Abstract
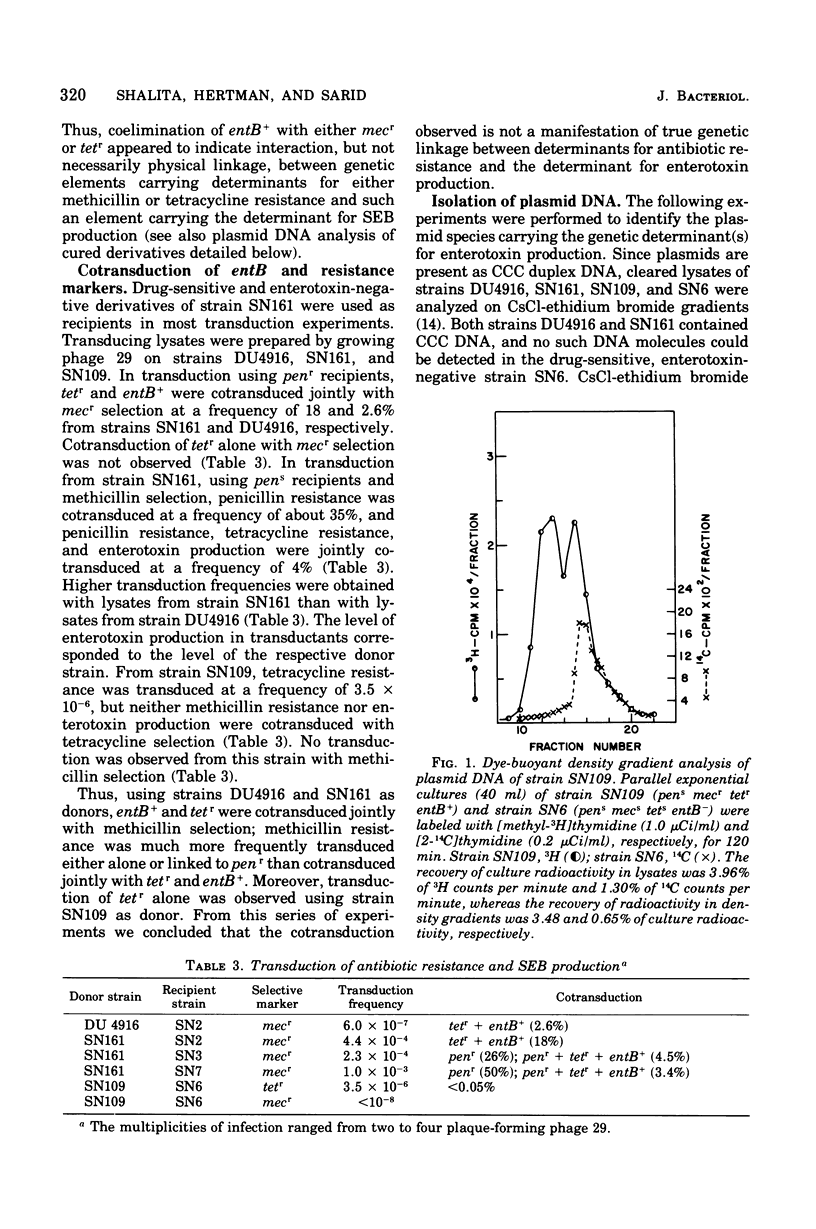
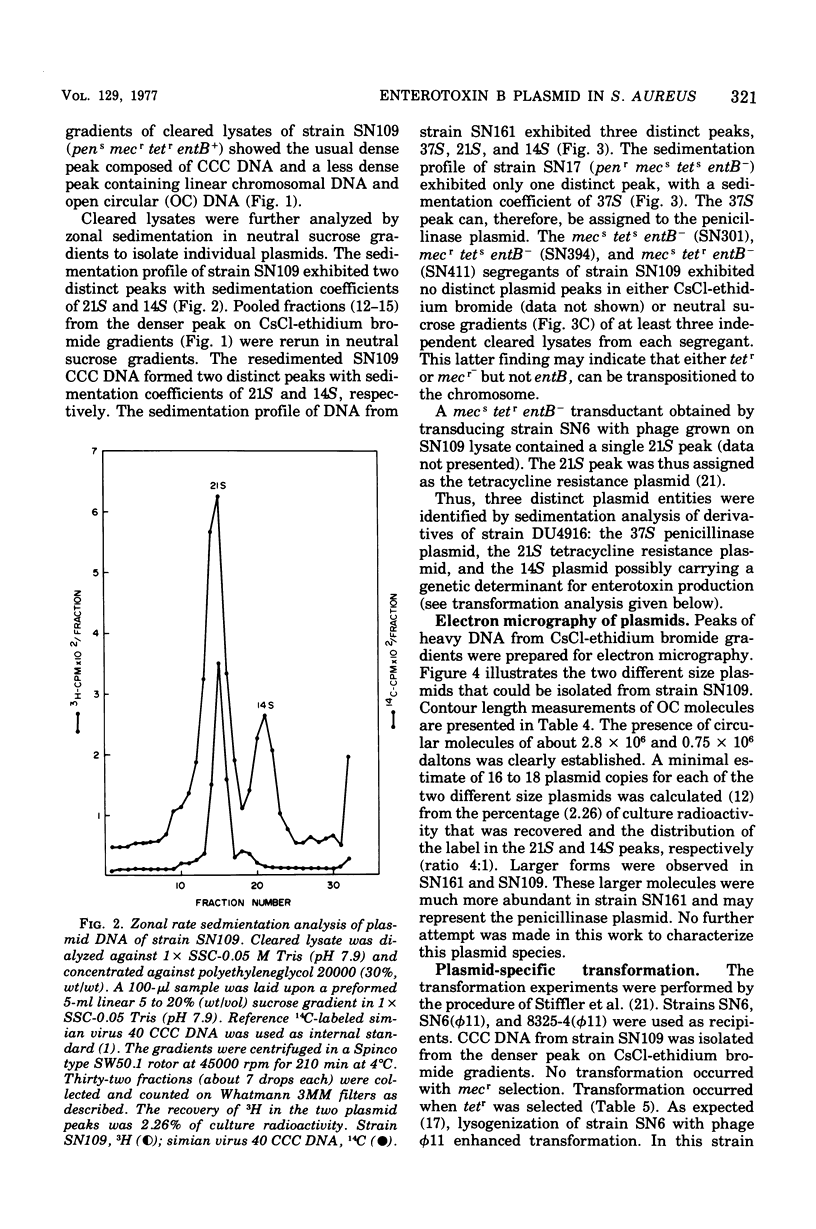
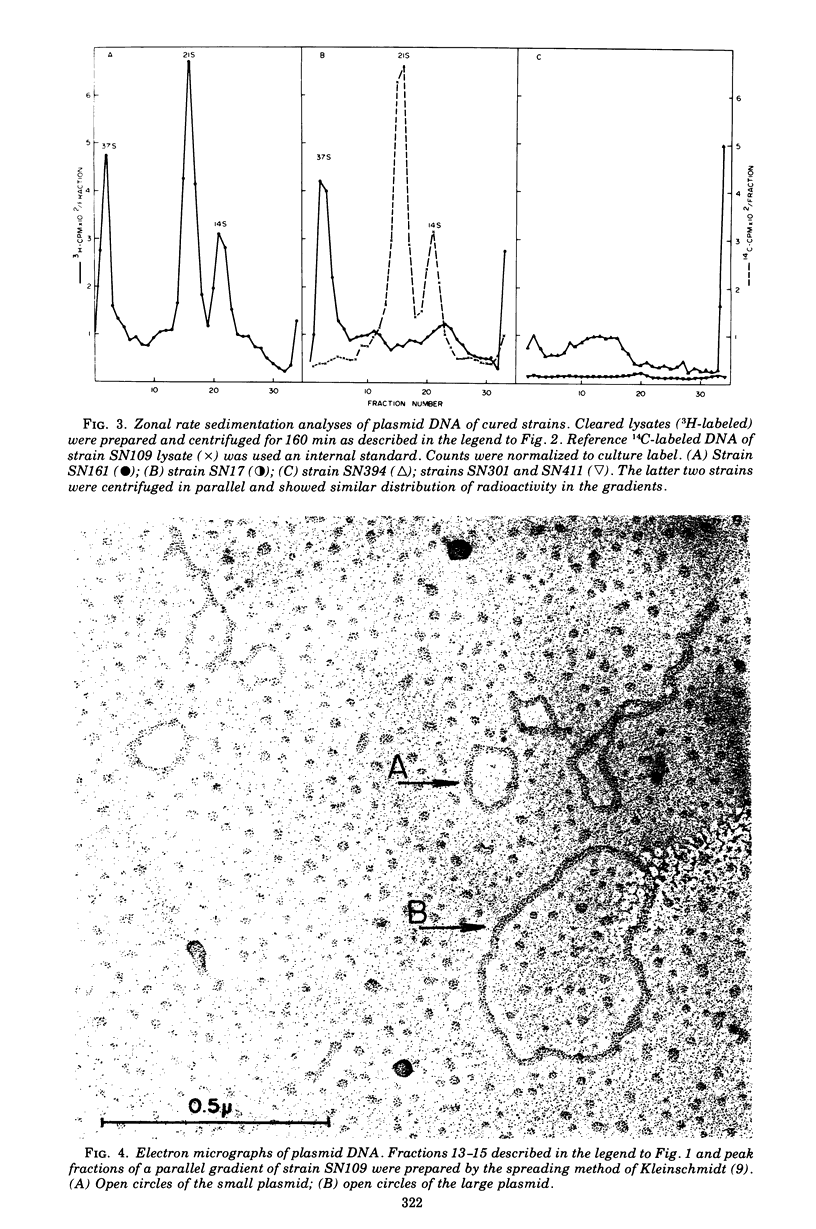
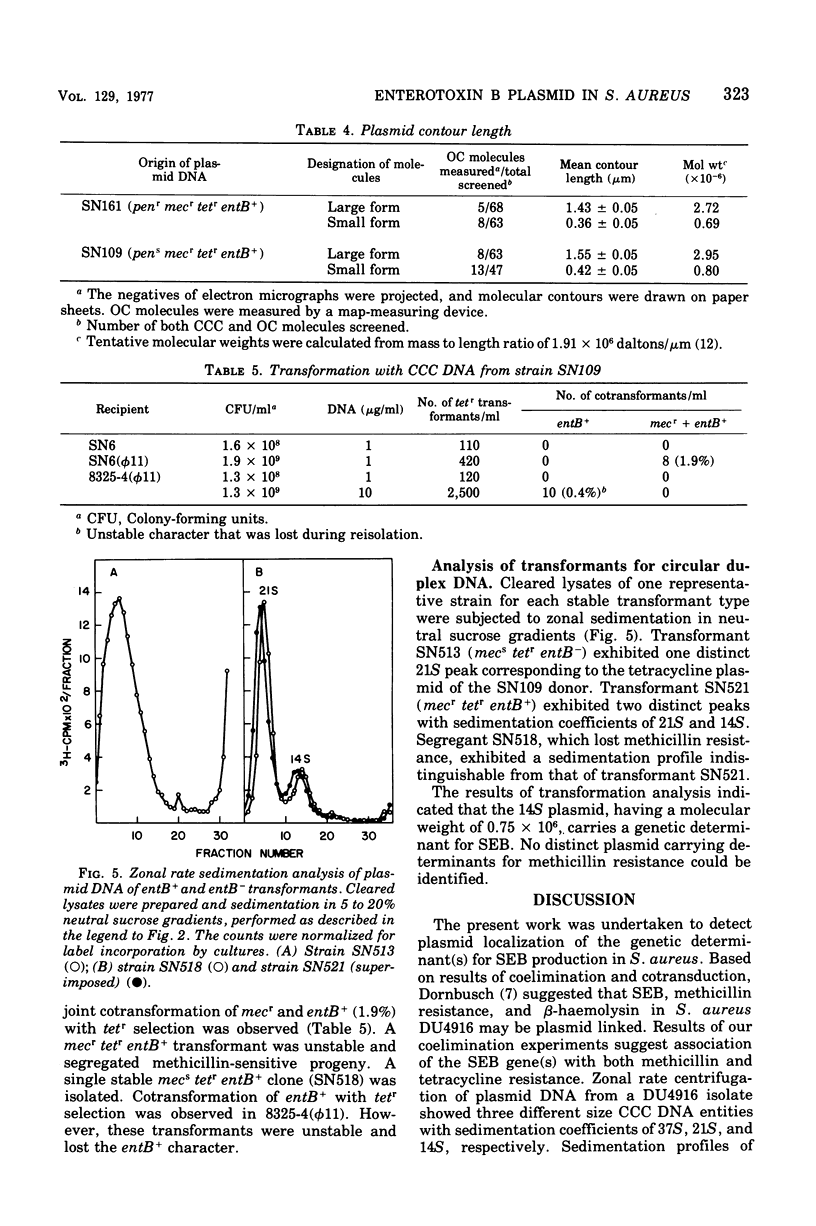
Genetic analysis and molecular characterization of plasmid deoxyribonucleic acid (DNA) was performed in a toxigenic isolate of Staphylococcus aureus strain DU4916. Elimination, transduction, and transformation experiments provided us with a series of derivatives similar except for the presence or absence of genes mediating resistance to penicillin (penr), methicillin (mecr), and tetracycline (tetr) and enterotoxin type B (SEB) production (entB+). The derivatives were examined for the presence of a plasmid species which encodes for SEB production. Two distinct species of covalently closed circular DNA of about 2.8 X 10(6) and 0.75 X 10(6) daltons were identified in an ethidium bromide-cured, penicillinase-negative (pens) isolate, SN109 (mecr tetr emtB+). Further segregation of either methicillin resistance or tetracycline resistance or of both together resulted in the loss of SEB production and the disappearance of both plasmids. Transduction from strain SN109 showed that determinants for tetracycline resistance are carried by the 2.8 X 10(6) dalton plasmid. Transformation with covalently closed circular DNA from strain SN109 yielded mecs tetr entB- transformants harboring the tetracycline resistance plasmid alone and mecr tetr entB+ transformants harboring both the tetracycline resistance and the 0.75 X 10(6)-dalton plasmid. Further segregation of methicillin resistance in transformants was not associated with any change in plasmid DNA. The results indicate that a genetic determinant for SEB production is carried by the 0.75 X 10(6)-dalton plasmid. It is possible, however, that this plasmid cannot be maintained in the host independently from the tetracycline resistance plasmid. Methicillin resistance in the strains examined could not be ascribed to any of the covalently closed circular DNA components resolved in strain DU4916.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderer F. A., Schlumberger H. D., Koch M. A., Frank H., Eggers H. J. Structure of simian virus 40. II. Symmetry and components of the virus particle. Virology. 1967 Jul;32(3):511–523. doi: 10.1016/0042-6822(67)90303-0. [DOI] [PubMed] [Google Scholar]
- Baldwin J. N., Strickland R. H., Cox M. F. Some properties of the beta-lactamase genes in Staphylococcus epidermidis. Appl Microbiol. 1969 Oct;18(4):628–630. doi: 10.1128/am.18.4.628-630.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouanchaud D. H., Scavizzi M. R., Chabbert Y. A. Elimination by ethidium bromide of antibiotic resistance in enterobacteria and staphylococci. J Gen Microbiol. 1968 Dec;54(3):417–425. doi: 10.1099/00221287-54-3-417. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes R. C. Molecular structure of bacterial plasmids. Bacteriol Rev. 1972 Sep;36(3):361–405. doi: 10.1128/br.36.3.361-405.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornbusch K. Genetic aspects of methicillin resistance and toxin production in a strain of Staphylococcus aureus. Ann N Y Acad Sci. 1971 Jun 11;182:91–97. doi: 10.1111/j.1749-6632.1971.tb30647.x. [DOI] [PubMed] [Google Scholar]
- Dornbusch K., Hallander H. O., Löfquist F. Extrachromosomal control of methicillin resistance and toxin production in Staphylococcus aureus. J Bacteriol. 1969 May;98(2):351–358. doi: 10.1128/jb.98.2.351-358.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkow S., Tompkins L. S., Silver R. P., Guerry P., Le Blanc D. J. The problems of drug-resistant pathogenic bacteria. The replication of R-factor DNA in Escherichia coli K-12 following conjugation. Ann N Y Acad Sci. 1971 Jun 11;182:153–171. doi: 10.1111/j.1749-6632.1971.tb30654.x. [DOI] [PubMed] [Google Scholar]
- Lacey R. W. Staphylococcus aureus strain DU4916--an atypical methicillin-resistant isolate? J Gen Microbiol. 1974 Sep;84(1):1–10. doi: 10.1099/00221287-84-1-1. [DOI] [PubMed] [Google Scholar]
- Meynell G. G. Dodecylamine in the isolation of bacterial DNA. Biochim Biophys Acta. 1971 Jun 17;240(1):37–38. doi: 10.1016/0005-2787(71)90509-0. [DOI] [PubMed] [Google Scholar]
- Novick R. P., Bouanchaud D. The problems of drug-resistant pathogenic bacteria. Extrachromosomal nature of drug resistance in Staphylococcus aureus. Ann N Y Acad Sci. 1971 Jun 11;182:279–294. doi: 10.1111/j.1749-6632.1971.tb30664.x. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SILVERMAN S. J. SEROLOGICAL ASSAY OF CULTURE FILTRATES FOR STAPHYLOCOCCUS ENTEROTOXIN. J Bacteriol. 1963 Apr;85:955–956. doi: 10.1128/jb.85.4.955-956.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman S. J., Knott A. R., Howard M. Rapid, sensitive assay for staphylococcal enterotoxin and a comparison of serological methods. Appl Microbiol. 1968 Jul;16(7):1019–1023. doi: 10.21236/ad0838753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström J. E., Löfdahl S., Philipson L. Transformation reveals a chromosomal locus of the gene(s) for methicillin resistance in Staphylococcus aureus. J Bacteriol. 1975 Sep;123(3):905–915. doi: 10.1128/jb.123.3.905-915.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöström J. E., Philipson L. Role of the phi 11 phage genome in competence of Staphylococcus aureus. J Bacteriol. 1974 Jul;119(1):19–32. doi: 10.1128/jb.119.1.19-32.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonstein S. A., Baldwin J. N. Nature of the elimination of the penicillinase plasmid from Staphylococcus aureus by surface-active agents. J Bacteriol. 1972 Jul;111(1):152–155. doi: 10.1128/jb.111.1.152-155.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiffler P. W., Sweeney H. M., Cohen S. Absence of circular plasmid deoxyribonucleic acid attributable to a genetic determinant for methicillin resistance in Staphylococcus aureus. J Bacteriol. 1973 Nov;116(2):771–777. doi: 10.1128/jb.116.2.771-777.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiffler P. W., Sweeney H. M., Cohen S. Co-transduction of plasmids mediating resistance to tetracycline and chloramphenicol in Staphylococcus aureus. J Bacteriol. 1974 Nov;120(2):934–934. doi: 10.1128/jb.120.2.934-944.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weirether F. J., Lewis E. E., Rosenwald A. J., Lincoln R. E. Rapid quantitative serological assay of staphylococcal enterotoxin B. Appl Microbiol. 1966 Mar;14(2):284–291. doi: 10.1128/am.14.2.284-291.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]




