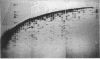
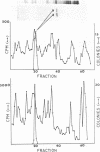
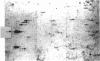
Abstract
With two-dimensional restriction enzyme analysis we have been able to cleave the Bacillus subtilis genome and resolve the resulting deoxyribonucleic acid (DNA) segments into discrete bands on agarose gels. A general procedure for gene purification has been developed by coupling multidimensional restriction analysis with a biological assay for gene detection. The organization of ribosomal ribonucleic acid (rRNA) genes was studied by hybridizing 16S and 23S rRNA probes to the two-dimensional DNA banding patterns.
Full text
PDF


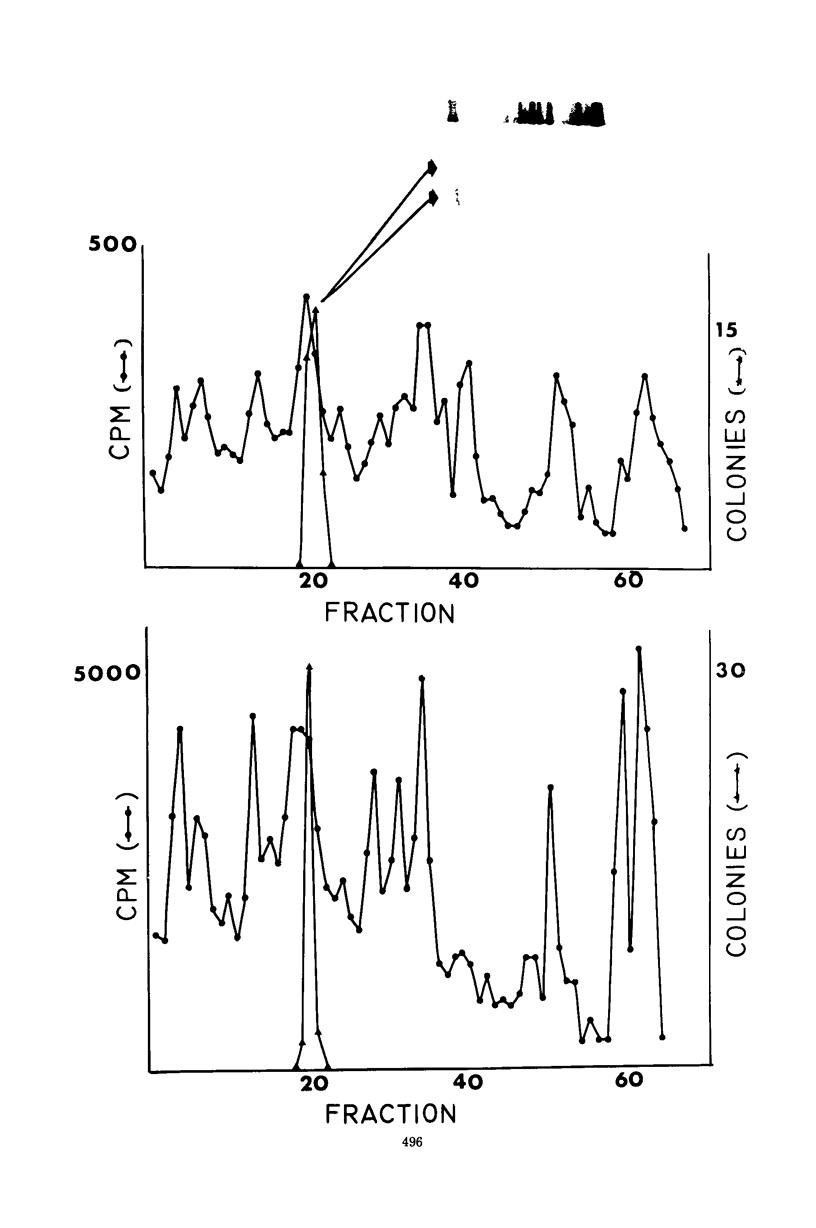
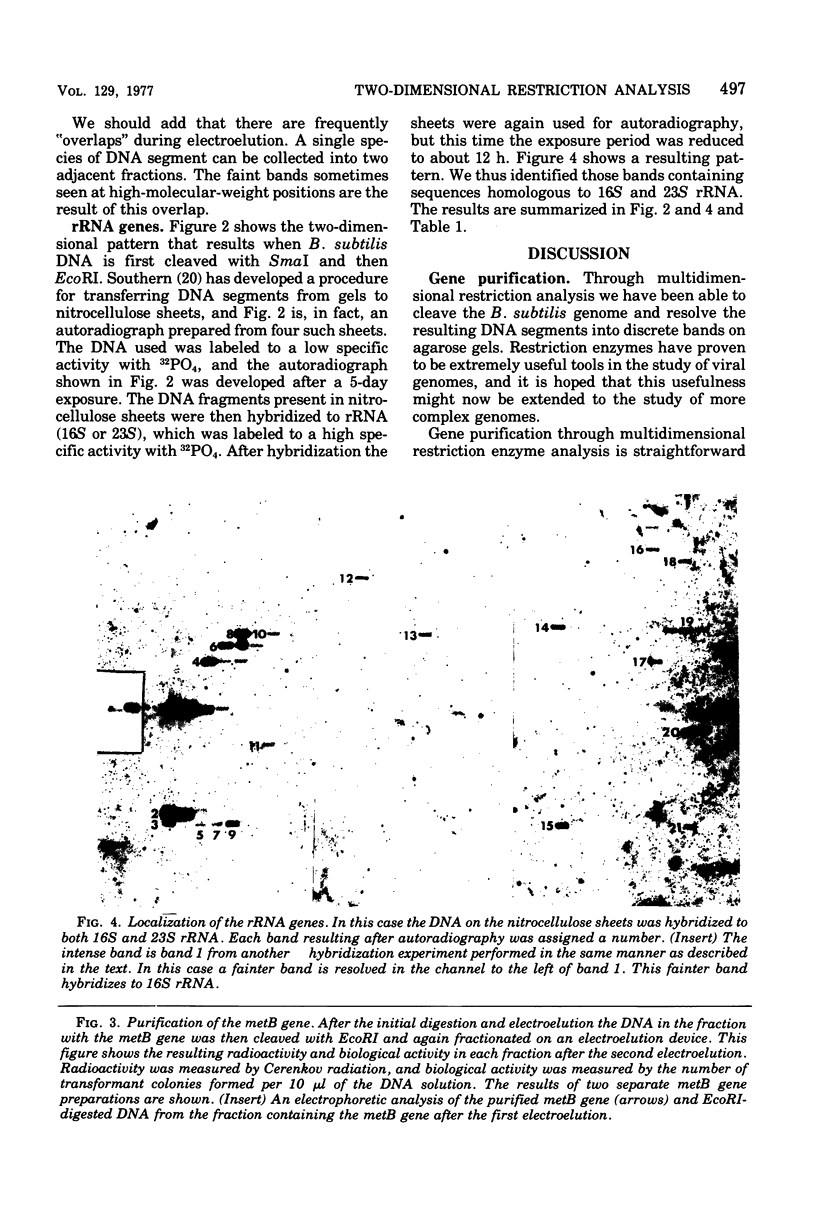



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumberg D. D., Malamy M. H. Evidence for the presence of nontranslated T7 late mRNA in infected F'(PIF+) episome-containing cells. J Virol. 1974 Feb;13(2):378–385. doi: 10.1128/jvi.13.2.378-385.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bott K. F., Wilson G. A. Metabolic and nutritional factors influencing the development of competence for transfection of Bacillus subtilis. Bacteriol Rev. 1968 Dec;32(4 Pt 1):370–378. [PMC free article] [PubMed] [Google Scholar]
- Carlton B. C., Whitt D. D. The isolation and genetic characterization of mutants of the tryptophan system of Bacillus subtilis. Genetics. 1969 Jul;62(3):445–460. doi: 10.1093/genetics/62.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow L. T., Davidson N. Electron microscope mapping of the distribution of ribosomal genes of the Bacillus subtilis chromosome. J Mol Biol. 1973 Apr 5;75(2):265–279. doi: 10.1016/0022-2836(73)90020-x. [DOI] [PubMed] [Google Scholar]
- Colli W., Smith I., Oishi M. Physical linkage between 5 s, 16 s and 23 s ribosomal RNA genes in Bacillus subtilis. J Mol Biol. 1971 Feb 28;56(1):117–127. doi: 10.1016/0022-2836(71)90088-x. [DOI] [PubMed] [Google Scholar]
- Edgell M. H., Hutchison C. A., 3rd, Sclair M. Specific endonuclease R fragments of bacteriophage phiX174 deoxyribonucleic acid. J Virol. 1972 Apr;9(4):574–582. doi: 10.1128/jvi.9.4.574-582.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith J. D. Visualization of prokaryotic DNA in a regularly condensed chromatin-like fiber. Proc Natl Acad Sci U S A. 1976 Feb;73(2):563–567. doi: 10.1073/pnas.73.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick R. M., Elkana Y., Ehrlich S. D., Lederberg J. Electrophoretic separation of Bacillus subtilis genes. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2207–2211. doi: 10.1073/pnas.72.6.2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee A. S., Sinsheimer R. L. A continuous electroelution method for the recovery of DNA restriction enzyme fragments. Anal Biochem. 1974 Aug;60(2):640–644. doi: 10.1016/0003-2697(74)90279-6. [DOI] [PubMed] [Google Scholar]
- Littauer U. Z., Inouye H. Regulation of tRNA. Annu Rev Biochem. 1973;42:439–470. doi: 10.1146/annurev.bi.42.070173.002255. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Specificity in bacterial protein synthesis: role of initiation factors and ribosomal subunits. Nature. 1970 May 23;226(5247):705–707. doi: 10.1038/226705a0. [DOI] [PubMed] [Google Scholar]
- Losick R., Sonenshein A. L. Change in the template specificity of RNA polymerase during sporulation of Bacillus subtilis. Nature. 1969 Oct 4;224(5214):35–37. doi: 10.1038/224035a0. [DOI] [PubMed] [Google Scholar]
- Lovett P. S., Bramucci M. G. Plasmid deoxyribonucleic acid in Bacillus subtilis and Bacillus pumilus. J Bacteriol. 1975 Oct;124(1):484–490. doi: 10.1128/jb.124.1.484-490.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettijohn D. E., Hecht R. RNA molecules bound to the folded bacterial genome stabilize DNA folds and segregate domains of supercoiling. Cold Spring Harb Symp Quant Biol. 1974;38:31–41. doi: 10.1101/sqb.1974.038.01.006. [DOI] [PubMed] [Google Scholar]
- Potter S. S., Newbold J. E. Multidimensional restriction enzyme analysis of complex genomes. Anal Biochem. 1976 Apr;71(2):452–458. doi: 10.1016/s0003-2697(76)80011-5. [DOI] [PubMed] [Google Scholar]
- Rutter W. J., Pictet R. L., Morris P. W. Toward molecular mechanisms of developmental processes. Annu Rev Biochem. 1973;42:601–646. doi: 10.1146/annurev.bi.42.070173.003125. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Smith I., Dubnau D., Morrell P., Marmur J. Chromosomal location of DNA base sequences complementary to transfer RNA and to 5 s, 16 s and 23 s ribosomal RNA in Bacillus subtilis. J Mol Biol. 1968 Apr 14;33(1):123–140. doi: 10.1016/0022-2836(68)90285-4. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Sterlini J. M., Mandelstam J. Commitment to sporulation in Bacillus subtilis and its relationship to development of actinomycin resistance. Biochem J. 1969 Jun;113(1):29–37. doi: 10.1042/bj1130029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Thuring R. W., Sanders J. P., Borst P. A freeze-squeeze method for recovering long DNA from agarose gels. Anal Biochem. 1975 May 26;66(1):213–220. doi: 10.1016/0003-2697(75)90739-3. [DOI] [PubMed] [Google Scholar]