Abstract
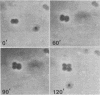
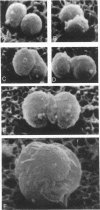
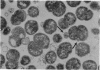
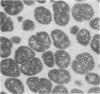
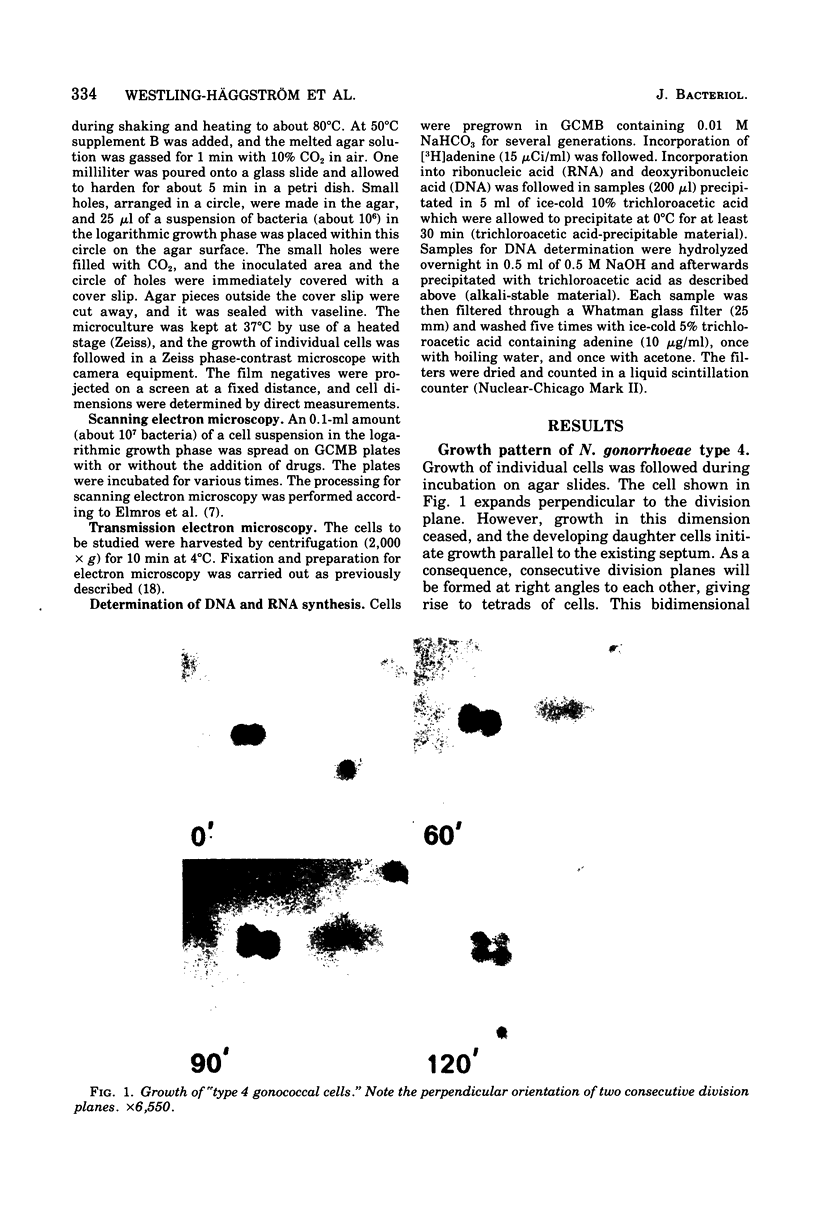
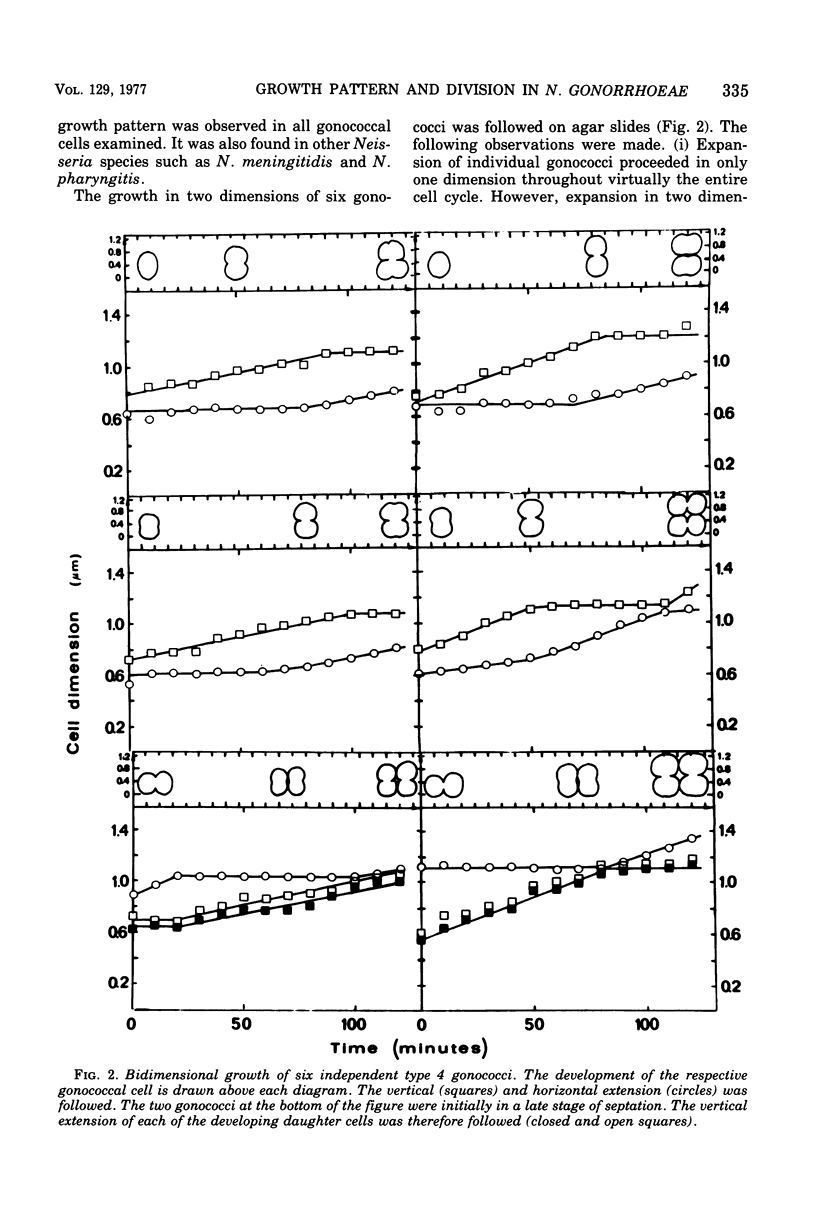
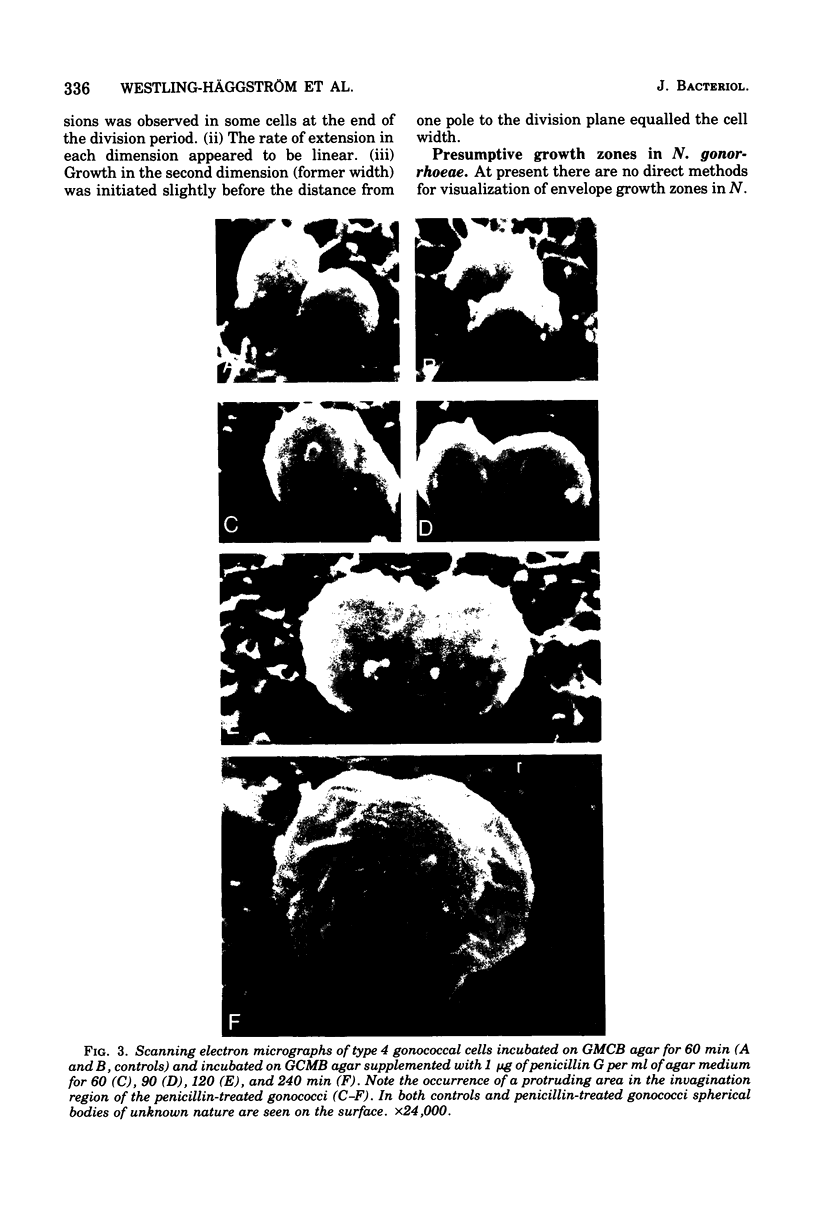
The gram-negative coccus Neisseria gonorrhoeae was found to grow regularly in at least two dimensions. Growth proceeded at a linear rate sequentially in each dimension. Growth in the second dimension (former width) was initiated slightly before the pole-division plane distance equalled the cell width. Penicillin treatment localized presumptive growth zones to the existing septum region. It was suggested that new growth zones were always formed perpendicular to the longitudinal axis created in the incipient daughter cells of a dividing coccus. Neither penicillin nor nalidixic acid induced filaments of N. gonorrhoeae. Such structures could nevertheless be formed in the rod-shaped species Neisseria elongata. N. gonorrhoeae divides by septation; however, complete septal structures with separated cytoplasms were rather infrequent. It is proposed that N. gonorrhoeae be regarded as a short rod which always extends parallel to the actual longitudinal axis and which never undergoes a rod-sphere-rod transition.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bloom G. D., Gumpert J., Normark S., Schuhmann E., Taubeneck U., Westling B. Morphology and growth pattern of a rod-negative envB mutant of Escherichia coli K12. Z Allg Mikrobiol. 1974;14(6):465–477. doi: 10.1002/jobm.3630140603. [DOI] [PubMed] [Google Scholar]
- Bovre K., Fuglesang J. E., Henriksen S. D. Neisseria elongata. Presentation of new isolates. Acta Pathol Microbiol Scand B Microbiol Immunol. 1972;80(6):919–922. doi: 10.1111/j.0365-5563.1973.tb00020.x. [DOI] [PubMed] [Google Scholar]
- Bovre K., Holten E. Neisseria elongata sp.nov., a rod-shaped member of the genus Neisseria. Re-evaluation of cell shape as a criterion in classification. J Gen Microbiol. 1970 Jan;60(1):67–75. doi: 10.1099/00221287-60-1-67. [DOI] [PubMed] [Google Scholar]
- Burdett I. D., Murray R. G. Electron microscope study of septum formation in Escherichia coli strains B and B-r during synchronous growth. J Bacteriol. 1974 Sep;119(3):1039–1056. doi: 10.1128/jb.119.3.1039-1056.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett I. D., Murray R. G. Septum formation in Escherichia coli: characterization of septal structure and the effects of antibiotics on cell division. J Bacteriol. 1974 Jul;119(1):303–324. doi: 10.1128/jb.119.1.303-324.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donachie W. D., Begg K. J. Growth of the bacterial cell. Nature. 1970 Sep 19;227(5264):1220–1224. doi: 10.1038/2271220a0. [DOI] [PubMed] [Google Scholar]
- Elmros T., Hörstedt P., Winblad B. Scanning electron microscopic study of virulent and avirulent colonies of Neisseria gonorrhoeae. Infect Immun. 1975 Sep;12(3):630–637. doi: 10.1128/iai.12.3.630-637.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FITZ-JAMES P. THIN SECTIONS OF DIVIDING NEISSERIA GONORRHOEAE. J Bacteriol. 1964 Jun;87:1477–1482. doi: 10.1128/jb.87.6.1477-1482.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOSS W. A., DEITZ W. H., COOK T. M. MECHANISM OF ACTION OF NALIDIXIC ACID ON ESCHERICHIA COLI. J Bacteriol. 1964 Oct;88:1112–1118. doi: 10.1128/jb.88.4.1112-1118.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Model for cell wall growth of Streptococcus faecalis. J Bacteriol. 1970 Feb;101(2):643–648. doi: 10.1128/jb.101.2.643-648.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann B., Messer W., Schwarz U. Regulation of polar cap formation in the life cycle of Escherichia coli. J Supramol Struct. 1972;1(1):29–37. doi: 10.1002/jss.400010105. [DOI] [PubMed] [Google Scholar]
- James R., Haga J. Y., Pardee A. B. Inhibition of an early event in the cell division cycle of Escherichia coli by FL1060, an amidinopenicillanic acid. J Bacteriol. 1975 Jun;122(3):1283–1292. doi: 10.1128/jb.122.3.1283-1292.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubitschek H. E. Evidence for the generality of linear cell growth. J Theor Biol. 1970 Jul;28(1):15–29. doi: 10.1016/0022-5193(70)90061-5. [DOI] [PubMed] [Google Scholar]
- Matsuzawa H., Hayakawa K., Sato T., Imahori K. Characterization and genetic analysis of a mutant of Escherichia coli K-12 with rounded morphology. J Bacteriol. 1973 Jul;115(1):436–442. doi: 10.1128/jb.115.1.436-442.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauck J., Chan L., Glaser L., Williamson J. Mode of cell wall growth of Bacillus megaterium. J Bacteriol. 1972 Jan;109(1):373–378. doi: 10.1128/jb.109.1.373-378.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S., Boman H. G., Bloom G. D. Cell division in a chain-forming envA mutant of Escherichia coli K12. Fine structure of division sites and effects of EDTA, lysozyme and ampicillin. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(5):651–664. doi: 10.1111/j.1699-0463.1971.tb00093.x. [DOI] [PubMed] [Google Scholar]
- Normark S. Mutation in Escherichia coli K-12 mediating spherelike envelopes and changes tolerance to ultraviolet irradiation and some antibiotics. J Bacteriol. 1969 Jun;98(3):1274–1277. doi: 10.1128/jb.98.3.1274-1277.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J. T., Burman L. FL-1060: a new penicillin with a unique mode of action. Biochem Biophys Res Commun. 1973 Apr 16;51(4):863–868. doi: 10.1016/0006-291x(73)90006-5. [DOI] [PubMed] [Google Scholar]
- Pritchard R. H. Review lecture on the growth and form of a bacterial cell. Philos Trans R Soc Lond B Biol Sci. 1974 Feb 21;267(886):303–336. doi: 10.1098/rstb.1974.0003. [DOI] [PubMed] [Google Scholar]
- Reyn A., Birch-Andersen A., Berger U. Fine structure and taxonomic position of Neisseria haemolysans (Thjotta and Boe 1938) or Gemella haemolysans (Berger 1960). Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78(3):375–389. doi: 10.1111/j.1699-0463.1970.tb04317.x. [DOI] [PubMed] [Google Scholar]
- Ryter A., Hirota Y., Schwarz U. Process of cellular division in Escherichia coli growth pattern of E. coli murein. J Mol Biol. 1973 Jun 25;78(1):185–195. doi: 10.1016/0022-2836(73)90437-3. [DOI] [PubMed] [Google Scholar]
- Ryter A., Shuman H., Schwartz M. Intergration of the receptor for bacteriophage lambda in the outer membrane of Escherichia coli: coupling with cell division. J Bacteriol. 1975 Apr;122(1):295–301. doi: 10.1128/jb.122.1.295-301.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G. Control of cell length in Bacillus subtilis. J Bacteriol. 1975 Jul;123(1):7–19. doi: 10.1128/jb.123.1.7-19.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz U., Asmus A., Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969 May 14;41(3):419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]
- Schwarz U., Ryter A., Rambach A., Hellio R., Hirota Y. Process of cellular division in Escherichia coli: differention of growth zones in the Sacculus. J Mol Biol. 1975 Nov 15;98(4):749–759. doi: 10.1016/s0022-2836(75)80008-8. [DOI] [PubMed] [Google Scholar]
- Talley R. S., Baugh C. L. Effects of bicarbonate on growth of Neisseria gonorrhoeae: replacement of gaseous CO2 atmosphere. Appl Microbiol. 1975 Apr;29(4):469–471. doi: 10.1128/am.29.4.469-471.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward C. B., Glaser D. A. Correlation between rate of cell growth and rate of DNA synthesis in Escherichia coli B-r. Proc Natl Acad Sci U S A. 1971 May;68(5):1061–1064. doi: 10.1073/pnas.68.5.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaritsky A., Pritchard R. H. Changes in cell size and shape associated with changes in the replication time of the chromosome of Escherichia coli. J Bacteriol. 1973 May;114(2):824–837. doi: 10.1128/jb.114.2.824-837.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]