Abstract
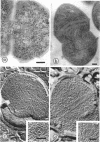
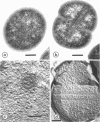
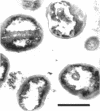
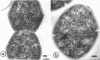
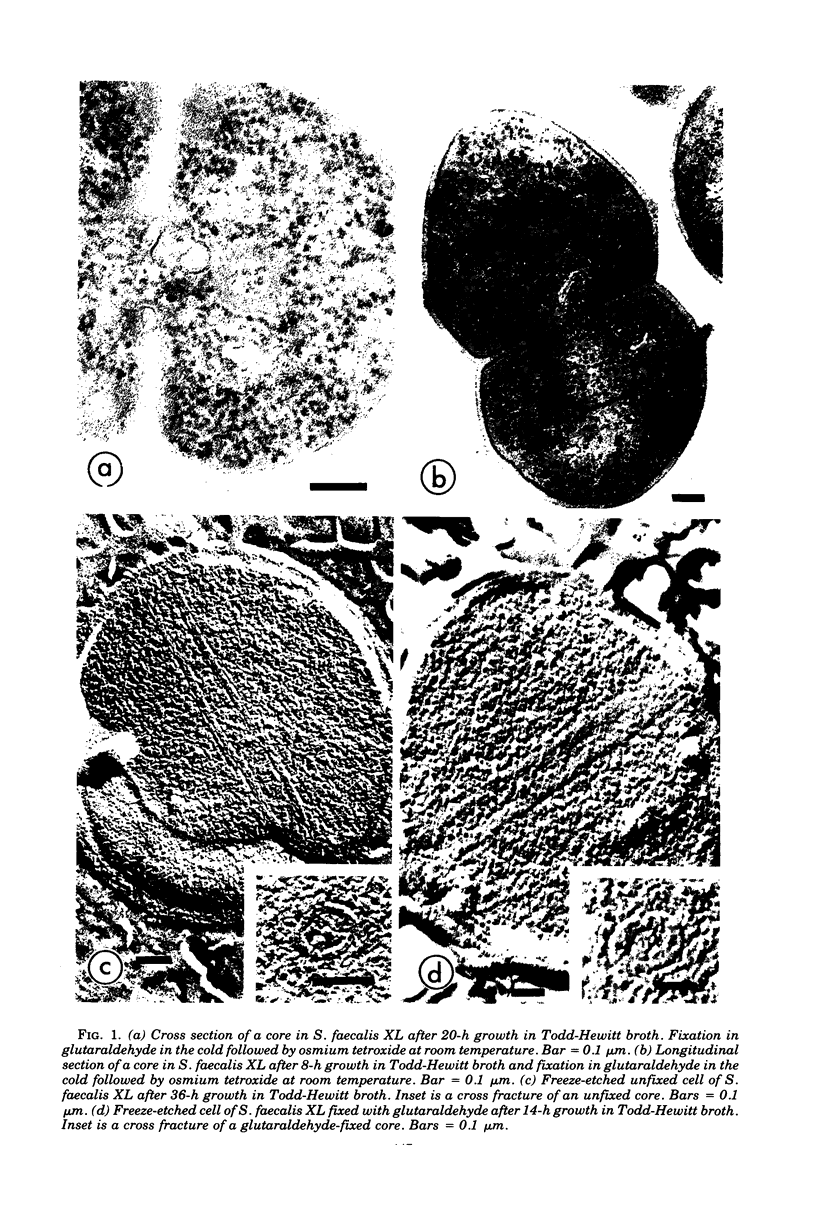
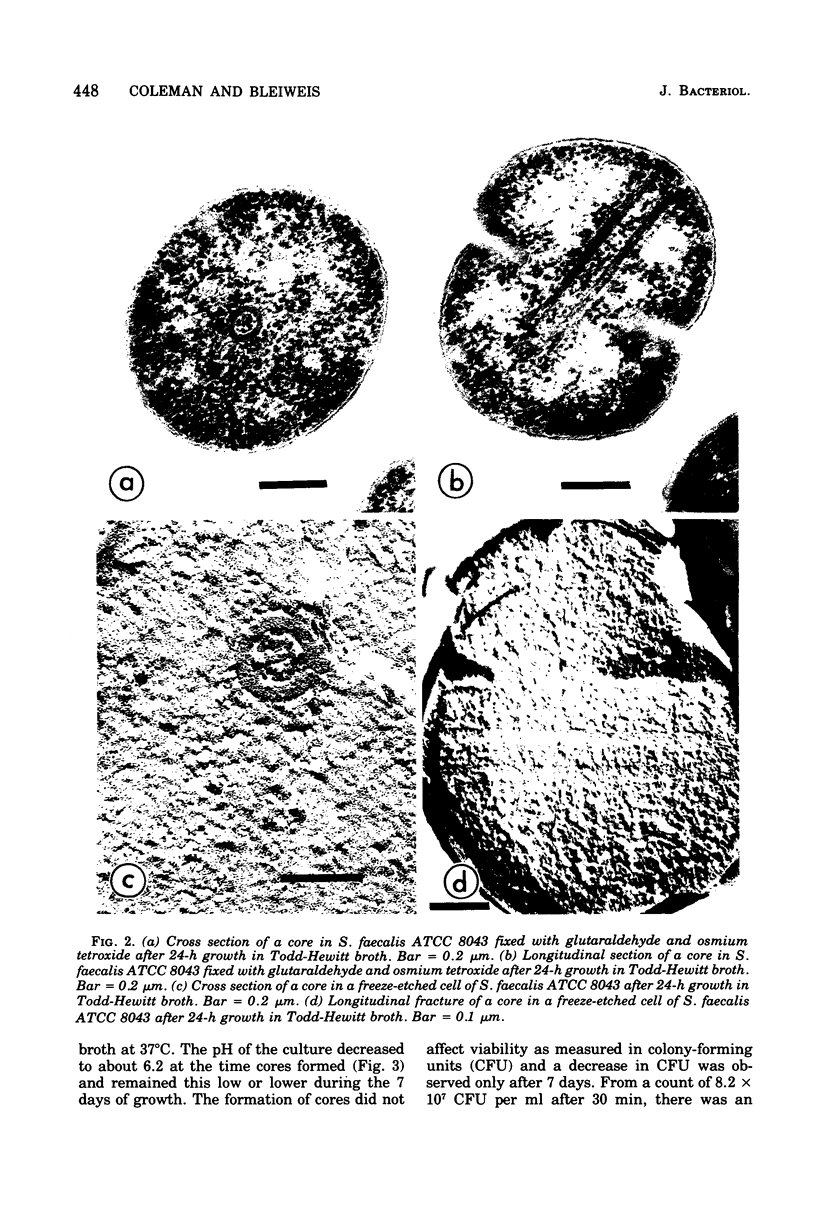
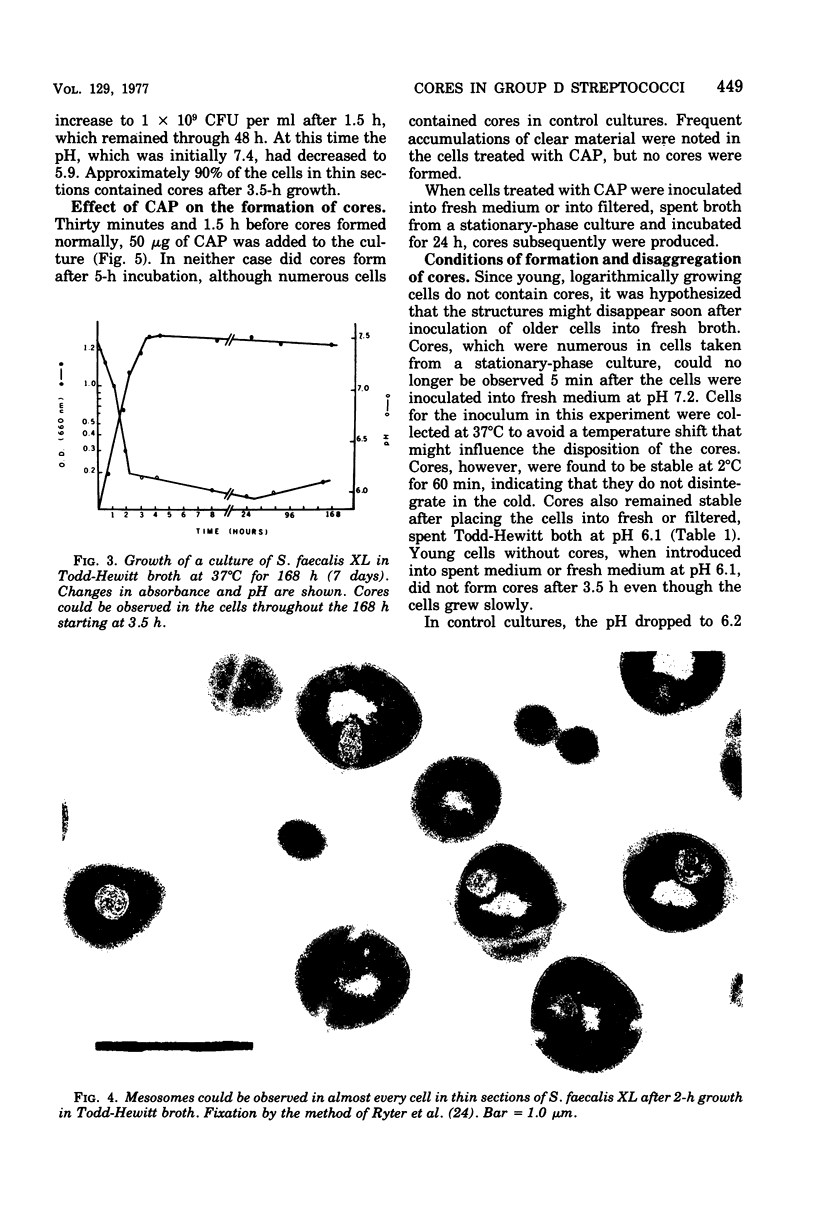
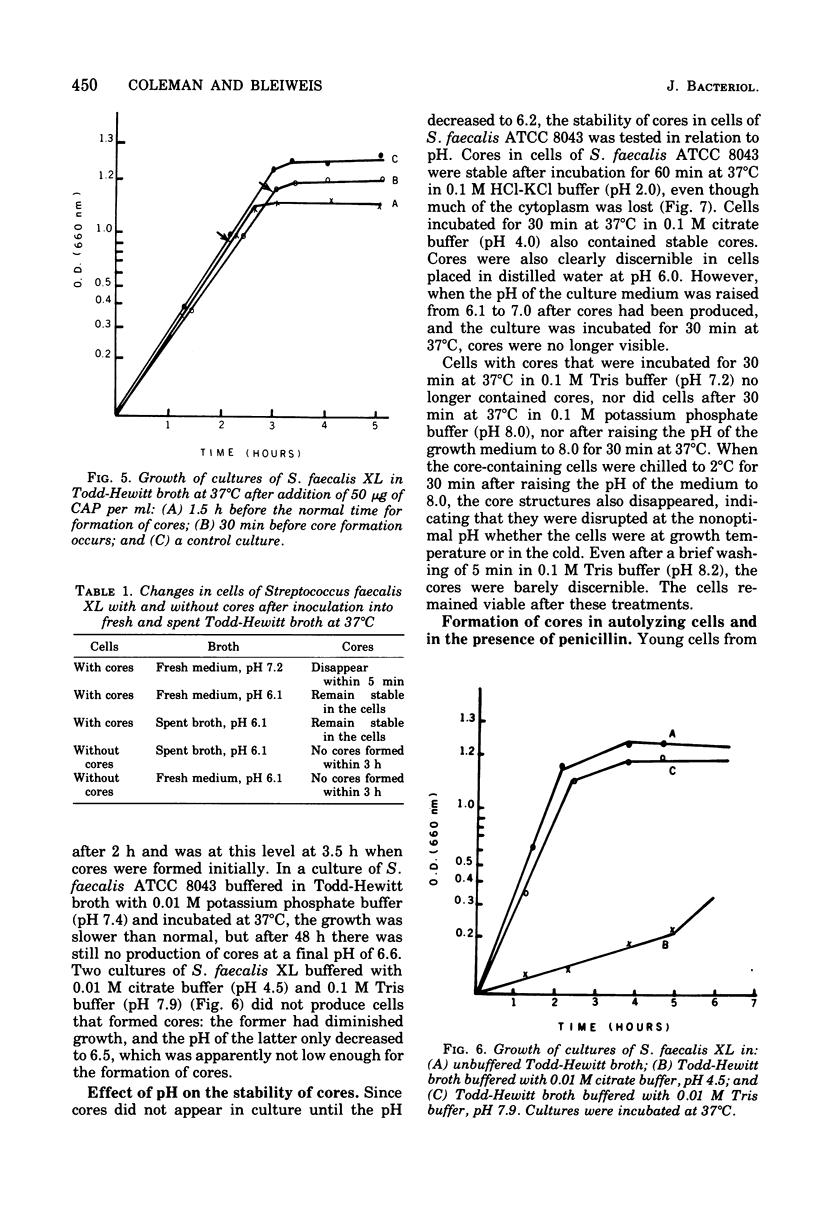
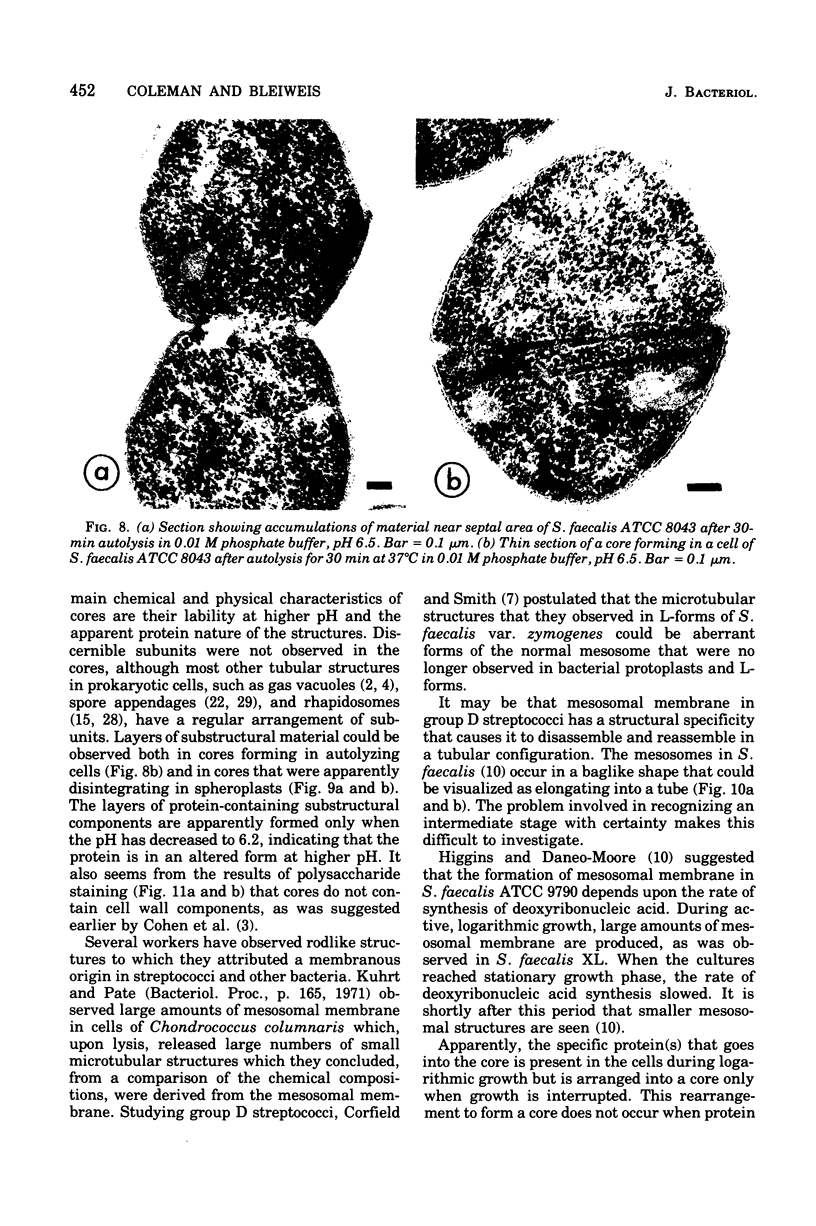
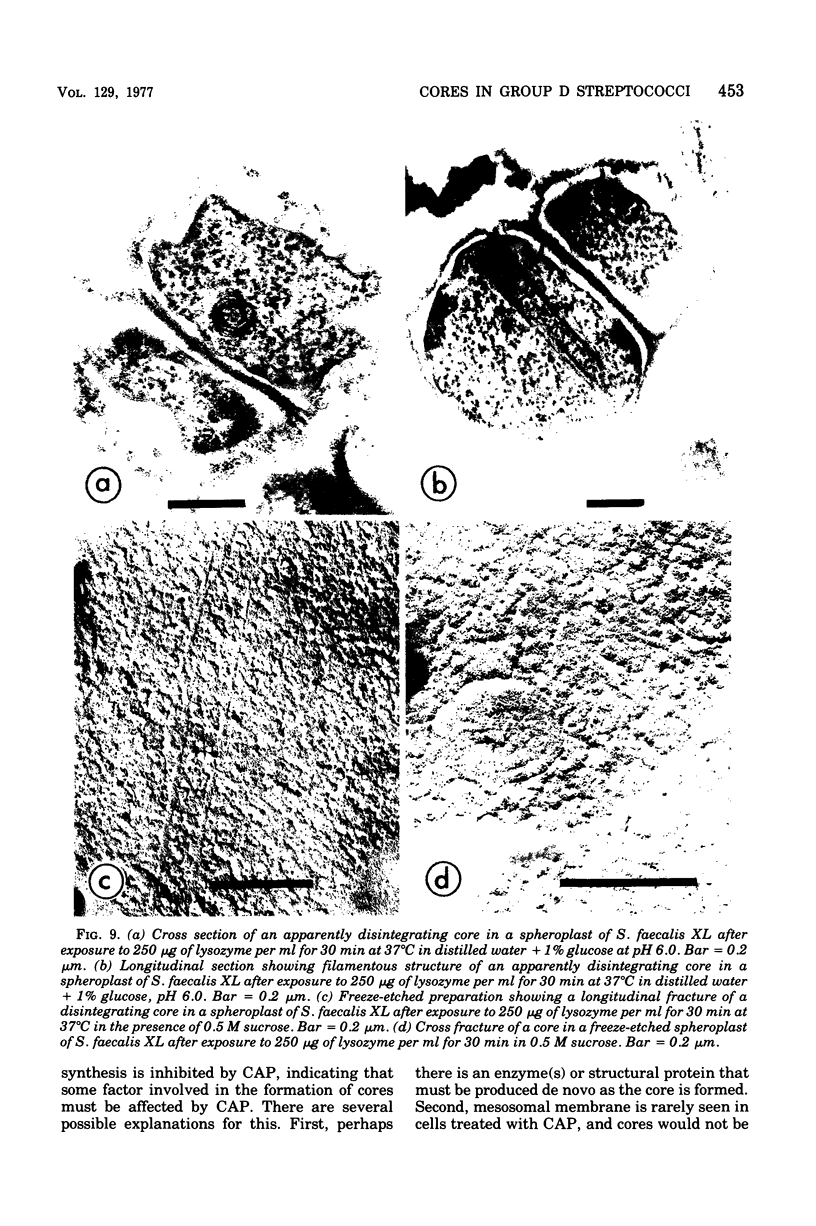
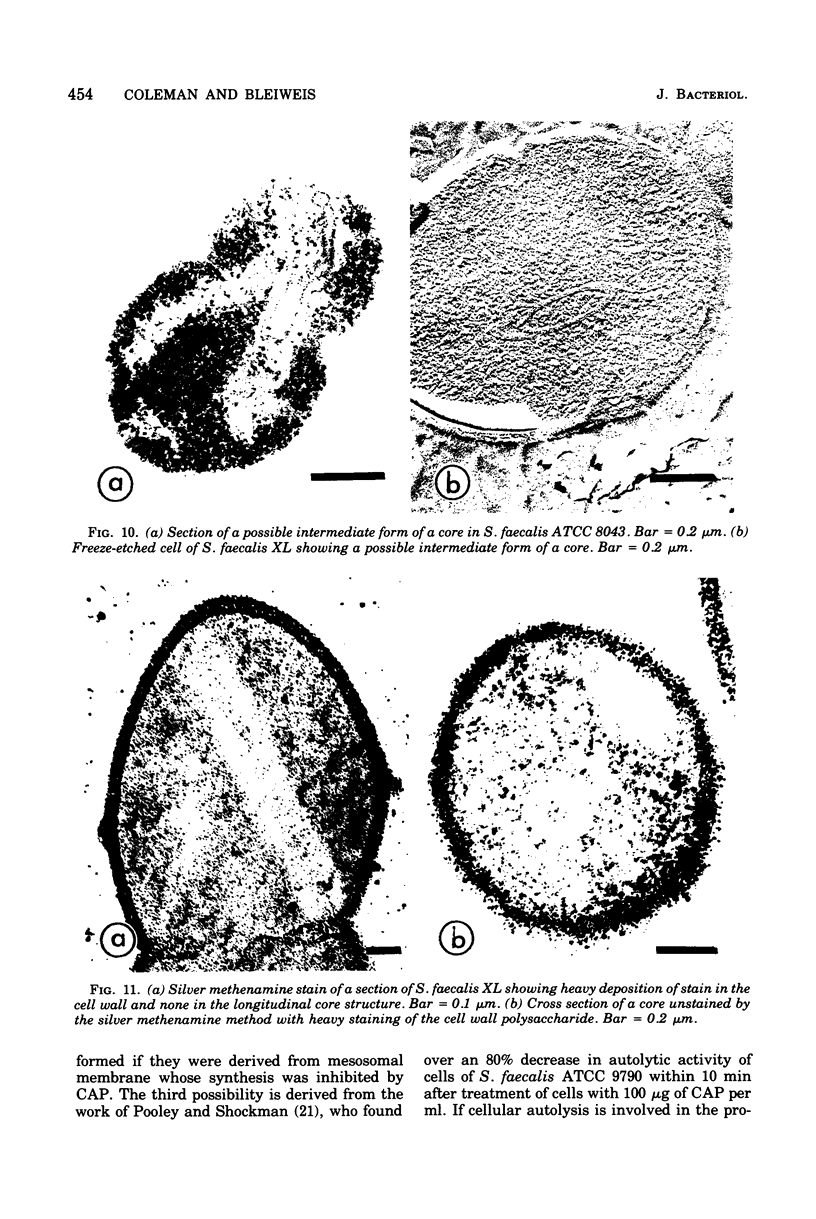
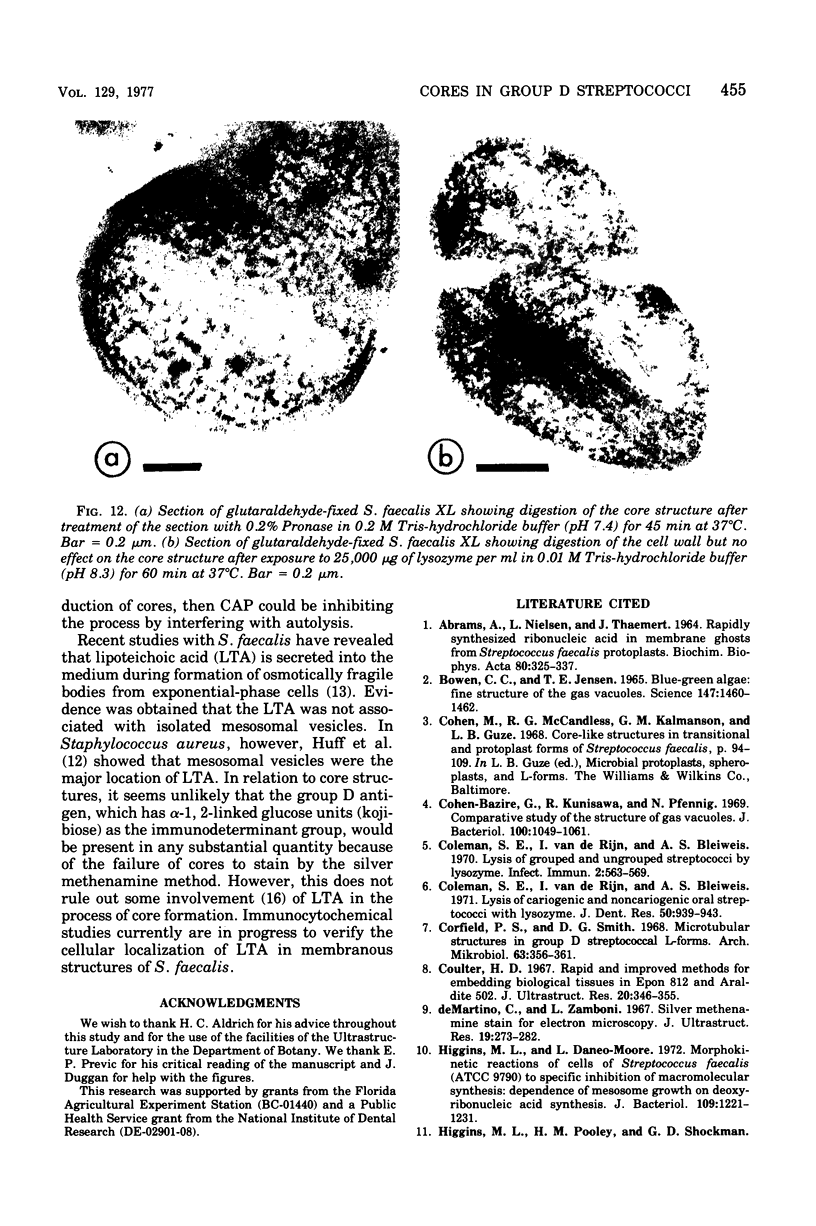
Cores are large, rod-shaped structures that have been found almost exclusively in group D streptococci, measure 0.1 to 0.16 mum in diameter, and extend the width or length of cells. This study has shown that cores are produced in the cells at a reproducible point in early stationary growth after extensive mesosomal formation and after the pH has dropped below 6.5. When cells containing cores were introduced into a fresh medium with a pH above 6.5, the structures disappeared within 5 min. The structures were not found in young, logarithmically growing cells but formed in these cells upon autolysis or treatment with penicillin. Cores that were forming or disintegrating appeared to have a lamellar substructure. When chloramphenicol was added to the medium before the culture reached stationary phase, no cores were found in the cells. Cytochemical studies indicated that cores contain protein and are not composed of cell wall material or other polysaccharides that contain 1,2-glycol groups.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMS A., NIELSEN L., THAEMERT J. RAPIDLY SYNTHESIZED RIBONUCLEIC ACID IN MEMBRANE GHOSTS FROM STREPTOCOCCUS FECALIS PROTOPLASTS. Biochim Biophys Acta. 1964 Feb 17;80:325–337. doi: 10.1016/0926-6550(64)90104-5. [DOI] [PubMed] [Google Scholar]
- Bowen C. C., Jensen T. E. Blue-Green Algae: Fine Structure of the Gas Vacuoles. Science. 1965 Mar 19;147(3664):1460–1462. doi: 10.1126/science.147.3664.1460. [DOI] [PubMed] [Google Scholar]
- Cohen-Bazire G., Kunisawa R., Pfennig N. Comparative study of the structure of gas vacuoles. J Bacteriol. 1969 Nov;100(2):1049–1061. doi: 10.1128/jb.100.2.1049-1061.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman S. E., Van de Rijn I., Bleiweis A. S. Lysis of cariogenic and noncariogenic oral streptococci with lysozyme. J Dent Res. 1971 Jul-Aug;50(4):939–943. doi: 10.1177/00220345710500042601. [DOI] [PubMed] [Google Scholar]
- Coleman S. E., van de Rijn I., Bleiweis A. S. Lysis of grouped and ungrouped streptococci by lysozyme. Infect Immun. 1970 Nov;2(5):563–569. doi: 10.1128/iai.2.5.563-569.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corfield P. S., Smith D. G. Microtubular structures in group D streptococcal L-forms. Arch Mikrobiol. 1968;63(4):356–361. doi: 10.1007/BF00412121. [DOI] [PubMed] [Google Scholar]
- Coulter H. D. Rapid and improved methods fr embedding biological tissues in Epon 812 and Araldite 502. J Ultrastruct Res. 1967 Oct 31;20(5):346–355. doi: 10.1016/s0022-5320(67)80104-7. [DOI] [PubMed] [Google Scholar]
- De Martino C., Zamboni L. Silver methenamine stain for electron microscopy. J Ultrastruct Res. 1967 Aug;19(3):273–282. doi: 10.1016/s0022-5320(67)80221-1. [DOI] [PubMed] [Google Scholar]
- Higgins M. L., Daneo-Moore L. Morphokinetic reaction of cells of Streptococcus faecalis (ATCC 9790) to specific inhibition of macromolecular synthesis: dependence of mesosome growth on deoxyribonucleic acid synthesis. J Bacteriol. 1972 Mar;109(3):1221–1231. doi: 10.1128/jb.109.3.1221-1231.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff E., Cole R. M., Theodore T. S. Lipoteichoic acid localization in mesosomal vesicles of Staphylococcus aureus. J Bacteriol. 1974 Oct;120(1):273–281. doi: 10.1128/jb.120.1.273-281.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph R., Shockman G. D. Cellular localization of lipoteichoic acid in Streptococcus faecalis. J Bacteriol. 1975 Jun;122(3):1375–1386. doi: 10.1128/jb.122.3.1375-1386.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduc E. H., Bernhard W. Recent modifications of the glycol methacrylate embedding procedure. J Ultrastruct Res. 1967 Jul;19(1):196–199. doi: 10.1016/s0022-5320(67)80068-6. [DOI] [PubMed] [Google Scholar]
- MOLLENHAUER H. H. PLASTIC EMBEDDING MIXTURES FOR USE IN ELECTRON MICROSCOPY. Stain Technol. 1964 Mar;39:111–114. [PubMed] [Google Scholar]
- McCandless R. G., Cohen M., Kalmanson G. M., Guze L. B. Cores, microbial organelles possibly specific to group D streptococci. J Bacteriol. 1968 Oct;96(4):1400–1412. doi: 10.1128/jb.96.4.1400-1412.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCandless R. G., Hensley T. J., Cohen M., Kalmanson G. M., Guze L. B. A study of specificity of cores fro group D streptococci. J Gen Microbiol. 1971 Nov;68(3):357–365. doi: 10.1099/00221287-68-3-357. [DOI] [PubMed] [Google Scholar]
- Pooley H. M., Shockman G. D. Relationship between the location of autolysin, cell wall synthesis, and the development of resistance to cellular autolysis in Streptococcus faecalis after inhibition of protein synthesis. J Bacteriol. 1970 Aug;103(2):457–466. doi: 10.1128/jb.103.2.457-466.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope L., Yolton D. P., Rode L. J. Appendages of Clostridium bifermentans spores. J Bacteriol. 1967 Oct;94(4):1206–1215. doi: 10.1128/jb.94.4.1206-1215.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- Shockman G. D., Martin J. T. Autolytic enzyme system of Streptococcus faecalis. IV. Electron microscopic observations of autolysin and lysozyme action. J Bacteriol. 1968 Nov;96(5):1803–1810. doi: 10.1128/jb.96.5.1803-1810.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T. Presence of rhapidosomes in various species of bacteria and their morphological characteristics. J Bacteriol. 1967 Nov;94(5):1746–1756. doi: 10.1128/jb.94.5.1746-1756.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yolton D. P., Pope L., Williams M. G., Rode L. J. Further electron microscope characterization of spore appendages of Clostridium bifermentans. J Bacteriol. 1968 Jan;95(1):231–238. doi: 10.1128/jb.95.1.231-238.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]