Abstract
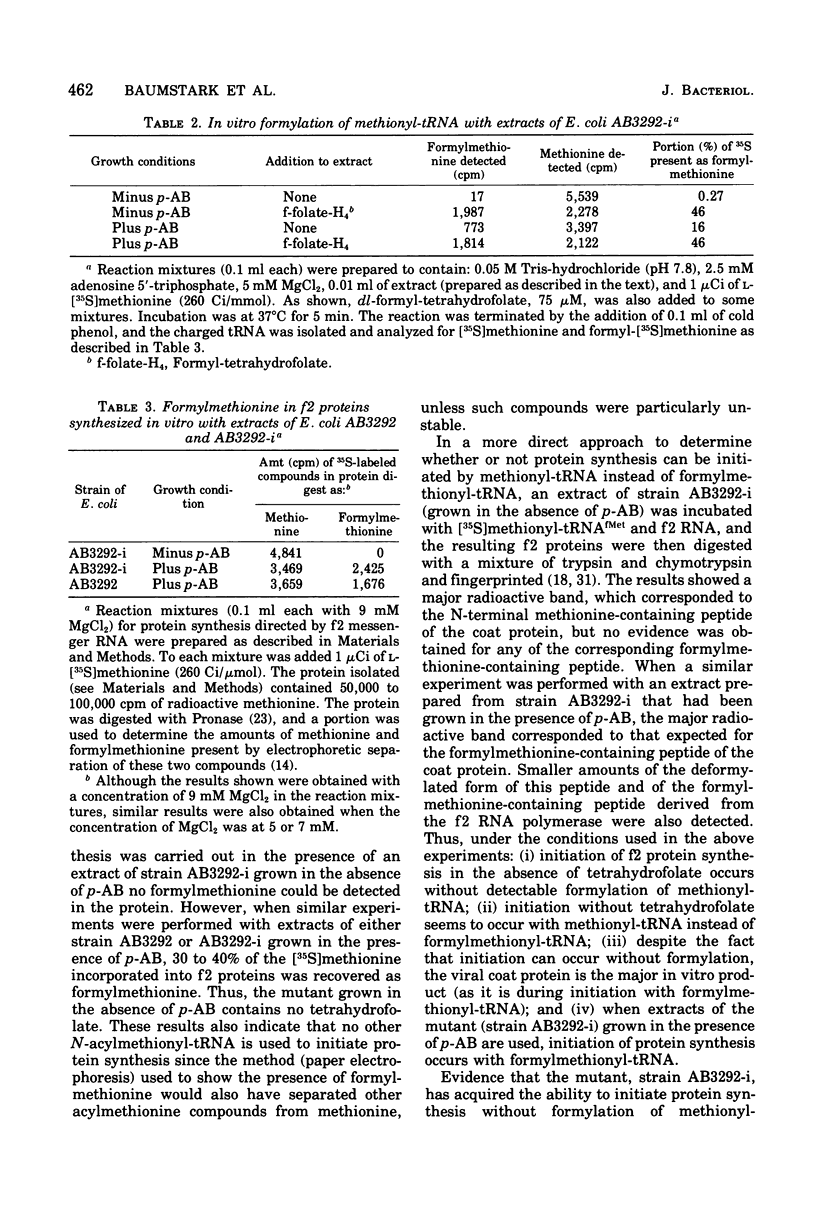
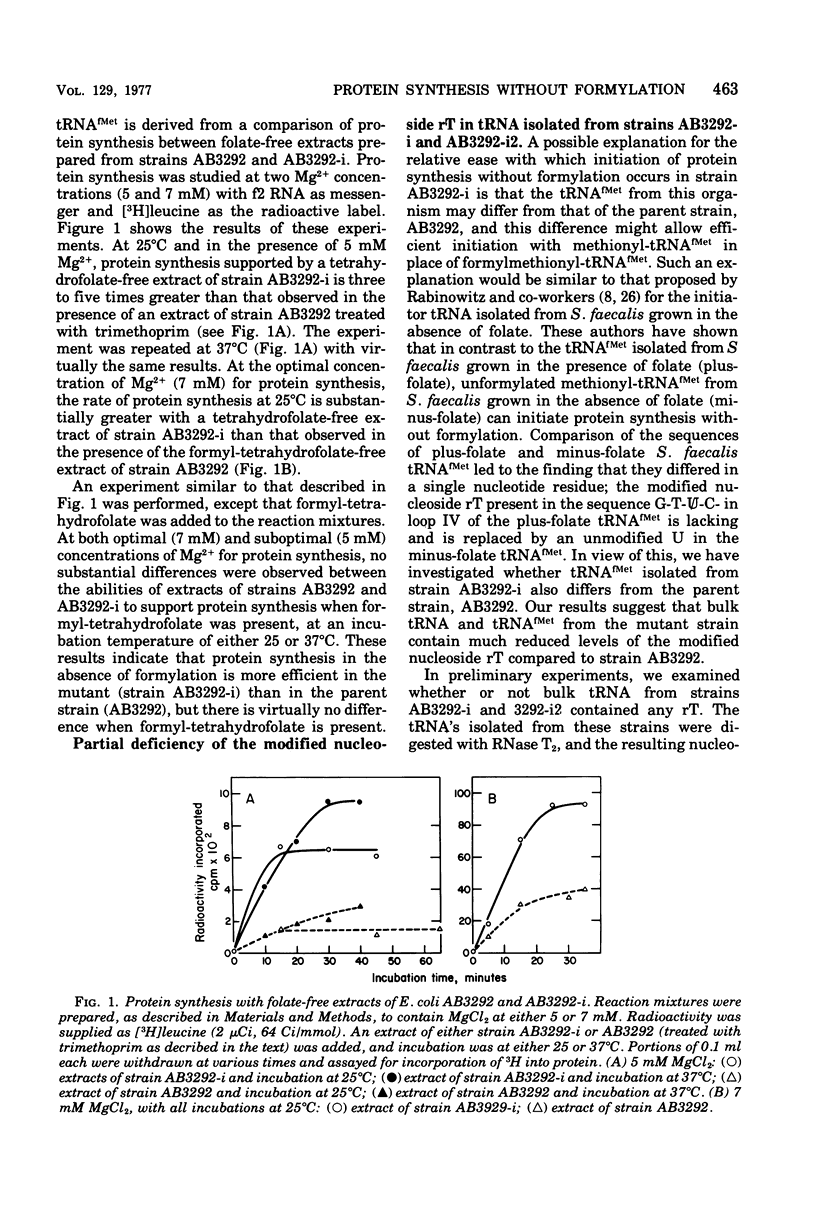
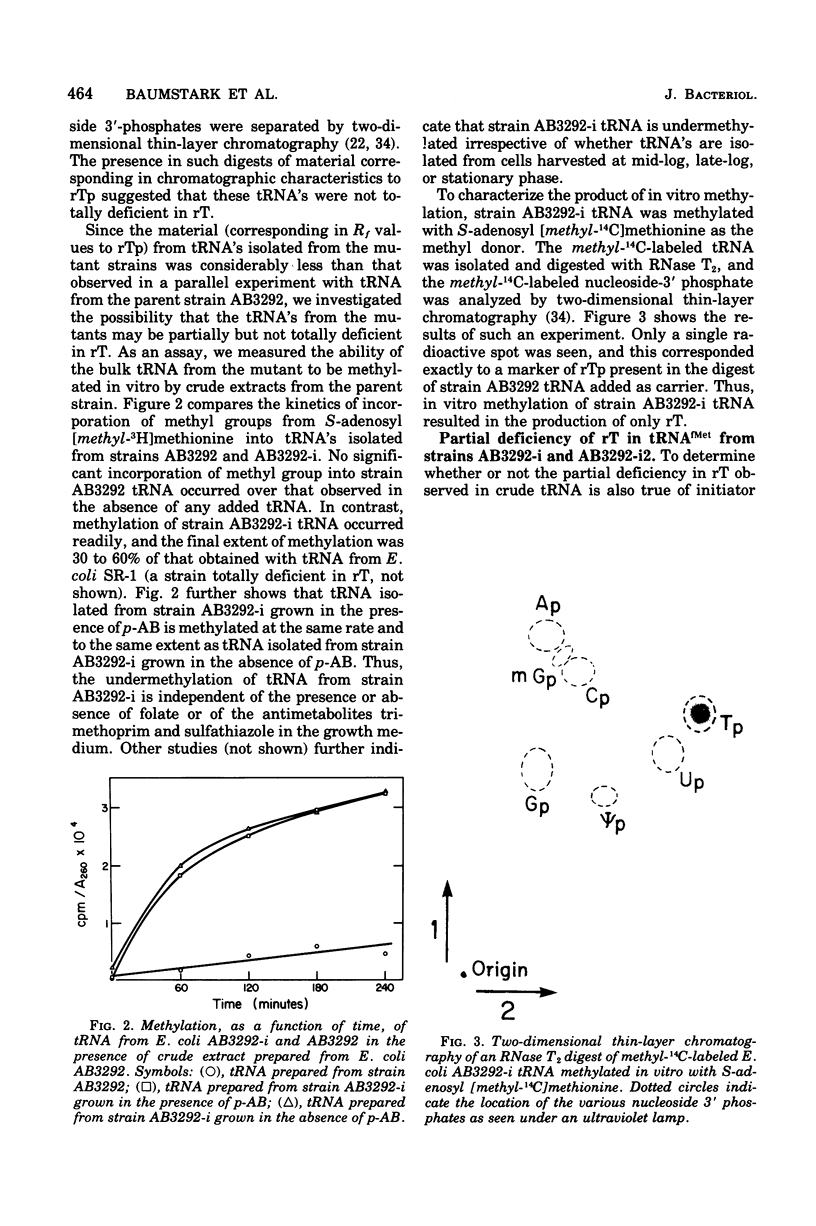
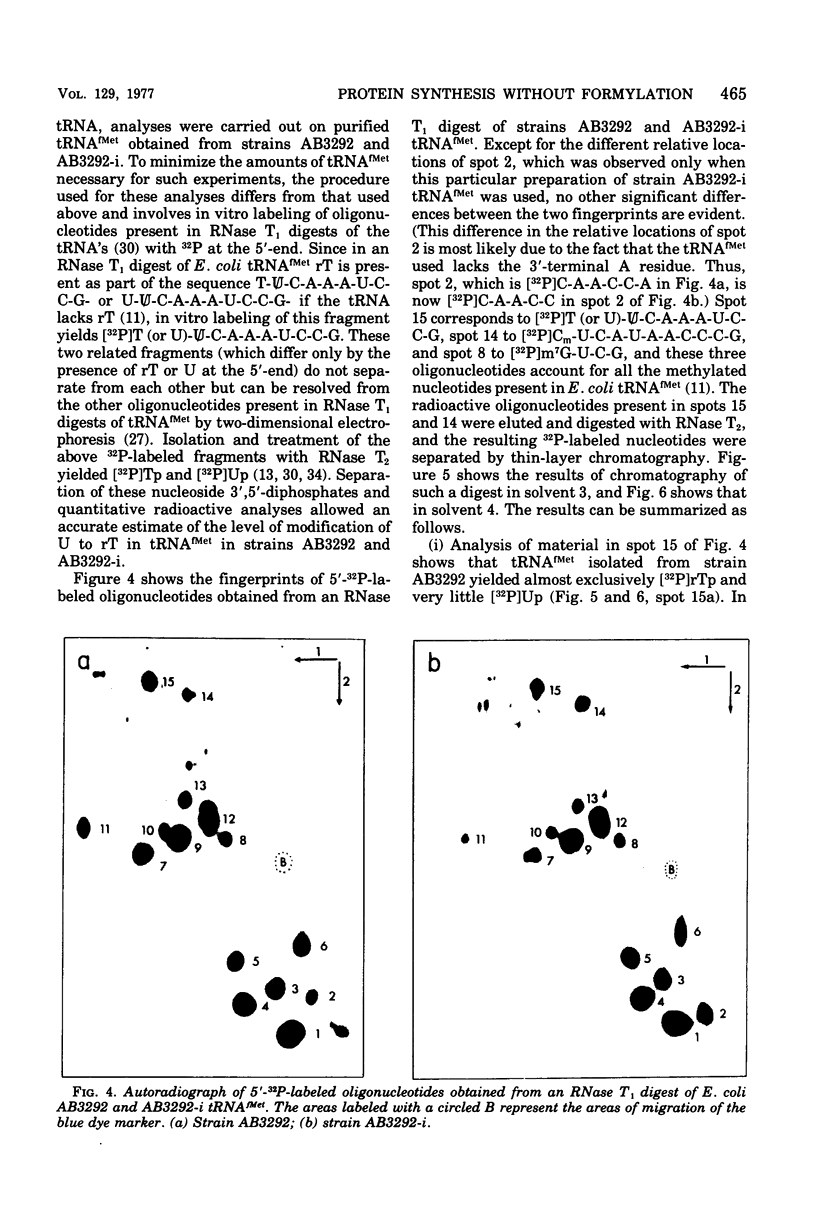
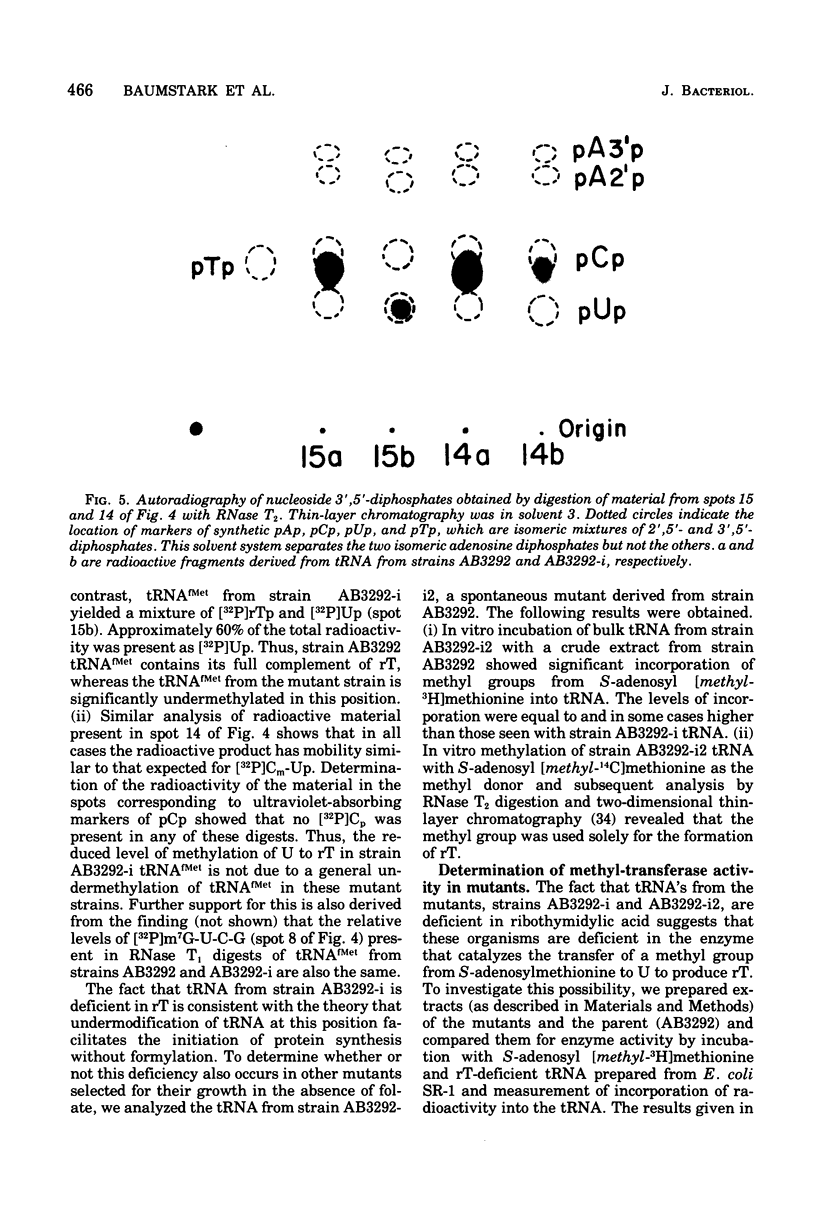
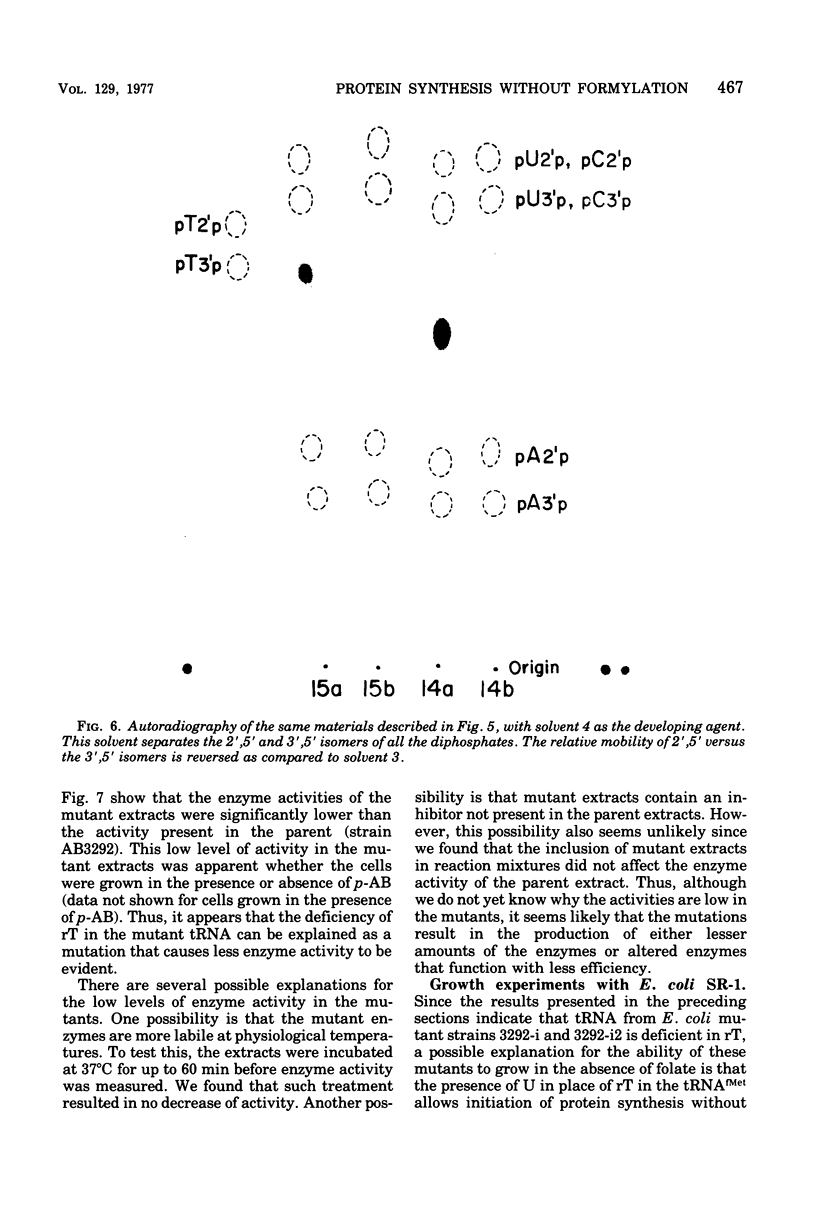
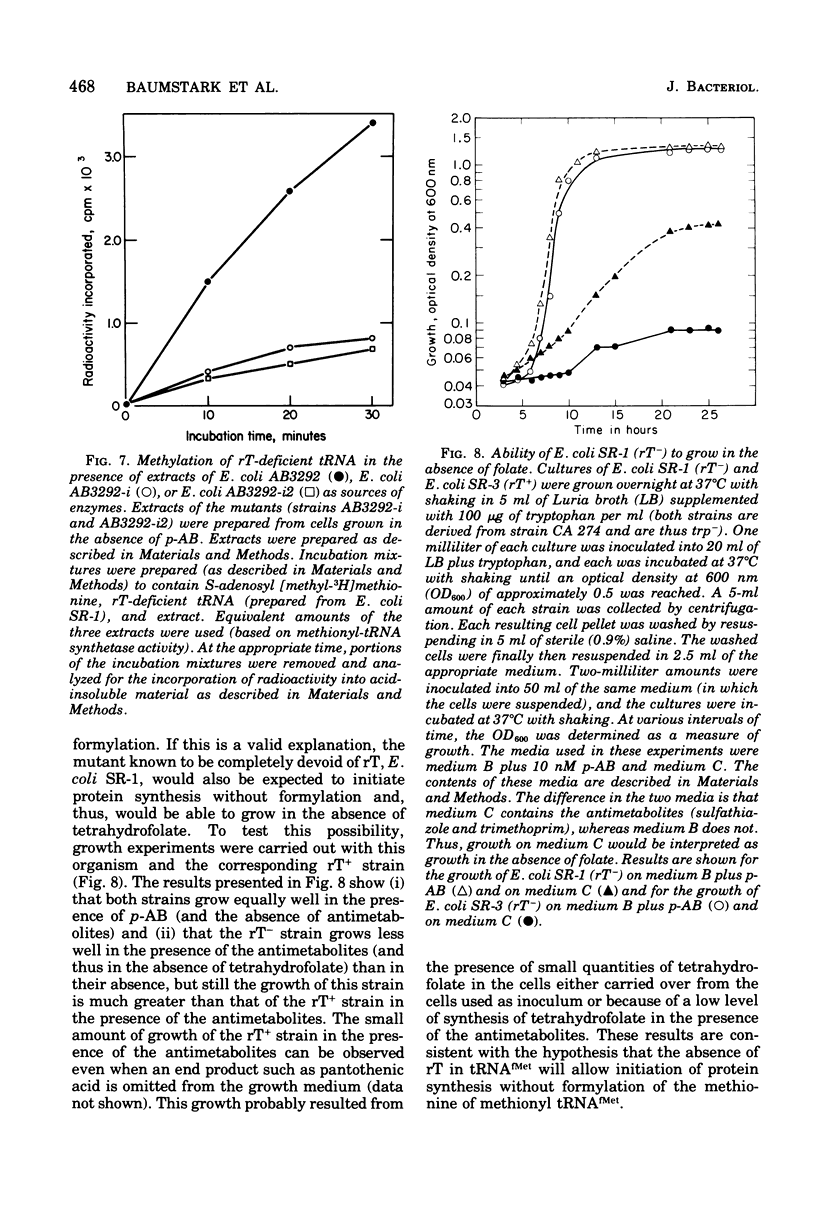
Starting from a p-aminobenzoate-requiring strain of Escherichia coli (E. coli K-12 AB3292), we have isolated mutants that can grow in the absence of p-aminobenzoate (and thus tetrahydrofolate). The following lines of evidence suggest that at least one of these mutants is capable of initiating protein synthesis without formylation of methionyl-transfer ribonucleic acid (methionyl-tRNAfMet). (i) tRNA isolated (and charged in vivo with [35S]methionine) from this mutant grown in a p-aminobenzoate-free medium contained less than 0.4% of the total methionine charged to the tRNA as formylmethionine. However, when the mutant was grown in the presence of p-aminobenzoate, 40 to 50% of the total [35S]methionine was detected as formylmethionine. (ii) Extracts of the mutant grown in the absence of p-aminobenzoate contained no formyl-tetrahydrofolate, but such extracts did contain formylatable methionyl-tRNA and a functional transformylase. (iii) Tetrahydrofolate-free extracts of the mutant were capable of supporting protein synthesis with viral RNA (from f2) as messenger, but the resulting synthesized proteins contained no formylmethionine, and methionine residues were detected where formylmethionine residues are normally found. In the presence of formyl-tetrahydrofolate, use of a similar extract resulted in the detection of 30 to 40% of the total polypeptide methionine as formylmethionine. (iv) Initiation of protein synthesis in vitro occurred more readily with formyl-tetrahydrofolate-free extracts of the mutant than with similar extracts prepared from the parent strain. However, in the presence of formyl-tetrahydrofolate, initiation of protein synthesis proceeded equally well with both kinds of extracts. tRNA from this mutant and another spontaneously derived mutant was found to be partially deficient in the modified nucleoside ribothymidine (rT). Analysis of extracts showed that the mutants contained decreased levels of the methylase that results in the formation of ribothymidine. In vivo studies with an independently isolated rT− strain suggest that the lack of rT in tRNA facilitates the growth of E. coli under conditions where protein synthesis is forced to take place without formylation.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Capecchi M. R. N-formylmethionyl-sRNA as the initiator of protein synthesis. Proc Natl Acad Sci U S A. 1966 Jan;55(1):147–155. doi: 10.1073/pnas.55.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björk G. R., Isaksson L. A. Isolation of mutants of Escherichia coli lac king 5-methyluracil in transfer ribonucleic acid or 1-methylguanine in ribosomal RNA. J Mol Biol. 1970 Jul 14;51(1):83–100. doi: 10.1016/0022-2836(70)90272-x. [DOI] [PubMed] [Google Scholar]
- Björk G. R., Neidhardt F. C. Physiological and biochemical studies on the function of 5-methyluridine in the transfer ribonucleic acid of Escherichia coli. J Bacteriol. 1975 Oct;124(1):99–111. doi: 10.1128/jb.124.1.99-111.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark B. F., Marcker K. A. The role of N-formyl-methionyl-sRNA in protein biosynthesis. J Mol Biol. 1966 Jun;17(2):394–406. doi: 10.1016/s0022-2836(66)80150-x. [DOI] [PubMed] [Google Scholar]
- Danchin A. Does formylation of initiator tRNA act as a regulatory signal in E. coli? FEBS Lett. 1973 Aug 15;34(2):327–332. doi: 10.1016/0014-5793(73)80823-3. [DOI] [PubMed] [Google Scholar]
- Delk A. S., Rabinowitz J. C. Biosynthesis of ribosylthymine in the transfer RNA of Streptococcus faecalis: a folate-dependent methylation not involving S-adenosylmethionine. Proc Natl Acad Sci U S A. 1975 Feb;72(2):528–530. doi: 10.1073/pnas.72.2.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delk A. S., Rabinowitz J. C. Partial nucleotide sequence of a prokaryote initiator tRNA that functions in its non-formylated form. Nature. 1974 Nov 8;252(5479):106–109. doi: 10.1038/252106a0. [DOI] [PubMed] [Google Scholar]
- Dickerman H. W., Steers E., Jr, Redfield B. G., Weissbach H. Methionyl soluble ribonucleic acid transformylase. I. Purification and partial characterization. J Biol Chem. 1967 Apr 10;242(7):1522–1525. [PubMed] [Google Scholar]
- Dube S. K., Marcker K. A., Clark B. F., Cory S. Nucleotide sequence of N-formyl-methionyl-transfer RNA. Nature. 1968 Apr 20;218(5138):232–233. doi: 10.1038/218232a0. [DOI] [PubMed] [Google Scholar]
- Eisenstadt J., Lengyel P. Formylmethionyl-tRNA dependence of amino acid incorporation in extracts of trimethoprim-treated Escherichia coli. Science. 1966 Oct 28;154(3748):524–527. [PubMed] [Google Scholar]
- Gillum A. M., Urquhart N., Smith M., RajBhandary U. L. Nucleotide sequence of salmon testes and salmon liver cytoplasmic initiator tRNA. Cell. 1975 Nov;6(3):395–405. doi: 10.1016/0092-8674(75)90189-0. [DOI] [PubMed] [Google Scholar]
- Goldman E., Lodish H. F. Inhibition of replication of ribonucleic acid bacteriophage f2 by superinfection with bacteriophage T4. J Virol. 1971 Oct;8(4):417–429. doi: 10.1128/jvi.8.4.417-429.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey R. J. Growth and initiation of protein synthesis in Escherichia coli in the presence of trimethoprim. J Bacteriol. 1973 Apr;114(1):309–322. doi: 10.1128/jb.114.1.309-322.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodish H. F. Bacteriophage f2 RNA: control of translation and gene order. Nature. 1968 Oct 26;220(5165):345–350. doi: 10.1038/220345a0. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Independent translation of the genes of bacteriophage f2 RNA. J Mol Biol. 1968 Mar 28;32(3):681–685. doi: 10.1016/0022-2836(68)90351-3. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Secondary structure of bacteriophage f2 ribonucleic acid and the initiation of in vitro protein biosynthesis. J Mol Biol. 1970 Jun 28;50(3):689–702. doi: 10.1016/0022-2836(70)90093-8. [DOI] [PubMed] [Google Scholar]
- Nishimura S. Minor components in transfer RNA: their characterization, location, and function. Prog Nucleic Acid Res Mol Biol. 1972;12:49–85. [PubMed] [Google Scholar]
- Pine M. J., Gordon B., Sarimo S. S. Protein initiation without folate in Streptococcus faecium. Biochim Biophys Acta. 1969 Apr 22;179(2):439–447. doi: 10.1016/0005-2787(69)90052-5. [DOI] [PubMed] [Google Scholar]
- RajBhandary U. L., Ghosh H. P. Studies on polynucleotides. XCI. Yeast methionine transfer ribonucleic acid: purification, properties, and terminal nucleotide sequences. J Biol Chem. 1969 Mar 10;244(5):1104–1113. [PubMed] [Google Scholar]
- SARIN P. S., ZAMECNIK P. C. ON THE STABILITY OF AMINOACYL-S-RNA TO NUCLEOPHILIC CATALYSIS. Biochim Biophys Acta. 1964 Dec 16;91:653–655. doi: 10.1016/0926-6550(64)90018-0. [DOI] [PubMed] [Google Scholar]
- Samuel C. E., D'Ari L., Rabinowitz J. C. Evidence against the folate-mediated formylation of formyl-accepting methionyl transfer ribonucleic acid in Streptococcus faecalis R. J Biol Chem. 1970 Oct 10;245(19):5115–5121. [PubMed] [Google Scholar]
- Samuel C. E., Rabinowitz J. C. Initiation of protein synthesis by folate-sufficient and folate-deficient Streptococcus faecalis R. Biochemical and biophysical properties of methionine transfer ribonucleic acid. J Biol Chem. 1974 Feb 25;249(4):1198–1206. [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Simsek M., RajBhandary U. L. The primary structure of yeast initiator transfer ribonucleic acid. Biochem Biophys Res Commun. 1972 Oct 17;49(2):508–515. doi: 10.1016/0006-291x(72)90440-8. [DOI] [PubMed] [Google Scholar]
- Simsek M., Ziegenmeyer J., Heckman J., Rajbhandary U. L. Absence of the sequence G-T-psi-C-G(A)- in several eukaryotic cytoplasmic initiator transfer RNAs. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1041–1045. doi: 10.1073/pnas.70.4.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spremulli L. L., Agris P. F., Brown G. M., Rajbhandary U. L. Escherichia coli formylmethionine tRNA: methylation of specific guanine and adenine residues catalyzed by HeLa cells tRNA methylases and the effect of these methylations on its biological properties. Arch Biochem Biophys. 1974 May;162(1):22–37. doi: 10.1016/0003-9861(74)90101-5. [DOI] [PubMed] [Google Scholar]
- Thach R. E., Dewey K. F., Brown J. C., Doty P. Formylmethionine codon AUG as an initiator of polypeptide synthesis. Science. 1966 Jul 22;153(3734):416–418. doi: 10.1126/science.153.3734.416. [DOI] [PubMed] [Google Scholar]
- Walker R. T., RajBhandary U. L. Formylatable methionine transfer RNA from Mycoplasma: purification and comparison of partial nucleotide sequences with those of other prokaryotic initiator tRNAs. Nucleic Acids Res. 1975 Jan;2(1):61–78. doi: 10.1093/nar/2.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. T., RajBhandary U. L. Studies on polynucleotides. CI. Escherichia coli tyrosine and formylmethionine transfer ribonucleic acids: effect of chemical modification of 4-thiouridine to uridine on their biological properties. J Biol Chem. 1972 Aug 10;247(15):4879–4892. [PubMed] [Google Scholar]
- Webster R. E., Engelhardt D. L., Zinder N. D. In vitro protein synthesis: chain initiation. Proc Natl Acad Sci U S A. 1966 Jan;55(1):155–161. doi: 10.1073/pnas.55.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White B. N., Bayley S. T. Methionine transfer RNAs from the extreme halophile, Halobacterium cutirubrum. Biochim Biophys Acta. 1972 Jul 31;272(4):583–587. doi: 10.1016/0005-2787(72)90513-8. [DOI] [PubMed] [Google Scholar]
- Yang S., Reinitz E. R., Gefter M. L. Role of modifications in tyrosine transfer RNA. II. Ribothymidylate-deficient tRNA. Arch Biochem Biophys. 1973 Jul;157(1):55–62. doi: 10.1016/0003-9861(73)90389-5. [DOI] [PubMed] [Google Scholar]







