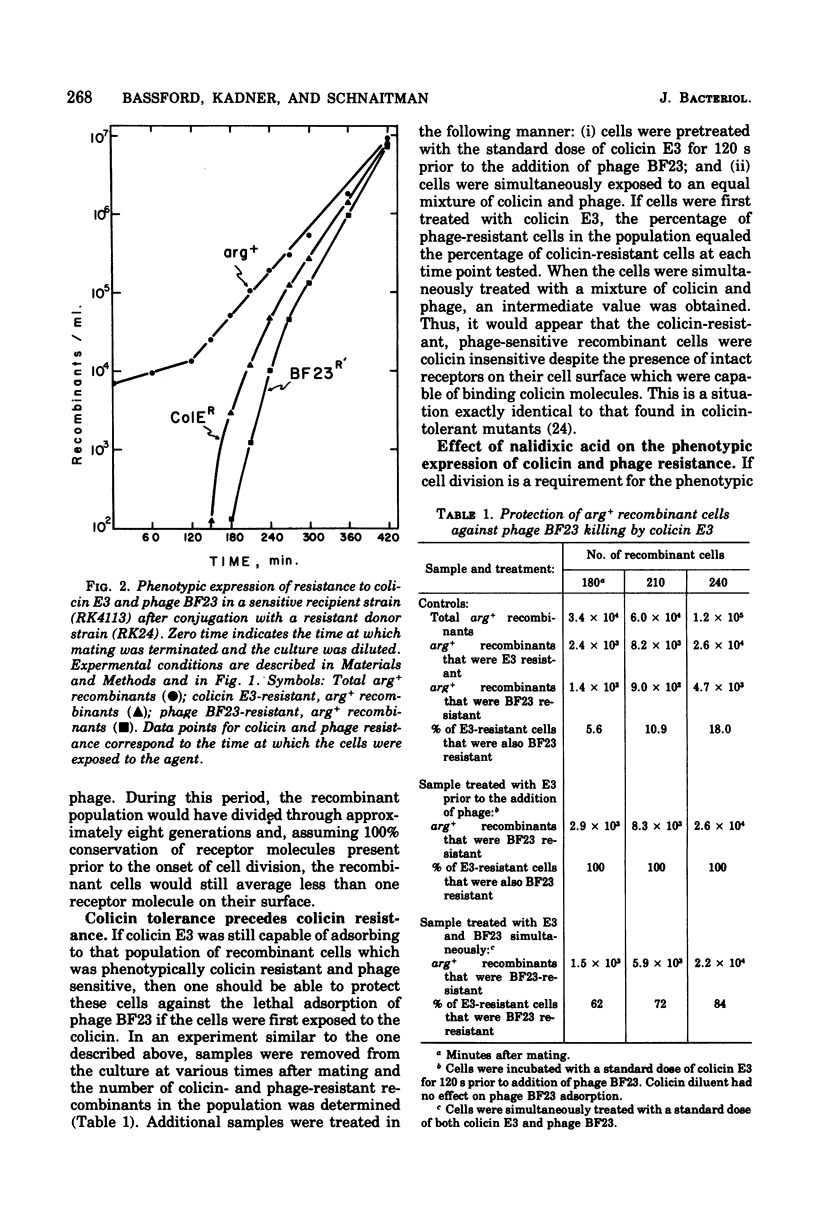
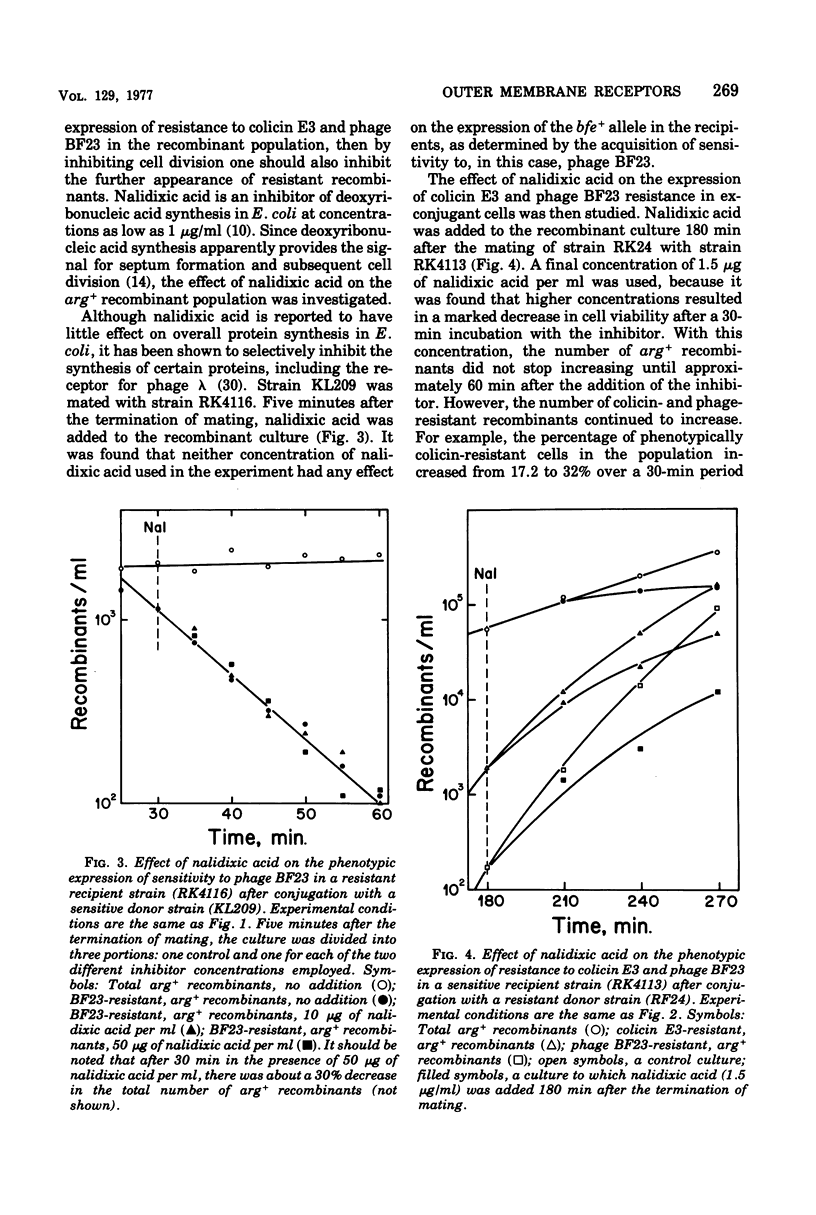
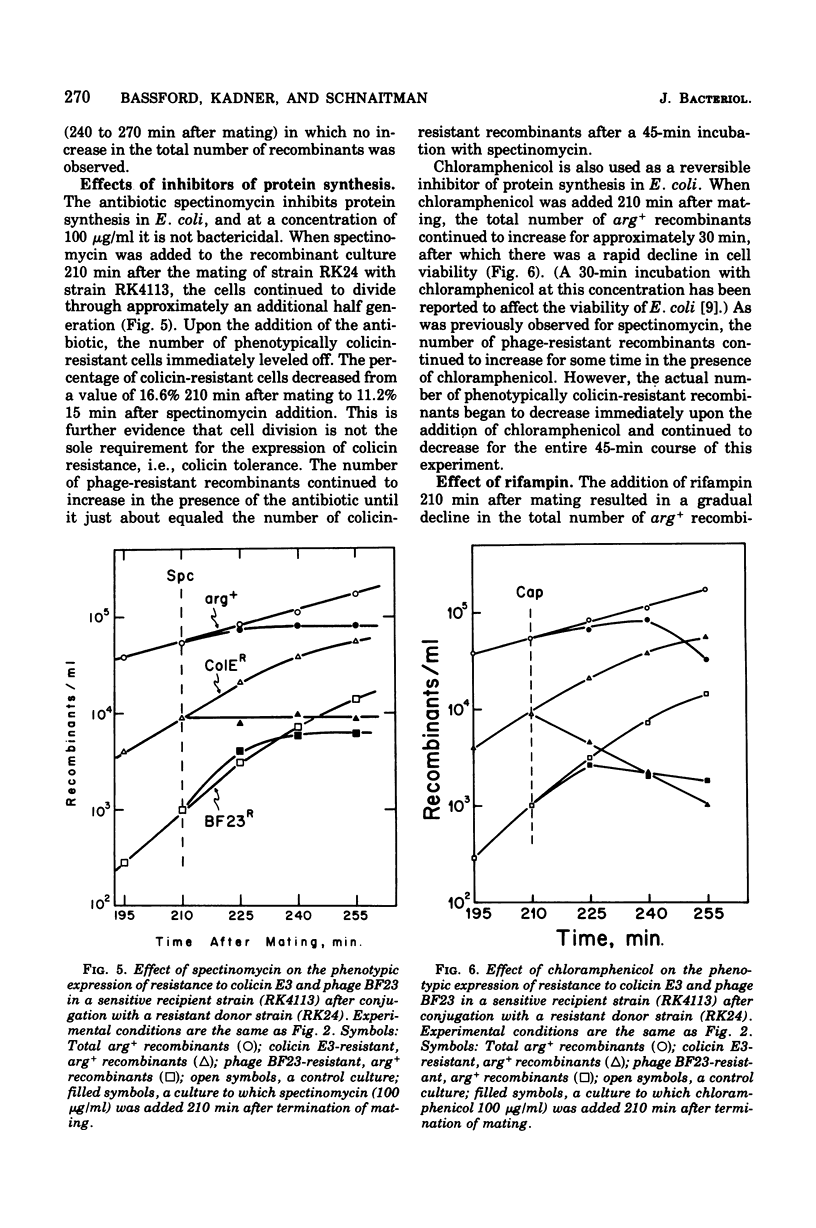
The bfe locus codes for the cell surface receptor for vitamin B12, the E colicins, and bacteriophage BF23 in the Escherichia coli outer membrane. When the bfe+ allele, which is closely linked to the argH locus, was introduced into an argH bfe recipient by conjugation, arg+ recombinant cells rapidly and simultaneously acquired sensitivity to colicin E3 and phage BF23. In the reciprocal experiment introducing bfe into an argH bfe+ recipient, it was found that colicin E3-resistant, arg+ cells began to appear shortly after the arg+ recombinant population began to divide. This was far earlier than would have been predicted on the basis of 220 receptors per haploid cell. Moreover, there was a lag between the appearance of colicin resistance and the appearance of resistance to killing by phage BF23, and hence a period of time during which some arg+ recombinant cells were sensitive to the phage but resistant to the colicin. Colicin E3 added to cells during this period of time protected against phage killing, indicating that the colicin-resistant cells still had receptors capable of binding colicin on their surface. The modification of the phenotypic expression of colicin and phage resistance by inhibitors of deoxyribonucleic acid, ribonucleic acid, and protein synthesis was also investigated. The results obtained indicate that the receptor protein coded for by the bfe locus can exist on the cell surface in several different functional states.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer M. E. Adsorption of bacteriophages to adhesions between wall and membrane of Escherichia coli. J Virol. 1968 Apr;2(4):346–356. doi: 10.1128/jvi.2.4.346-356.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E. Areas of adhesion between wall and membrane of Escherichia coli. J Gen Microbiol. 1968 Oct;53(3):395–404. doi: 10.1099/00221287-53-3-395. [DOI] [PubMed] [Google Scholar]
- Bradbeer C., Woodrow M. L., Khalifah L. I. Transport of vitamin B12 in Escherichia coli: common receptor system for vitamin B12 and bacteriophage BF23 on the outer membrane of the cell envelope. J Bacteriol. 1976 Mar;125(3):1032–1039. doi: 10.1128/jb.125.3.1032-1039.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton R. S. Genetic analysis of Escherichia coli K12 mutants resistant to bacteriophage BF23 and the E-group colicins. Mol Gen Genet. 1971;113(2):154–156. doi: 10.1007/BF00333188. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies J. K., Reeves P. Genetics of resistance to colicins in Escherichia coli K-12: cross-resistance among colicins of group A. J Bacteriol. 1975 Jul;123(1):102–117. doi: 10.1128/jb.123.1.102-117.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Masi D. R., White J. C., Schnaitman C. A., Bradbeer C. Transport of vitamin B12 in Escherichia coli: common receptor sites for vitamin B12 and the E colicins on the outer membrane of the cell envelope. J Bacteriol. 1973 Aug;115(2):506–513. doi: 10.1128/jb.115.2.506-513.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworsky P., Schaechter M. Effect of rifampin on the structure and membrane attachment of the nucleoid of Escherichia coli. J Bacteriol. 1973 Dec;116(3):1364–1374. doi: 10.1128/jb.116.3.1364-1374.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guota R. S. Killing and lysis of Echerichia coli in the presence of choloramphenicol: relation to cellular magensim. Antimicrob Agents Chemother. 1975 Jun;7(6):748–753. doi: 10.1128/aac.7.6.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hantke K., Braun V. Membrane receptor dependent iron transport in Escherichia coli. FEBS Lett. 1975 Jan 1;49(3):301–305. doi: 10.1016/0014-5793(75)80771-x. [DOI] [PubMed] [Google Scholar]
- Hirashima A., Childs G., Inouye M. Differential inhibitory effects of antibiotics on the biosynthesis of envelope proteins of Escherichia coli. J Mol Biol. 1973 Sep 15;79(2):373–389. doi: 10.1016/0022-2836(73)90012-0. [DOI] [PubMed] [Google Scholar]
- James R. Identification of an outer membrane protein of Escherichia coli, with a role in the coordination of deoxyribonucleic acid replication and cell elongation. J Bacteriol. 1975 Nov;124(2):918–929. doi: 10.1128/jb.124.2.918-929.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasper P., Whitney E., Silver S. Genetic locus determining resistance to phage BF23 and colicins E 1 , E 2 and E 3 in Escherichia coli. Genet Res. 1972 Jun;19(3):305–312. doi: 10.1017/s0016672300014555. [DOI] [PubMed] [Google Scholar]
- Kadner R. J., Bassford P. J., Jr Relation of cell growth and colicin tolerance to vitamin B12 uptake in Escherichia coli. J Bacteriol. 1977 Jan;129(1):254–264. doi: 10.1128/jb.129.1.254-264.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadner R. J., Liggins G. L. Transport of vitamin B12 in Escherichia coli: genetic studies. J Bacteriol. 1973 Aug;115(2):514–521. doi: 10.1128/jb.115.2.514-521.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klainer A. S., Russell R. R. Effect of the inhibition of protein synthesis on the Escherichia coli cell envelope. Antimicrob Agents Chemother. 1974 Aug;6(2):216–224. doi: 10.1128/aac.6.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leal J., Marcovich H. Expression of T6 sensitivity in Escherichia coli K12F minus bacteria after mating with HFR cells. Mol Gen Genet. 1974 Jun 27;130(4):345–359. doi: 10.1007/BF00333874. [DOI] [PubMed] [Google Scholar]
- Leal J., Marcovich H. Segregation of phage receptors T 6 during cell division in Escherichia coli K 12. Ann Inst Pasteur (Paris) 1971 Apr;120(4):467–474. [PubMed] [Google Scholar]
- Lindberg A. A. Bacteriophage receptors. Annu Rev Microbiol. 1973;27:205–241. doi: 10.1146/annurev.mi.27.100173.001225. [DOI] [PubMed] [Google Scholar]
- Low B. Rapid mapping of conditional and auxotrophic mutations in Escherichia coli K-12. J Bacteriol. 1973 Feb;113(2):798–812. doi: 10.1128/jb.113.2.798-812.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacGregor C. H. Solubilization of Escherichia coli nitrate reductase by a membrane-bound protease. J Bacteriol. 1975 Mar;121(3):1102–1110. doi: 10.1128/jb.121.3.1102-1110.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matzura H., Broda P. Sensitization of Escherichia coli to actinomycin D by the arrest of protein synthesis. J Bacteriol. 1968 Nov;96(5):1877–1879. doi: 10.1128/jb.96.5.1877-1879.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabet S. F., Schnaitman C. A. Purification and properties of the colicin E3 receptor of Escherichia coli. J Biol Chem. 1973 Mar 10;248(5):1797–1806. [PubMed] [Google Scholar]
- Shuman H., Schwartz M. The effect of nalidixic acid on the expression of some genes in Escherichia coli K-12. Biochem Biophys Res Commun. 1975 May 5;64(1):204–209. doi: 10.1016/0006-291x(75)90239-9. [DOI] [PubMed] [Google Scholar]
- Szmelcman S., Hofnung M. Maltose transport in Escherichia coli K-12: involvement of the bacteriophage lambda receptor. J Bacteriol. 1975 Oct;124(1):112–118. doi: 10.1128/jb.124.1.112-118.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayne R., Neilands J. B. Evidence for common binding sites for ferrichrome compounds and bacteriophage phi 80 in the cell envelope of Escherichia coli. J Bacteriol. 1975 Feb;121(2):497–503. doi: 10.1128/jb.121.2.497-503.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K. R., Alberts B. M., Benzinger R., Lawhorne L., Treiber G. Rapid bacteriophage sedimentation in the presence of polyethylene glycol and its application to large-scale virus purification. Virology. 1970 Mar;40(3):734–744. doi: 10.1016/0042-6822(70)90218-7. [DOI] [PubMed] [Google Scholar]


